Screening the Extract of Laportea bulbifera (Sieb. et Zucc.) Wedd. Based on Active Component Content, Its Antioxidant Capacity and Exploration of Hepatoprotective Activity in Rats
Abstract
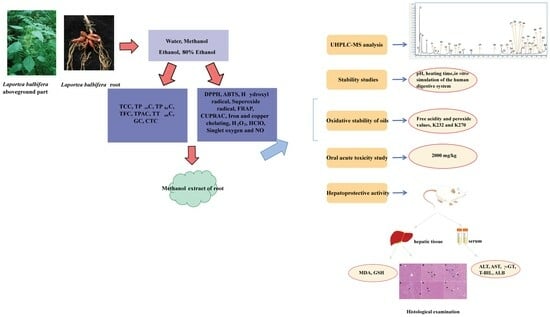
:1. Introduction
2. Results and Discussion
2.1. Qualitative Phytochemical Analysis
2.2. Yields
2.3. Quantitative Phytochemical Analysis
2.3.1. Total Carbohydrate Content (TCC)
2.3.2. Total Protein Content (TProC)
2.3.3. Total Phenolic Content (TPheC)
2.3.4. Total Flavonoid Content (TFC)
2.3.5. Total Phenolic Acid Content (TPAC)
2.3.6. Total Tannin Content (TTanC), Gallotannin Content (GC) and Condensed Tannin Content (CTC)
2.4. Antioxidant Activity In Vitro
2.4.1. DPPH and 2,2’-Azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) Diammonium Salt Cation Radicals (ABTS) Tests
2.4.2. Hydroxyl Radicals and Superoxide Radicals Tests
2.4.3. Ferric-Reducing Antioxidant Power (FRAP) and Cupric Ion Reducing Antioxidant Capacity (CUPRAC) Tests
2.4.4. Metal Chelating Tests
2.4.5. Hydrogen Peroxide (H2O2) and Singlet Oxygen Tests
2.4.6. Hypochlorous Acid (HClO) Tests
2.4.7. Nitric Oxide (NO) Tests
2.5. UHPLC-MS Analysis
2.6. Stability Studies of Methanol Extract of LBR
2.7. Oxidative Stability Studies of Oils
Primarily Oxidation and Polymerization
2.8. Oral Acute Toxicity Study
2.9. Hepatoprotective Activity
3. Material and Methods
3.1. Materials
3.2. Methods
3.2.1. Qualitative Phytochemical Analysis
3.2.2. Quantitative Phytochemical Analysis
3.2.3. Antioxidant Activity Assays
3.2.4. Stability Studies of Methanol Extract
3.2.5. Oxidative Stability of Oils
3.2.6. Oral Acute Toxicity Study
3.2.7. Hepatoprotective Experiments
3.2.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Flora of China Editorial Committee of Chinese Academy of Sciences. The Flora of China; Science Press: Beijing, China, 1995; Volume 23, pp. 31–32. [Google Scholar]
- Feng, J.X.; Sun, Y.; Xia, G.Q.; Li, L.; Zang, H. Research progress on chemical constituents and pharmacological effects of Laportea plants. Ginseng Res. 2023, 35, 38–42. [Google Scholar] [CrossRef]
- Fu, L.G.; Chen, T.Q.; Lang, K.Y.; Hong, T.; Lin, Q. Higher Plants of China; Qingdao Publishing House: Qingdao, China, 2000; Volume 4. [Google Scholar]
- The Editorial Committee of Chinese Herbals. Chinese Herbals; Shanghai Scientific & Technical Publishers: Shanghai, China, 1999; Volume 4. [Google Scholar]
- Wang, M.M.; Li, Y.N.; Ming, W.K.; Wu, P.F.; Yi, P.; Gong, Z.P.; Hao, X.J.; Yuan, C.M. Bioassay-guided isolation of human carboxylesterase 2 inhibitory and antioxidant constituents from Laportea bulbifera: Inhibition interactions and molecular mechanism. Arabian J. Chem. 2022, 15, 103723. [Google Scholar] [CrossRef]
- Chen, Y.; Zou, S.; Xu, W.; Sun, Q.; Yun, L. Spectrum-effect relationship of antioxidant and anti-inflammatory activities of Laportea bulbifera based on multivariate statistical analysis. Biomed. Chromatogr. 2020, 34, 4734. [Google Scholar] [CrossRef]
- Luo, X.; Li, L.L.; Zhang, S.S.; Lu, J.L.; Zeng, Y.; Zhang, H.Y.; Xiang, M. Therapeutic effects of total coumarins from Urtica dentata Hand on collagen-induced arthritis in balb/c mice. J. Ethnopharmacol. 2011, 138, 523–529. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Lu, Q.; Xing, D.; Zhang, R. Exploring carbohydrates for therapeutics: A review on future directions. Front. Pharmacol. 2021, 12, 756724. [Google Scholar] [CrossRef] [PubMed]
- Badshah, S.L.; Faisal, S.; Muhammad, A.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Antiviral activities of flavonoids. Biomed. Pharmacother. 2021, 140, 111596. [Google Scholar] [CrossRef]
- Lu, X.; Zhao, Y.; Li, B.; Feng, W.; Qi, J.; Feng, B. Phytochemical, chemotaxonomic and bioinformatics study on Laportea bulbifera (Urticaceae). Chem. Biodivers. 2022, 19, 202200070. [Google Scholar] [CrossRef]
- Tan, M.; Nawaz, M.A.; Buckow, R. Functional and food application of plant proteins—A review. Food Rev. Int. 2023, 39, 2428–2456. [Google Scholar] [CrossRef]
- Di, L.C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and human health: The role of bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef]
- Wang, J.; Xu, J.; Gong, X.; Yang, M.; Zhang, C.; Li, M. Biosynthesis, chemistry, and pharmacology of polyphenols from Chinese Salvia species: A review. Molecules 2019, 24, 155. [Google Scholar] [CrossRef]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, X.; Li, B.; Yu, D.Y.; Feng, B.M. Flavonoids from Laportea bulbifera and their anti-N1 neuraminidase activities. J. Shenyang Pharm. Univ. 2018, 35, 931–935, 942. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, Y.; Xue, J.; Yuan, J.; Cai, Z.; Wu, N.; Zou, L.; Yin, S.; Yang, W.; Liu, X.; et al. Quality evaluation of Tetrastigmae radix from two different habitats based on simultaneous determination of multiple bioactive constituents combined with multivariate statistical analysis. Molecules 2022, 27, 4813. [Google Scholar] [CrossRef]
- Tang, J.; Wu, D.; Chen, S.Y.; Li, Y.; Li, J.; Gong, Z.P.; Li, W.W.; Lan, Y.Y.; Wang, Y.L. Identification of chemical compositions in Laportea bulbifera by UPLC-ESI-Q-TOF-MS. Chin. J. Exp. Tradit. Med. Formulae 2018, 24, 67–72. [Google Scholar] [CrossRef]
- Das, A.K.; Islam, M.N.; Faruk, M.O.; Ashaduzzaman, M.; Dungani, R. Review on tannins: Extraction processes, applications and possibilities. S. Afr. J. Bot. 2020, 135, 58–70. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, Y.; Zhou, Y.; Liu, Z.; Liu, R. Pharmacological mechanism of natural drugs and their active ingredients in the treatment of arrhythmia via calcium channel regulation. Biomed. Pharmacother. 2023, 160, 114413. [Google Scholar] [CrossRef]
- Zhang, J.; Hou, X.; Ahmad, H.; Zhang, H.; Zhang, L.; Wang, T. Assessment of free radicals scavenging activity of seven natural pigments and protective effects in AAPH-challenged chicken erythrocytes. Food Chem. 2014, 145, 57–65. [Google Scholar] [CrossRef]
- Mahlangeni, N.T.; Moodley, R.; Jonnalagadda, S.B. Nutritional value, antioxidant and antidiabetic properties of nettles (Laportea alatipes and Obetia tenax). Sci. Rep. 2020, 10, 9762. [Google Scholar] [CrossRef] [PubMed]
- Peteros, N.P.; Uy, M.M. Antioxidant and cytotoxic activities and phytochemical screening of four Philippine medicinal plants. J. Med. Plants Res. 2010, 4, 407–414. [Google Scholar]
- Gulcin, I. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [PubMed]
- Oloyede, G.K.; Oyelola, M.S. Chrysen-2-ol derivative from west Indian wood nettle Laportea aestuans (L.) Chew inhibits oxidation and microbial growth in vitro. Excli J. 2013, 12, 894–906. [Google Scholar] [PubMed]
- Omotosho, O.E.; Ogunlade, D. Protective effect of Larportea aestuans extract on diclofenac-induced oxidative stress in the brain of male wistar rats. FASEB J. 2018, 32, 538.13. [Google Scholar] [CrossRef]
- Tunnisa, F.; Nur, F.D.; Afriyanti, A.; Rosalina, D.; Ana, S.M.; Darmawan, N.; Dewi, Y.N. Antioxidant and antidiabetic compounds identification in several Indonesian underutilized Zingiberaceae spices using SPME-GC/MS-based volatilomics and in silico methods. Food Chem. X 2022, 14, 100285. [Google Scholar] [CrossRef] [PubMed]
- Lomozová, Z.; Hrubša, M.; Conte, P.F.; Papastefanaki, E.; Moravcová, M.; Catapano, M.C.; Proietti, S.I.; Karlíčková, J.; Kučera, R.; Macáková, K.; et al. The effect of flavonoids on the reduction of cupric ions, the copper-driven fenton reaction and copper-triggered haemolysis. Food Chem. 2022, 394, 133461. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Yu, M.; Tian, Z.; Cui, Y.; Deng, D.; Rong, T.; Liu, Z.; Song, M.; Li, Z.; Ma, X.; et al. Exogenous glutathione protects IPEC-J2 cells against oxidative stress through a mitochondrial mechanism. Molecules 2022, 27, 2416. [Google Scholar] [CrossRef]
- Kim, S.Y.; Park, J.W. Cellular defense against singlet oxygen-induced oxidative damage by cytosolic NADP+-dependent isocitrate dehydrogenase. Free Radic. Res. 2003, 37, 309–316. [Google Scholar] [CrossRef]
- Joachim, D. Wound cleansing: Benefits of hypochlorous acid. J. Wound Care 2020, 29, S4–S8. [Google Scholar] [CrossRef]
- Rana, T. Influence and implications of the molecular paradigm of nitric oxide underlying inflammatory reactions of the gastrointestinal tract of dog: A major hallmark of inflammatory bowel disease. Inflamm. Bowel Dis. 2022, 28, 1280–1288. [Google Scholar] [CrossRef]
- Guan, R.; Van, L.Q.; Yang, H.; Zhang, D.; Gu, H.; Yang, Y.; Sonne, C.; Lam, S.S.; Zhong, J.; Zhu, J.; et al. A review of dietary phytochemicals and their relation to oxidative stress and human diseases. Chemosphere 2021, 271, 129499. [Google Scholar] [CrossRef]
- Richez, C.; Morel, J.; Cornec, D.; Daïen, C.; Goupille, P.; Lazaro, E.; Lequerré, T.; Nocturne, G.; De, L.V.; Le, G.B.; et al. Practical management of patients on Janus kinase inhibitor (JAKi) therapy: Practical fact sheets drawn up by the rheumatism and inflammation club (CRI), a group endorsed by the French society for rheumatology (SFR). Jt. Bone Spine 2019, 86, eS2–eS103. [Google Scholar] [CrossRef]
- Kewlani, P.; Singh, L.; Singh, B.; Bhatt, I.D. Sustainable extraction of phenolics and antioxidant activities from Prinsepia utilis byproducts for alleviating aging and oxidative stress. Sustain. Chem. Pharm. 2022, 29, 100791. [Google Scholar] [CrossRef]
- Ismail, B.B.; Pu, Y.; Guo, M.; Ma, X.; Liu, D. LC-MS/QTOF identification of phytochemicals and the effects of solvents on phenolic constituents and antioxidant activity of baobab (Adansonia digitata) fruit pulp. Food Chem. 2019, 277, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, A.; Hu, S.; Ares, I.; Martínez-Larrañaga, M.R.; Wang, X.; Martínez, M.; Anadón, A.; Martínez, M.A. Synthetic phenolic antioxidants: Metabolism, hazards and mechanism of action. Food Chem. 2021, 353, 129488. [Google Scholar] [CrossRef]
- Sultana, B.; Anwar, F.; Przybylski, R. Antioxidant potential of corncob extracts for stabilization of corn oil subjected to microwave heating. Food Chem. 2007, 104, 997–1005. [Google Scholar] [CrossRef]
- Oloyede, G.K. Toxicity, antimicrobial and antioxidant activities of methyl salicylate dominated essential oils of Laportea aestuans (Gaud). Arabian J. Chem. 2016, 9, S840–S845. [Google Scholar] [CrossRef]
- Michalopoulos, G.K.; Bhushan, B. Liver regeneration: Biological and pathological mechanisms and implications. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 40–55. [Google Scholar] [CrossRef] [PubMed]
- Chinnappan, R.; Mir, T.A.; Alsalameh, S.; Makhzoum, T.; Adeeb, S.; Al-Kattan, K.; Yaqinuddin, A. Aptasensors are conjectured as promising ALT and AST diagnostic tools for the early diagnosis of acute liver injury. Life 2023, 13, 1273. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Yang, Y.; Tang, L.; Wan, J.; Dai, J.; Li, L.; Huang, J.; Shen, Y.; Lin, L.; Gong, X.; et al. Therapeutic benefits of apocynin in mice with lipopolysaccharide/d-galactosamine-induced acute liver injury via suppression of the late stage pro-apoptotic AMPK/JNK pathway. Biomed. Pharmacother. 2020, 125, 110020. [Google Scholar] [CrossRef]
- Elizabeth, O.O.; Olawumi, O.O. Therapeutic effect of methanolic extract of Laportea aestuans (L.) Chew, on oxidative stress in the brain of male wistar rats. In AIP Conference Proceedings 2018; AIP Publishing: Melville, NY, USA, 2018; Volume 1954, p. 040008. [Google Scholar] [CrossRef]
- Chen, M.H.; He, X.; Sun, H.; Sun, Y.; Li, L.; Zhu, J.Y.; Xia, G.Q.; Guo, X.; Zang, H. Phytochemical analysis, UPLC-ESI-Orbitrap-MS analysis, biological activity, and toxicity of extracts from Tripleurospermum limosum (Maxim.) Pobed. Arabian J. Chem. 2022, 15, 103797. [Google Scholar] [CrossRef]
- Sun, H.; Chen, M.H.; He, X.; Sun, Y.; Feng, J.X.; Guo, X.; Zhu, J.Y.; Xia, G.Q.; Zang, H. Phytochemical analysis and in vitro and in vivo antioxidant properties of Plagiorhegma dubia Maxim as a medicinal crop for diabetes treatment. Arabian J. Chem. 2023, 16, 104788. [Google Scholar] [CrossRef]
- Wang, S.; Bi, Y.; Quan, W.; Christie, P. Growth and metabolism of dark septate endophytes and their stimulatory effects on plant growth. Fungal Biol. 2022, 126, 674–686. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.W.; Wong, M.Y.; Lam, W.; Cheng, Y.C.; Che, C.M.; Ng, K.M. Molecular histology analysis by matrix-assisted laser desorption/ionization imaging mass spectrometry using gold nanoparticles as matrix. Rapid Commun. Mass Spectrom. 2011, 25, 3690–3696. [Google Scholar] [CrossRef]
- Papadi, G.; Wesseling, S.; Troganis, A.N.; Vervoort, J.; Rietjens, I.M.C.M. Induction of EpRE-mediated gene expression by a series of mediterranean botanicals and their constituents. J. Ethnopharmacol. 2019, 240, 111940. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Liu, S.; Qiu, J.; Lin, H.; Li, D.; Jiang, J. Identification and quality evaluation of Chinese rice wine using UPLC-PDA-QTOF/MS with dual-column separation. Phytomedicine 2023, 108, 154498. [Google Scholar] [CrossRef]
- Yao, Y.; Yu, Y.C.; Cai, M.R.; Zhang, Z.Q.; Bai, J.; Wu, H.M.; Li, P.; Zhao, T.T.; Ni, J.; Yin, X.B. UPLC-MS/MS method for the determination of the herb composition of Tangshen formula and the in vivo pharmacokinetics of its metabolites in rat plasma. Phytochem. Anal. 2022, 33, 402–426. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.X. Study on the Mechanism of Anti-Hyperlipidemia Effects and Its Pharmacodynamics Substantial Foundation of Laportea bulbifera. Ph.D. Thesis, Hubei University of Chinese Medicine, Wuhan, China, 2018. [Google Scholar]
- Han, H.Y. Study on the Anti-Inflammatory Material Basis and Preliminary Metabolism In Vivo of the Ethnic Medicine Laportea bulbifera. Ph.D. Thesis, Beijing University of Chinese Medicine, Beijing, China, 2018. [Google Scholar]
- Widelski, J.; Okińczyc, P.; Suśniak, K.; Malm, A.; Paluch, E.; Sakipov, A.; Zhumashova, G.; Ibadullayeva, G.; Sakipova, Z.; Korona-Glowniak, I. Phytochemical profile and antimicrobial potential of propolis samples from Kazakhstan. Molecules 2023, 28, 2984. [Google Scholar] [CrossRef]
- Yang, M.C.; Choi, S.Z.; Lee, S.O.; Chung, A.K.; Nam, J.H.; Lee, K.H.; Lee, K.R. Flavonoid constituents and their antioxidant activity of Laportea bulbifera Weddell. Korean J. Pharmacogn. 2003, 34, 18–24. [Google Scholar]
- Zhang, X.; Wang, L.; Li, X.; Zhang, S.; Hou, H.; Zhang, M.; He, Q.; Liu, K. Preparation and characterization of young Prunus persica fruit fraction and its anti-inflammatory effect on a transgenic zebrafish model. Nat. Prod. Res. 2022, 36, 5048–5052. [Google Scholar] [CrossRef]
- Lee, J.P.; Kang, M.G.; Lee, J.Y.; Oh, J.M.; Baek, S.C.; Leem, H.H.; Park, D.; Cho, M.L.; Kim, H. Potent inhibition of acetylcholinesterase by sargachromanol I from Sargassum siliquastrum and by selected natural compounds. Bioorg. Chem. 2019, 89, 103043. [Google Scholar] [CrossRef]
- Xu, J.F.; Li, F.S.; Feng, Z.M.; Jiang, J.S.; Zhang, P.C. A new sesquiterpenoid from Mallotus apelta. Chem. Nat. Compd. 2011, 47, 218–219. [Google Scholar] [CrossRef]
- Yang, D.; Jia, X.; Xie, H. Heptyl vicianoside and methyl caramboside from sour star fruit. Nat. Prod. Res. 2019, 33, 1233–1236. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.M.; Wen, H.C.; Dou, D.Q. Chemical constituents from Laportea bulbifera, a Zhuang medicine. Chin. Pharm. J. 2019, 54, 773–776. [Google Scholar]
- Pandey, G.; Koley, S.; Talukdar, R.; Sahani, P.K. Cross-dehydrogenating coupling of aldehydes with amines/R-OTBS ethers by visible-light photoredox catalysis: Synthesis of amides, esters, and ureas. Org. Lett. 2018, 20, 5861–5865. [Google Scholar] [CrossRef]
- Marchetti, P.M.; Richardson, S.M.; Kariem, N.M.; Campopiano, D.J. Synthesis of N-acyl amide natural products using a versatile adenylating biocatalyst. MedChemComm 2019, 10, 1192–1196. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, Y.; Feng, B.M.; Yu, D.Y.; Li, B. Research of chemical composition in Laportea bulbifera. In Abstracts of the 30th Annual Meeting of the Chinese Chemical Society-Chapter 9: Organic Chemistry; Chinese Chemical Society: Dalian, China, 2016. [Google Scholar]
- Zhang, Z.; Xu, H.; Zhao, H.; Geng, Y.; Ren, Y.; Guo, L.; Shi, J.; Xu, Z. Edgeworthia gardneri (Wall.) Meisn. water extract improves diabetes and modulates gut microbiota. J. Ethnopharmacol. 2019, 239, 111854. [Google Scholar] [CrossRef]
- Herbert, M.B.; Marx, V.M.; Pederson, R.L.; Grubbs, R.H. Concise syntheses of insect pheromones using z-selective cross metathesis. Angew. Chem. Int. Ed. Engl. 2013, 52, 310–314. [Google Scholar] [CrossRef] [PubMed]
- Torres-Benítez, A.; Ortega-Valencia, J.E.; Sanchez, M.; Divakar, P.K.; Simirgiotis, M.J.; Gómez-Serranillos, M.P. Metabolomic profiling, antioxidant and enzyme inhibition properties and molecular docking analysis of Antarctic lichens. Molecules 2022, 27, 8086. [Google Scholar] [CrossRef]
- Saeed, M.M.; Fernández-Ochoa, Á.; Saber, F.R.; Sayed, R.H.; Cádiz-Gurrea, M.L.; Elmotayam, A.K.; Leyva-Jiménez, F.J.; Segura-Carretero, A.; Nadeem, R.I. The potential neuroprotective effect of Cyperus esculentus L. extract in scopolamine-induced cognitive impairment in rats: Extensive biological and metabolomics approaches. Molecules 2022, 27, 7118. [Google Scholar] [CrossRef]
- Pan, X.Q.; Irwin, A.J.; Leonard, M.; Welsby, D. Choline and ethanolamine decompose lipid hydroperoxides into hydroxyl lipids. J. Am. Oil Chem. Soc. 2010, 87, 1235–1245. [Google Scholar] [CrossRef]
- Xue, Y.; Guo, C.; Hu, F.; Liu, J.; Mao, S. Hepatic metabolic profile reveals the adaptive mechanisms of ewes to severe undernutrition during late gestation. Metabolites 2018, 8, 85. [Google Scholar] [CrossRef]
- Lee, J.; Jung, Y.; Shin, J.H.; Kim, H.K.; Moon, B.C.; Ryu, D.H.; Hwang, G.S. Secondary metabolite profiling of Curcuma species grown at different locations using GC/TOF and UPLC/Q-TOF MS. Molecules 2014, 19, 9535–9551. [Google Scholar] [CrossRef] [PubMed]













| Phytochemical | Type of Test | Sample Solution of LBAP | Sample Solution of LBR | ||||
|---|---|---|---|---|---|---|---|
| Water | Methanol | Petroleum Ether | Water | Methanol | Petroleum Ether | ||
| Proteins/amino acids | 1. Ninhydrin test | + | ○ | ○ | + | ○ | ○ |
| 2. Biuret test | + | ○ | ○ | + | ○ | ○ | |
| Carbohydrates | 1. Fehling’s test | + | ○ | ○ | + | ○ | ○ |
| 2. Benedict’s test | + | ○ | ○ | + | ○ | ○ | |
| 3. Molisch’s test | + | ○ | ○ | + | ○ | ○ | |
| Phenolics | 1. FeCl3 test | + | ○ | ○ | + | ○ | ○ |
| 2. FeCl3-K3[Fe(CN)6] test | + | ○ | ○ | + | ○ | ○ | |
| 3. Diazotization test | + | ○ | ○ | + | ○ | ○ | |
| Organic acids | 1. Blue litmus paper test | + | ○ | ○ | + | ○ | ○ |
| 2. Bromocresol green test | + | ○ | ○ | + | ○ | ○ | |
| Tannins | 1. FeCl3 test | + | ○ | ○ | + | ○ | ○ |
| 2. Bromine water test | + | ○ | ○ | + | ○ | ○ | |
| 3. Lead acetate test | + | ○ | ○ | + | ○ | ○ | |
| 4. Lime water test | + | ○ | ○ | + | ○ | ○ | |
| Flavonoids | 1. Shinoda test | ○ | + | ○ | ○ | – | ○ |
| 2. Alkaline reagent test | ○ | + | ○ | ○ | – | ○ | |
| 3. AlCl3 test | ○ | + | ○ | ○ | – | ○ | |
| 4. Lead acetate test | ○ | + | ○ | ○ | – | ○ | |
| Saponins | 1. Foam test | – | ○ | ○ | – | ○ | ○ |
| Steroids and triterpenoids | 1. Liebermann–Burchard test | ○ | + | ○ | ○ | + | ○ |
| 2. Salkowski test | ○ | + | ○ | ○ | + | ○ | |
| Terpenoids | 1. CHCl3-H2SO4 test | ○ | ○ | – | ○ | ○ | – |
| 2. Vanillin-H2SO4 test | ○ | ○ | – | ○ | ○ | – | |
| Alkaloids | 1. Bertrad’s reagent | ○ | + | ○ | ○ | + | ○ |
| 2. Dragendorff’s reagent | ○ | + | ○ | ○ | + | ○ | |
| 3. Mayer’s reagent | ○ | + | ○ | ○ | + | ○ | |
| Anthraquinones | 1. Borntrager’s test | ○ | – | ○ | ○ | – | ○ |
| 2. Magnesium acetate test | ○ | – | ○ | ○ | – | ○ | |
| Coumarins and lactones | 1. Hydroxamic acid iron test | ○ | – | ○ | ○ | + | ○ |
| 2. Diazotization test | ○ | – | ○ | ○ | + | ○ | |
| 3. Fluorescence test | ○ | – | ○ | ○ | + | ○ | |
| Volatile oils and fats | 1. Phosphomolybdic acid test | ○ | ○ | + | ○ | ○ | + |
| 2. Vanillin-H2SO4 test | ○ | ○ | + | ○ | ○ | + | |
| 3. Sudan test III | ○ | ○ | + | ○ | ○ | + | |
| 4. Sudan test IV | ○ | ○ | + | ○ | ○ | + | |
| Cardiac glycosides | 1. Kedde test | ○ | – | ○ | ○ | – | ○ |
| 2. Raymond test | ○ | – | ○ | ○ | – | ○ | |
| 3. Legal test | ○ | – | ○ | ○ | – | ○ | |
| Cyanogenic glycosides | 1. Prussian blue test | – | ○ | ○ | – | ○ | ○ |
| Medicament Portions | Extracting Solvents | Yields (%, w/w) |
|---|---|---|
| Aboveground part | Water | 16.60 ± 1.21 c,d |
| Methanol | 10.40 ± 1.01 f,g | |
| Ethanol | 7.16 ± 0.93 g | |
| 80% Ethanol | 12.03 ± 1.00 e,f | |
| Root | Water | 14.30 ± 1.29 d,e |
| Methanol | 24.90 ± 2.03 a | |
| Ethanol | 21.86 ± 2.98 a,b | |
| 80% Ethanol | 19.16 ± 2.97 b |
| Medicament Portions | Extracting Solvents | TCC (mg GE/g Extract) | TProC (mg BSAE/g Extract) | TPheC (mg GAE/g Extract) | TFC (mg QE/g Extract) | TPAC (mg CAE/g Extract) | TTanC (mg TAE/g Extract) | GC (mg GAE/g Extract) | CTC (mg GAE/g Extract) |
|---|---|---|---|---|---|---|---|---|---|
| Aboveground part | Water | 118.71 ± 1.48 g | 440.23 ± 7.57 b | 32.70 ± 0.72 f | 4.17 ± 0.18 d | 4.85 ± 0.33 d | 27.95 ± 0.16 g | 12.27 ± 0.03 d | 15.09 ± 0.11 g |
| Methanol | 172.38 ± 3.68 e | N.T. | 50.79 ± 0.90 e | 69.53 ± 1.25 a | 12.22 ± 1.20 b | 49.14 ± 0.50 e | NONE | 26.63 ± 0.43 e | |
| Ethanol | 98.54 ± 0.51 h | N.T. | 23.69 ± 0.23 g | 30.65 ± 0.59 c | 6.31 ± 0.22 c,d | 22.30 ± 0.29 h | 7.52 ± 1.87 e | 12.40 ± 0.21 h | |
| 80% Ethanol | 142.65 ± 1.10 f | N.T. | 35.21 ± 0.42 f | 58.96 ± 0.30 b | 8.46 ± 0.44 c | 33.88 ± 0.06 f | 12.64 ± 0.26 d | 18.70 ± 0.17 f | |
| Root | Water | 466.11 ± 4.43 d | 2032.86 ± 2.55 a | 98.62 ± 0.31 d | NONE | 8.46 ± 0.65 c | 95.83 ± 0.39 d | 44.18 ± 2.06 c | 55.23 ± 0.74 d |
| Methanol | 686.14 ± 3.53 a | N.T. | 313.83 ± 4.16 a | NONE | 13.85 ± 0.87 b | 304.86 ± 3.34 a | 58.91 ± 1.10 a | 188.70 ± 0.43 a | |
| Ethanol | 505.41 ± 4.57 c | N.T. | 302.70 ± 1.79 b | NONE | 17.85 ± 3.05 a | 268.29 ± 2.69 b | 61.27 ± 0.77 a | 171.06 ± 0.67 b | |
| 80% Ethanol | 641.36 ± 1.46 b | N.T. | 272.90 ± 4.00 c | NONE | 18.46 ± 2.16 a | 246.24 ± 0.83 c | 52.55 ± 0.77 b | 154.89 ± 1.55 c |
| Medicament Portions | Extracting Solvents | DPPH (IC50, μg/mL) | ABTS (IC50, μg/mL) | Hydroxyl Radicals (IC50, μg/mL) | Superoxide Radicals (%, 2143 μg/mL) |
|---|---|---|---|---|---|
| Aboveground part | Water | 30.96 ± 1.21 c | >500 e | >2500 f | NONE |
| Methanol | 52.59 ± 4.34 e | 42.73 ± 3.77 c | 1451.16 ± 46.23 e | 20.94 ± 2.02 b | |
| Ethanol | 123.31 ± 0.73 f | 25.16 ± 1.80 b | 281.49 ± 2.07 d | NONE | |
| 80% Ethanol | 43.79 ± 3.81 d | 36.65± 5.35 b,c | NONE | NONE | |
| Root | Water | 4249.55 ± 265.80 g | 222.61 ± 10.50 d | NONE | 15.66 ± 4.06 b |
| Methanol | 2.63 ± 0.11 a | 3.00± 0.16 a | 176.81 ± 6.71 b,c | 19.76 ± 7.78 b | |
| Ethanol | 2.63 ± 0.14 a | 2.68 ± 0.15 a | 163.20 ± 14.58 b | 22.49 ± 1.44 b | |
| 80% Ethanol | 3.48 ± 0.06 a | 2.97 ± 0.13 a | 212.83 ± 8.82 c | 16.05 ± 1.08 b | |
| Standard antioxidant | Trolox | 2.03 ± 0.13 a | 3.78 ± 0.13 a | 97.52 ± 1.37 a | N.T. |
| BHT | 9.34 ± 0.12 b | 4.72 ± 0.04 a | 208.14 ± 2.53 c | N.T. | |
| Curcumin | N.T. | N.T. | N.T. | 58.23 ± 1.12 a |
| Medicament Portions | Extracting Solvents | TEACFRAP | TEACCUPRAC | Iron Chelating (IC50, μg/mL) | Copper Chelating (IC50, μg/mL) |
|---|---|---|---|---|---|
| Aboveground part | Water | 0.11 ± 0.00 g | NONE | 1525.61 ± 88.02 c | 430.80 ± 39.49 d |
| Methanol | 0.14 ± 0.00 f | 0.03 ± 0.01 d | 1123.73 ± 64.92 b | 738.06 ± 45.59 f | |
| Ethanol | 0.16 ± 0.00 e | 0.04 ± 0.01 d | 1573.85 ± 55.72 c | 2027.70 ± 128.13 g | |
| 80% Ethanol | 0.16 ± 0.00 e | 0.03 ± 0.00 d | 1028.78 ± 77.95 b | 612.80 ± 1.05 e | |
| Root | Water | 0.11 ± 0.00 g | NONE | >2500 d | 463.23 ± 56.75 d |
| Methanol | 0.55 ± 0.01 c | 0.87 ± 0.07 b | >2500 d | 249.26 ± 10.61 c | |
| Ethanol | 0.63 ± 0.01 b | 0.86 ± 0.04 b | >2500 d | 144.96 ± 4.38 a,b | |
| 80% Ethanol | 0.51 ± 0.01 d | 0.69 ± 0.05 c | >2500 d | 187.92 ± 6.08 b,c | |
| Standard antioxidant | Trolox | 1.00 ± 0.01 a | 1.00 ± 0.01 a | N.T. | N.T. |
| EDTANa2 | N.T. | N.T. | 2.28 ± 0.11 a | 41.58 ± 1.09 a |
| Medicament Portions | Extracting Solvents | H2O2 (IC50, μg/mL) | Singlet Oxygen (%, 2000 μg/mL) | HClO (IC50, μg/mL) |
|---|---|---|---|---|
| Aboveground part | Water | >2500 e | NONE | NONE |
| Methanol | >2500 e | NONE | NONE | |
| Ethanol | >2500 e | NONE | >990 c | |
| 80% Ethanol | >2500 e | NONE | NONE | |
| Root | Water | 1033.64 ± 26.35 d | NONE | 80.62 ± 12.67 b |
| Methanol | 67.74 ± 3.85 b,c | NONE | 24.27 ± 0.39 a | |
| Ethanol | 88.21 ± 10.80 c | NONE | 21.81 ± 1.22 a | |
| 80% Ethanol | 58.27 ± 3.70 b | NONE | 14.44 ± 0.92 a | |
| Standard antioxidant | Trolox | N.T. | N.T. | 12.81 ± 0.14 a |
| Lipoic acid | N.T. | N.T. | 26.53 ± 0.80 a | |
| Gallic acid | 20.04 ± 1.07 a | N.T. | N.T. | |
| Ferulic acid | N.T. | 95.3 ± 3.2 a | N.T. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, J.; Sun, Y.; Wei, Z.; Sun, H.; Li, L.; Zhu, J.; Xia, G.; Zang, H. Screening the Extract of Laportea bulbifera (Sieb. et Zucc.) Wedd. Based on Active Component Content, Its Antioxidant Capacity and Exploration of Hepatoprotective Activity in Rats. Molecules 2023, 28, 6256. https://doi.org/10.3390/molecules28176256
Feng J, Sun Y, Wei Z, Sun H, Li L, Zhu J, Xia G, Zang H. Screening the Extract of Laportea bulbifera (Sieb. et Zucc.) Wedd. Based on Active Component Content, Its Antioxidant Capacity and Exploration of Hepatoprotective Activity in Rats. Molecules. 2023; 28(17):6256. https://doi.org/10.3390/molecules28176256
Chicago/Turabian StyleFeng, Jiaxin, Yue Sun, Zhongbao Wei, Hui Sun, Li Li, Junyi Zhu, Guangqing Xia, and Hao Zang. 2023. "Screening the Extract of Laportea bulbifera (Sieb. et Zucc.) Wedd. Based on Active Component Content, Its Antioxidant Capacity and Exploration of Hepatoprotective Activity in Rats" Molecules 28, no. 17: 6256. https://doi.org/10.3390/molecules28176256
APA StyleFeng, J., Sun, Y., Wei, Z., Sun, H., Li, L., Zhu, J., Xia, G., & Zang, H. (2023). Screening the Extract of Laportea bulbifera (Sieb. et Zucc.) Wedd. Based on Active Component Content, Its Antioxidant Capacity and Exploration of Hepatoprotective Activity in Rats. Molecules, 28(17), 6256. https://doi.org/10.3390/molecules28176256