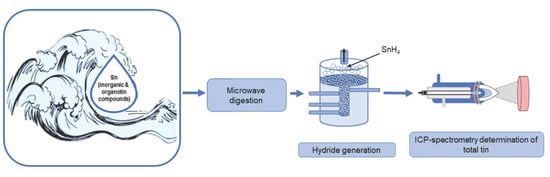
Possibilities and Limitations of ICP-Spectrometric Determination of the Total Content of Tin, Its Inorganic and Organic Speciations in Waters with Different Salinity Levels—Part 1: Determination of the Total Tin Content
Abstract
:1. Introduction
2. Results and Discussion
2.1. Conditions for the ICP-Spectrometric Determination of Tin
2.2. Influence of Chemical Forms of Tin in Water on the Analytical Signal in ICP-Spectrometric Determination
2.3. Microwave Sample Pretreatment of Waters of Different Salinity for the Determination of Total Tin
2.4. Hydride Generation as a Method of Concentration and Determination of Tin
2.4.1. Study of the Conditions for the Hydride Generation of Tin
2.4.2. Influence of Organotin Compounds in Water on the Hydride Generation of Tin
2.4.3. Influence of Water Salinity on the Determination of Tin via Hydride Generation
2.4.4. Effect of Transition Metals on the Determination of Tin Hydrides
2.5. Limits of Quantification of Tin and Analysis of Real Seawater Samples
3. Materials and Methods
3.1. Research Objects
3.2. Reagents
3.3. Instrumentation
3.3.1. Optimization of Operating Modes of Spectrometers
3.3.2. Influence of Matrix Components of Sea Waters on the Determination of Tin
3.3.3. Microwave Sample Pretreatment of Water of Different Salinity
3.3.4. Conditions for the Generation of Tin Hydrides
3.3.5. Limit of Quantification
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Cima, F. Tin: Environmental Pollution and Health Effects. In Encyclopedia of Environmental Health, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 351–359. [Google Scholar] [CrossRef]
- de Carvalho Oliveira, R.; Santelli, R.E. Occurrence and chemical speciation analysis of organotin compounds in the environment: A review. Talanta 2010, 82, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Leroy, M.J.F.; Quevauviller, P.; Donard, O.F.X.; Astruc, M. Determination of tin species in environmental samples (Technical Report). Pure Appl. Chem. 1998, 70, 2051–2064. [Google Scholar] [CrossRef]
- World Health Organization. Organotins in Drinking-Water: Background Document for Development of WHO Guidelines for Drinking-Water Quality. 2020. Available online: https://apps.who.int/iris/bitstream/handle/10665/338068/WHO-HEP-ECH-WSH-2020.7-eng.pdf?sequence=1&isAllowed=y (accessed on 28 April 2023).
- Cole, R.F.; Mills, G.A.; Parker, R.; Bolam, T.; Birchenough, A.; Kröger, S.; Fones, G.R. Trends in the analysis and monitoring of organotins in the aquatic environment. Trends Environ. Anal. Chem. 2015, 8, 1–11. [Google Scholar] [CrossRef] [Green Version]
- International Marine Organization. Anti-Fouling Systems 2002. Available online: https://www.imo.org/en/OurWork/Environment/Pages/Anti-fouling.aspx (accessed on 30 June 2022).
- Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First and Second Addenda 2022; Volume 614. Available online: https://www.who.int/publications/i/item/9789240045064 (accessed on 28 April 2023).
- Chen, B.; Wang, T.; Yin, Y.; He, B.; Jiang, G. Identification of photochemical methylation products of tin(ii) in aqueous solutions using headspace SPME coupled with GC-FPD or GC–MS. Anal. Methods 2012, 4, 2109–2114. [Google Scholar] [CrossRef]
- Kosyan, R.D.; Krylenko, M.V. Modern state and dynamics of the Sea of Azov coasts. Estuar. Coast. Shelf Sci. 2019, 224, 314–323. [Google Scholar] [CrossRef]
- Poulos, S.E. The Mediterranean and Black Sea Marine System: An overview of its physico-geographic and oceanographic characteristics. Earth-Sci. Rev. 2020, 200, 103004. [Google Scholar] [CrossRef]
- Karandashev, V.K.; Leikin, A.Y.; Khvostikov, V.A.; Kutseva, N.K.; Pirogova, S.V. Water analysis by inductively coupled plasma mass spectrometry. Inorg. Mater. 2016, 52, 1391–1404. [Google Scholar] [CrossRef]
- Nicolaï, M.; Rosin, C.; Tousset, N.; Nicolai, Y. Trace metals analysis in estuarine and seawater by ICP-MS using on line preconcentration and matrix elimination with chelating resin. Talanta 1999, 50, 433–444. [Google Scholar] [CrossRef]
- Pupyshev, A.A.; Epova, E.N. Spectral Interferences of Polyatomic Ions in Inductively Coupled Plasma Mass Spectrometry. Anal. Control 2001, 4, 335–369. [Google Scholar]
- Evans, E.H.; Giglio, J.J. Interferences in inductively coupled plasma mass spectrometry. A review. J. Anal. At. Spectrom. 1993, 8, 1–18. [Google Scholar] [CrossRef]
- Machado, R.C.; Amaral, C.D.B.; Schiavo, D.; Nóbrega, J.A.; Nogueira, A.R.A. Complex samples and spectral interferences in ICP-MS: Evaluation of tandem mass spectrometry for interference-free determination of cadmium, tin and platinum group elements. Microchem. J. 2017, 130, 271–275. [Google Scholar] [CrossRef]
- Brzezinska-Paudyn, A.; Van Loon, J.C. Determination of tin in environmental samples by graphite furnace atomic absorption and inductively coupled plasma-mass spectrometry. Fresenius Z. Anal. Chem. 1988, 331, 707–712. [Google Scholar] [CrossRef]
- Falk, H.; Geerling, R.; Hattendorf, B.; Krengel-Rothensee, K.; Schmidt, K.P. Capabilities and limits of ICP-MS for direct determination of element traces in saline solutions. Fresenius Z. Anal. Chem. 1997, 359, 352–356. [Google Scholar] [CrossRef]
- Wu, B.; Zhao, D.Y.; Jia, H.Y.; Zhang, Y.; Zhang, X.X.; Cheng, S.P. Preliminary Risk Assessment of Trace Metal Pollution in Surface Water from Yangtze River in Nanjing Section, China. Bull. Environ. Contam. Toxicol. 2009, 82, 405–409. [Google Scholar] [CrossRef]
- Biata, N.R.; Mashile, G.P.; Ramontja, J.; Mketo, N.; Nomngongo, P.N. Application of ultrasound-assisted cloud point extraction for preconcentration of antimony, tin and thallium in food and water samples prior to ICP-OES determination. J. Food Compos. Anal. 2019, 76, 14–21. [Google Scholar] [CrossRef]
- Feizbakhsh, A.; Panahi, H.A.; Makavipour, F.; Moniri, E.; Nezhati, M.N. Preconcentration of tin in environmental and biological samples by ion exchange using modified Amberlite XAD-2. Toxicol. Environ. Chem. 2013, 95, 1650–1658. [Google Scholar] [CrossRef]
- U.S. EPA. Method 3015A (SW-846): Microwave Assisted Acid Digestion of Aqueous Samples and Extracts, Revision 1. Washington, DC. 2007. Available online: https://www.epa.gov/esam/epa-method-3015a-microwave-assisted-acid-digestion-aqueous-samples-and-extracts (accessed on 21 May 2023).
- Harzdorf, C.; Janser, G.; Rinne, D.; Rogge, M. Application of microwave digestion to trace organoelement determination in water samples. Anal. Chim. Acta 1998, 374, 209–214. [Google Scholar] [CrossRef]
- Microwave Digestion of US EPA 3015 (Aqueous Samples). Available online: https://cem.com/media/contenttype/media/literature/MetNote_MARS6_US_EPA_/ (accessed on 21 May 2023).
- Currie, L.A.; Svehla, G. Nomenclature for the presentation of results of chemical analysis (IUPAC Recommendations 1994). Pure Appl. Chem. 1994, 66, 595–608. [Google Scholar] [CrossRef]
- Stauffer, M.T. Calibration and Validation of Analytical Methods—A Sampling of Current Approaches; IntechOpen: London, UK, 2018; 174p. [Google Scholar] [CrossRef]
- Bakirdere, S.; Aydin, F.; Bakirdere, E.G.; Titretir, S.; Akdeniz, I.; Aydin, I.; Yildirim, E.; Arslan, Y. From mg/kg to pg/kg Levels: A Story of Trace Element Determination: A Review. Appl. Spectrosc. Rev. 2011, 46, 38–66. [Google Scholar] [CrossRef]
- Ünal, S.; Yalçın, S. Development of a continuous flow hydride generation laser-induced breakdown spectroscopic system: Determination of tin in aqueous environments. Spectrochim. Acta Part B At. Spectrosc. 2010, 65, 750–757. [Google Scholar] [CrossRef] [Green Version]
- Welna, M.; Borkowska-Burnecka, J.; Popko, M. Ultrasound- and microwave-assisted extractions followed by hydride generation inductively coupled plasma optical emission spectrometry for lead determination in geological samples. Talanta 2015, 144, 953–959. [Google Scholar] [CrossRef]
- Kumar, A.R.; Riyazuddin, P. Chemical interferences in hydride-generation atomic spectrometry. TrAC Trends Anal. Chem. 2010, 29, 166–176. [Google Scholar] [CrossRef]
- Matusiewicz, H.; Ślachciński, M. Method development for simultaneous multi-element determination of hydride forming elements (As, Bi, Ge, Sb, Se, Sn) and Hg by microwave induced plasma-optical emission spectrometry using integrated continuous-microflow ultrasonic nebulizer-hydride generator sample introduction system. Microchem. J. 2010, 95, 213–221. [Google Scholar] [CrossRef]
- Pohl, P.; Jamroz, P.; Welna, M.; Szymczycha-Madeja, A.; Greda, K. Chemical-vapor generation of transition metals through the reaction with tetrahydroborate in recent achievements in analytical atomic spectrometry. TrAC Trends Anal. Chem. 2014, 59, 144–155. [Google Scholar] [CrossRef]
- Faías, S.; Rodíguez, R.E.; Ledesma, A.; Batistoni, D.A.; Smichowski, P. Assessment of acid media effects on the determination of tin by hydride generation-inductively coupled plasma atomic emission spectrometry. Microchem. J. 2002, 73, 79–88. [Google Scholar] [CrossRef]
- Pohl, P. Hydride generation—recent advances in atomic emission spectrometry. TrAC Trends Anal. Chem. 2004, 23, 87–101. [Google Scholar] [CrossRef]
- Pohl, P.; Sturgeon, R.E. Simultaneous determination of hydride- and non-hydride-forming elements by inductively coupled plasma optical emission spectrometry. TrAC Trends Anal. Chem. 2010, 29, 1376–1389. [Google Scholar] [CrossRef]
- Fornieles, A.C.; de Torres, A.G.; Alonso, E.I.V.; Pavón, J.M.C. Determination of antimony, bismuth and tin in natural waters by flow injection solid phase extraction coupled with online hydride generation inductively coupled plasma mass spectrometry. J. Anal. At. Spectrom. 2013, 28, 364–372. [Google Scholar] [CrossRef]
- D’Ulivo, A. Mechanisms of chemical vapor generation by aqueous tetrahydridoborate. Recent developments toward the definition of a more general reaction model. Spectrochim. Acta Part B At. Spectrosc. 2016, 119, 91–107. [Google Scholar] [CrossRef]
- Pitzalis, E.; Mascherpa, M.C.; Onor, M.; D’Ulivo, A. Mechanisms involved in stannane generation by aqueous tetrahydroborate(III): Role of acidity and l-cysteine. Spectrochim. Acta Part B At. Spectrosc. 2009, 64, 309–314. [Google Scholar] [CrossRef]
- Thomaidis, N.S.; Stasinakis, A.S.; Gatidou, G.; Morabito, R.; Massanisso, P.; Lekkas, T.D. Occurrence of Organotin Compounds in the Aquatic Environment of Greece. Wat. Air Soil Poll. 2006, 181, 201–210. [Google Scholar] [CrossRef]
- ASTM D1141-98(2021); Standard Practice for the Preparation of Substitute Ocean Water. Available online: https://www.astm.org/d1141-98r21.html (accessed on 28 April 2023).
- Howell, P.D.; Watts, P.; World Health Organization; Chemical Safety Team & International Programme on Chemical Safety. Tin and Inorganic Tin Compounds; World Health Organization: Geneva, Switzerland, 2005; 73p. [Google Scholar]
- Horne, R.A. Marine Chemistry: The Structure of Water and the Chemistry of the Hydrosphere, 1st ed.; Wiley-Interscience, A Division of John Wiley & Sons: Hoboken, NJ, USA, 1969; 568p. [Google Scholar]
- Alekin, O.A.; Lyakhin, Y.I. Ocean Chemistry; Gidrometeoizdat: Leningrad, Russia, 1984; 344p. [Google Scholar]
- Drever, J.I. The Geochemistry of Natural Waters, 2nd ed.; Prentice-Hall: Hoboken, NJ, USA, 1988; 437p. [Google Scholar]
- Beer, T. Water composition. In Environmental Oceanography, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 1983; pp. 83–89. [Google Scholar]
- Lewis, R.J. Sax’s Dangerous Properties of Industrial Materials, 9th ed.; Wiley: New York, NY, USA, 1996; 838p. [Google Scholar]
- Othmer, K. Kirk-Othmer Encyclopedia of Chemical Technology, 4th ed.; John Wiley and Sons: New York, NY, USA, 1997; 1264p. [Google Scholar]
- Larrañaga, M.D.; Lewis, J.R.; Lewis, R.A. Hawley’s Condensed Chemical Dictionary, 13th ed.; John Wiley and Sons: New York, NY, USA, 1997; 1082p. [Google Scholar]
- Lide, D.R. Handbook of Chemistry and Physics, 81st ed.; CRC Press LLC: Boca Raton, FL, USA, 2000; 2556p. [Google Scholar]
- Specification Sheet: iCAP 7400 ICP-OES. For Routine Analysis Requirements and Mid-Range Sample Throughput. Available online: https://assets.thermofisher.com/TFS-Assets/CMD/Specification-Sheets/PS-43230-ICP-OES-iCAP-7400-PS43230-EN.pdf (accessed on 13 April 2023).










| Type of Water | The Composition of the Oxidizer | Found, in Terms of Tin, μg/L | Recovery, % (Acceptance Criteria: 95% < R < 105%) | ||
|---|---|---|---|---|---|
| ICP-OES | ICP-MS | ICP-OES | ICP-MS | ||
| Deionized water | Without oxidizer | 2.2 ± 0.3 | 1.5 ± 0.3 | 44 | 30 |
| 1.2 mL HNO3 + 0.6 mL H2O2 | 3.9 ± 0.6 | 3.4 ± 0.5 | 78 | 68 | |
| 5.0 mL HNO3 | 4.9 ± 0.8 | 5.1 ± 0.7 | 98 | 101 | |
| 4.0 mL HNO3 + 1.0 mL HCl | 4.8 ± 0.7 | 4.9 ± 0.7 | 95 | 98 | |
| 2.5 mL HNO3 + 2.5 mL HCl | 4.8 ± 0.7 | 4.2 ± 0.6 | 96 | 84 | |
| 3.0 mL HNO3 + 2.0 mL H2O2 | 5.4 ± 0.8 | 5.3 ± 0.8 | 108 | 106 | |
| 1.0 mL HNO3 + 4.0 mL HCl | 5.1 ± 0.7 | 3.6 ± 0.5 | 101 | 72 | |
| Model water with a salinity of 6‰ | Without oxidizer | 2.2 ± 0.3 | 1.5 ± 0.2 | 44 | 29 |
| 1.2 mL HNO3 + 0.6 mL H2O2 | 3.8 ± 0.6 | 3.3 ± 0.5 | 75 | 67 | |
| 5.0 mL HNO3 | 4.7 ± 0.7 | 4.9 ± 0.7 | 95 | 97 | |
| 4.0 mL HNO3 + 1.0 mL HCl | 4.7 ± 0.7 | 4.7 ± 0.7 | 94 | 94 | |
| 2.5 mL HNO3 + 2.5 mL HCl | 4.4 ± 0.7 | 4.2 ± 0.6 | 89 | 85 | |
| 3.0 mL HNO3 + 2.0 mL H2O2 | 4.9 ± 0.7 | 4.9 ± 0.7 | 98 | 98 | |
| 1.0 mL HNO3 + 4.0 mL HCl | 4.8 ± 0.7 | 4.7 ± 0.7 | 97 | 94 | |
| Model water with a salinity of 18‰ | Without oxidizer | 2.3 ± 0.4 | 1.7 ± 0.3 | 46 | 35 |
| 1.2 mL HNO3 + 0.6 mL H2O2 | 3.6 ± 0.5 | 3.7 ± 0.6 | 71 | 75 | |
| 5.0 mL HNO3 | 4.8 ± 0.7 | 4.8 ± 0.7 | 97 | 96 | |
| 4.0 mL HNO3 + 1.0 mL HCl | 4.8 ± 0.7 | 4.8 ± 0.7 | 95 | 96 | |
| 2.5 mL HNO3 + 2.5 mL HCl | 4.6 ± 0.7 | 4.7 ± 0.7 | 93 | 94 | |
| 3.0 mL HNO3 + 2.0 mL H2O2 | 4.9 ± 0.7 | 4.9 ± 0.7 | 98 | 99 | |
| 1.0 mL HNO3 + 4.0 mL HCl | 4.4 ± 0.7 | 4.5 ± 0.7 | 88 | 90 | |
| Analyte | Added Total Tin Concentration, μg/L | Found, μg/L | Recovery, % (Acceptance Criteria: 95% < R < 105%) | ||
|---|---|---|---|---|---|
| ICP-OES | ICP-MS | ICP-OES | ICP-MS | ||
| Tin without microwave digestion determined | 0.10 | <0.05 | <0.02 | – | – |
| 0.50 | 0.17 ± 0.03 | 0.10 ± 0.02 | 34 | 20 | |
| 1.00 | 0.26 ± 0.05 | 0.19 ± 0.04 | 26 | 19 | |
| 5.00 | 1.3 ± 0.3 | 1.0 ± 0.2 | 26 | 20 | |
| Inorganic tin determined after mineralizate redissolution | 0.10 | 0.09 ± 0.02 | 0.11 ± 0.02 | 90 | 91 |
| 0.50 | 0.5 ± 0.1 | 0.5 ± 0.1 | 94 | 108 | |
| 1.00 | 1.0 ± 0.2 | 1.0 ± 0.2 | 99 | 105 | |
| 5.00 | 5 ± 1 | 5 ± 1 | 100 | 101 | |
| Total content of tin after microwave digestion and redissolution of mineralizate | 0.10 | 0.10 ± 0.02 | 0.11 ± 0.02 | 100 | 110 |
| 0.50 | 0.5 ± 0.1 | 0.4 ± 0.1 | 94 | 88 | |
| 1.00 | 1.0 ± 0.2 | 0.9 ± 0.2 | 98 | 90 | |
| 5.00 | 5 ± 1 | 5 ± 1 | 99 | 102 | |
| Masking Agent | Concentration of Masking Agent, g/L | Found Concentration of Tin, μg/L | Recovery, % (Acceptance Criteria: 95% < R < 105%) | ||
|---|---|---|---|---|---|
| ICP-OES | ICP-MS | ICP-OES | ICP-MS | ||
| EDTA | 0.0005 | 0.4 ± 0.1 | 0.5 ± 0.1 | 40 | 50 |
| 0.0010 | 0.6 ± 0.1 | 0.6 ± 0.1 | 60 | 60 | |
| 0.0015 | 0.5 ± 0.1 | 0.6 ± 0.1 | 50 | 60 | |
| 0.0020 | 0.5 ± 0.1 | 0.5 ± 0.1 | 50 | 50 | |
| L-cysteine | 0.50 | 0.9 ± 0.2 | 0.9 ± 0.2 | 90 | 90 |
| 0.75 | 1.0 ± 0.2 | 1.0 ± 0.2 | 100 | 100 | |
| 1.00 | 0.9 ± 0.2 | 0.9 ± 0.2 | 90 | 94 | |
| 1.25 | 0.8 ± 0.2 | 0.9 ± 0.2 | 80 | 90 | |
| C4H6O6 | 1.00 | 0.6 ± 0.1 | 0.6 ± 0.1 | 60 | 60 |
| 2.00 | 0.6 ± 0.1 | 0.6 ± 0.1 | 60 | 60 | |
| 3.00 | 0.6 ± 0.1 | 0.6 ± 0.1 | 60 | 60 | |
| 4.00 | 0.5 ± 0.1 | 0.5 ± 0.1 | 50 | 50 | |
| KI | 0.05 | 0.5 ± 0.1 | 0.5 ± 0.1 | 50 | 50 |
| 0.10 | 0.5 ± 0.1 | 0.5 ± 0.1 | 50 | 50 | |
| 0.50 | 0.5 ± 0.1 | 0.5 ± 0.1 | 50 | 50 | |
| 1.00 | 0.5 ± 0.1 | 0.4 ± 0.1 | 50 | 40 | |
| CH4N2S | 0.50 | 0.5 ± 0.1 | 0.5 ± 0.1 | 50 | 50 |
| 0.75 | 0.9 ± 0.2 | 0.8 ± 0.2 | 90 | 80 | |
| 1.00 | 0.7 ± 0.2 | 0.7 ± 0.2 | 70 | 70 | |
| 1.25 | 0.6 ± 0.1 | 0.6 ± 0.1 | 60 | 60 | |
| Object of Study | Limits of Quantification, μg/L | |||
|---|---|---|---|---|
| Direct Injection | Hydride Generation | |||
| ICP-OES | ICP-MS | ICP-OES | ICP-MS | |
| Deionized water | 0.32 | 0.03 | 0.05 | 0.03 |
| Model water with a salinity of 6‰ | 0.40 | 0.37 | 0.05 | 0.03 |
| Model water with a salinity of 18‰ | 0.47 | 0.45 | 0.05 | 0.03 |
| Sample Injection | Water Sample | Sample Analyte Concentration, μg/L | Introduced, μg/L | Found, μg/L | Recovery, % | ||||
|---|---|---|---|---|---|---|---|---|---|
| ICP-OES | ICP-MS | ICP-OES | ICP-MS | ICP-OES | ICP-MS | ICP-OES | ICP-MS | ||
| direct injection | Azov sea | <0.40 | <0.37 | 1.00 | 1.2 ± 0.2 | 1.2 ± 0.2 | 100 | 99 | |
| Black sea | <0.47 | <0.45 | 1.2 ± 0.2 | 1.3 ± 0.2 | 97 | 98 | |||
| hydride generation | Azov sea | 0.17 ± 0.03 | 0.16 ± 0.03 | 0.10 | 0.26 ± 0.05 | 0.27 ± 0.05 | 90 | 91 | |
| Black sea | 0.25 ± 0.05 | 0.23 ± 0.05 | 0.35 ± 0.07 | 0.34 ± 0.07 | 100 | 91 | |||
| Parameter | iCAP RQ Mass Spectrometer | iCAP-7400 Spectrometer (Axial Overview of Plasma) | |
|---|---|---|---|
| Analyte | 120Sn | Sn II 189.989 nm | |
| Applied power, W | 1300 | 1150 | |
| Argon flowrate, L/min | Plasma-forming (cooling) | 15 | 12 |
| Auxiliary | 0.80 | 0.50 | |
| Nebulizer | 1.10 | 0.50 | |
| Peristaltic pump speed, rpm | 40 | 50 | |
| Sample rate, mL/min | 0.4 | ||
| Parameter | iCAP RQ Mass Spectrometer | iCAP-7400 Spectrometer (Axial Overview of Plasma) | |
|---|---|---|---|
| Analyte | 120Sn | Sn II 189.989 nm | |
| Applied power, W | 1300 | 1150 | |
| Argon flowrate, L/min | Plasma-forming (cooling) | 15 | 12 |
| Auxiliary | 0.80 | 0.50 | |
| Nebulizer | 0.45 | ||
| Peristaltic pump speed, rpm | 60 | 30 | |
| Sample injection | Hydride system: oxidizer—0.10 mol/L HCl; reducing agent—0.50 mol/L NaBH4 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Temerdashev, Z.; Abakumov, P.; Bolshov, M.; Abakumova, D.; Pupyshev, A. Possibilities and Limitations of ICP-Spectrometric Determination of the Total Content of Tin, Its Inorganic and Organic Speciations in Waters with Different Salinity Levels—Part 1: Determination of the Total Tin Content. Molecules 2023, 28, 5967. https://doi.org/10.3390/molecules28165967
Temerdashev Z, Abakumov P, Bolshov M, Abakumova D, Pupyshev A. Possibilities and Limitations of ICP-Spectrometric Determination of the Total Content of Tin, Its Inorganic and Organic Speciations in Waters with Different Salinity Levels—Part 1: Determination of the Total Tin Content. Molecules. 2023; 28(16):5967. https://doi.org/10.3390/molecules28165967
Chicago/Turabian StyleTemerdashev, Zaual, Pavel Abakumov, Mikhail Bolshov, Darya Abakumova, and Alexander Pupyshev. 2023. "Possibilities and Limitations of ICP-Spectrometric Determination of the Total Content of Tin, Its Inorganic and Organic Speciations in Waters with Different Salinity Levels—Part 1: Determination of the Total Tin Content" Molecules 28, no. 16: 5967. https://doi.org/10.3390/molecules28165967
APA StyleTemerdashev, Z., Abakumov, P., Bolshov, M., Abakumova, D., & Pupyshev, A. (2023). Possibilities and Limitations of ICP-Spectrometric Determination of the Total Content of Tin, Its Inorganic and Organic Speciations in Waters with Different Salinity Levels—Part 1: Determination of the Total Tin Content. Molecules, 28(16), 5967. https://doi.org/10.3390/molecules28165967