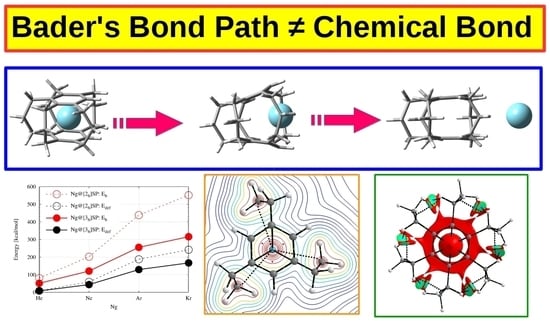
Bader’s Topological Bond Path Does Not Necessarily Indicate Stabilizing Interaction—Proof Studies Based on the Ng@[3n]cyclophane Endohedral Complexes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structures of the Considered [3]Cyclophanes
2.1.1. [3](1,2,3,4,5,6)
2.1.2. [3](1,2,3,4,5)
2.1.3. [3](1,2,3,5)
2.1.4. [3](1,2,4,5)
2.1.5. [3](1,3,5)
2.2. The Ng@[3]cyclophane Endohedral Complexes
2.2.1. Endohedral Complexes of the [2] and [3] Superphanes
2.2.2. Endohedral Complexes of the [2] and [3] One-Carbon Window Cyclophanes
2.3. Bader’s Bond Path Does Not Necessarily Indicate Stabilizing Interaction
2.3.1. QTAIM-Based Study
2.3.2. Spontaneous Escape of the Ng Atom from the Cage—Yet Another Proof
2.3.3. The NCI-Based Studies
3. Methodology
4. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| QTAIM | Quantum theory of atoms in molecules |
| BP | Bond path |
| BCP | Bond critical point |
| Ng | Noble gas atom |
| SP | Superphane |
| CP | Cyclophane |
| NCI | Noncovalent interaction index |
References
- Bader, R.F.W. Atoms in Molecules: A Quantum Theory; Oxford University Press: New York, NY, USA, 1990. [Google Scholar]
- Popelier, P.L.A. Atoms in Molecules: An Introduction; Longman: Singapore, 2000. [Google Scholar]
- Matta, C.F.; Boyd, R.J. The Quantum Theory of Atoms in Molecules; Wiley-VCH: Weinheim, Germany, 2007. [Google Scholar]
- Bader, R.F.W.; Anderson, S.G.; Duke, A.J. Quantum Topology of Molecular Charge Distributions. J. Am. Chem. Soc. 1979, 101, 1389–1395. [Google Scholar] [CrossRef]
- Bader, R.F.W.; Nguyen-Dang, T.T.; Tal, Y. A topological theory of molecular structure. Rep. Prog. Phys. 1981, 44, 893–948. [Google Scholar]
- Bader, R.F.W. Atoms in Molecules. Accounts Chem. Res. 1985, 18, 9–15. [Google Scholar]
- Bader, R.F.W. A Bond Path: A Universal Indicator of Bonded Interactions. J. Phys. Chem. A 1998, 102, 7314–7323. [Google Scholar] [CrossRef]
- Bader, R.F.W. A Quantum Theory of Molecular Structure and Its Appllcatlons. Chem. Rev. 1991, 91, 893–928. [Google Scholar] [CrossRef]
- Cioslowski, J.; Mixon, S.T.; Edwards, W.D. Weak Bonds in the Topological Theory of Atoms in Molecules. J. Am. Chem. Soc. 1991, 113, 1083–1085. [Google Scholar] [CrossRef]
- Cioslowski, J.; Mixon, S.T. Topological Properties of Electron Density in Search of Steric Interactions in Molecules: Electronic Structure Calculations on Ortho-Substituted Biphenyls. J. Am. Chem. Soc. 1992, 114, 4382–4387. [Google Scholar]
- Cerpa, E.; Krapp, A.; Vela, A.; Merino, G. The Implications of Symmetry of the External Potential on Bond Paths. Chem. Eur. J. 2008, 14, 10232–10234. [Google Scholar]
- Haaland, A.; Shorokhov, D.J.; Tverdova, N.V. Topological Analysis of Electron Densities: Is the Presence of an Atomic Interaction Line in an Equilibrium Geometry a Sufficient Condition for the Existence of a Chemical Bond? Chem. Eur. J. 2004, 10, 4416–4421. [Google Scholar]
- Strenalyuk, T.; Haaland, A. Chemical Bonding in the Inclusion Complex of He in Adamantane (He@adam): The Origin of the Barrier to Dissociation. Chem. Eur. J. 2008, 14, 10223–10226. [Google Scholar] [CrossRef]
- Poater, J.; Solà, M.; Bickelhaupt, F.M. A Model of the Chemical Bond Must Be Rooted in Quantum Mechanics, Provide Insight, and Possess Predictive Power. Chem. Eur. J. 2006, 12, 2902–2905. [Google Scholar] [CrossRef] [PubMed]
- Cerpa, E.; Krapp, A.; Flores-Moreno, R.; Donald, K.J.; Merino, G. Influence of Endohedral Confinement on the Electronic Interaction between He atoms: A He2@C20H20 Case Study. Chem. Eur. J. 2009, 15, 1985–1990. [Google Scholar] [PubMed]
- Krapp, A.; Frenking, G. Is This a Chemical Bond? A Theoretical Study of Ng2@C60 (Ng = He, Ne, Ar, Kr, Xe). Chem. Eur. J. 2007, 13, 8256–8270. [Google Scholar] [PubMed]
- Moran, D.; Woodcock, H.L.; Chen, Z.; Schaefer, H.F., III; Schleyer, P.V.R. On the Viability of Small Endohedral Hydrocarbon Cage Complexes: X@C4H4, X@C8H8, X@C8H14, X@C10H16, X@C12H12, and X@C16H16. J. Am. Chem. Soc. 2003, 125, 11442–11451. [Google Scholar] [PubMed]
- Cioslowski, J.; Edgington, L.; Stefanov, B.B. Steric Overcrowding in Perhalogenated Cyclohexanes, Dodecahedranes, and [60]Fulleranes. J. Am. Chem. Soc. 1995, 117, 10381–10384. [Google Scholar] [CrossRef]
- Tsirelson, V.G.; Zou, P.F.; Tang, T.-H.; Bader, R.F.W. Topological Definition of Crystal Structure: Determination of the Bonded Interactions in Solid Molecular Chlorine. Acta Cryst. 1995, A51, 143–153. [Google Scholar] [CrossRef]
- Bone, R.G.A.; Bader, R.F.W. Identifying and Analyzing Intermolecular Bonding Interactions in van der Waals Molecules. J. Phys. Chem. 1996, 100, 10892–10911. [Google Scholar] [CrossRef]
- Abramov, Y.A. Secondary Interactions and Bond Critical Points in Ionic Crystals. J. Phys. Chem. A 1997, 101, 5725–5728. [Google Scholar] [CrossRef]
- Vila, A.; Mosquera, R.A. On the perfluorination of alkyl ethers. An electron density study under the AIM approach. J. Mol. Struct. Theochem 2001, 546, 63–72. [Google Scholar] [CrossRef]
- Luaña, V.; Costales, A.; Mori-Sánchez, P.; Pendás, A.M. Ions in Crystals: The Topology of the Electron Density in Ionic Materials. 4. The Danburite (CaB2Si2O8) Case and the Occurrence of Oxide–Oxide Bond Paths in Crystals. J. Phys. Chem. B 2003, 107, 4912–4921. [Google Scholar]
- Matta, C.F.; Castillo, N.; Boyd, R.J. Characterization of a Closed-Shell Fluorine–Fluorine Bonding Interaction in Aromatic Compounds on the Basis of the Electron Density. J. Phys. Chem. A 2005, 109, 3669–3681. [Google Scholar]
- Pakiari, A.H.; Eskandari, K. Closed shell oxygen–oxygen bonding interaction based on electron density analysis. J. Mol. Struct. Theochem 2007, 806, 1–7. [Google Scholar] [CrossRef]
- Palusiak, M.; Grabowski, S.J. Do intramolecular halogen bonds exist? Ab initio calculations and crystal structures’ evidences. Struct. Chem. 2007, 18, 859–865. [Google Scholar] [CrossRef]
- Jabłoński, M. Energetic and Geometrical Evidence of Nonbonding Character of Some Intramolecular Halogen⋯Oxygen and Other Y⋯Y Interactions. J. Phys. Chem. A 2012, 116, 3753–3764. [Google Scholar]
- Jabłoński, M.; Palusiak, M. The halogen⋯oxygen interaction in 3-halogenopropenal revisited—The dimer model vs. QTAIM indications. Chem. Phys. 2013, 415, 207–213. [Google Scholar] [CrossRef]
- Dem’yanov, P.; Polestshuk, P. A Bond Path and an Attractive Ehrenfest Force Do Not Necessarily Indicate Bonding Interactions: Case Study on M2X2 (M = Li, Na, K; X = H, OH, F, Cl). Chem. Eur. J. 2012, 18, 4982–4993. [Google Scholar] [CrossRef]
- Tognetti, V.; Joubert, L. On the physical role of exchange in the formation of an intramolecular bond path between two electronegative atoms. J. Chem. Phys. 2013, 138, 024102. [Google Scholar] [CrossRef]
- Tognetti, V.; Joubert, L. On critical points and exchange-related properties of intramolecular bonds between two electronegative atoms. Chem. Phys. Lett. 2013, 579, 122–126. [Google Scholar] [CrossRef]
- Jabłoński, M. Hydride-Triel Bonds. J. Comput. Chem. 2018, 39, 1177–1191. [Google Scholar] [CrossRef]
- Jabłoński, M. Bond Paths Between Distant Atoms Do Not Necessarily Indicate Dominant Interactions. J. Comput. Chem. 2018, 39, 2183–2195. [Google Scholar] [CrossRef]
- Jabłoński, M. On the Uselessness of Bond Paths Linking Distant Atoms and on the Violation of the Concept of Privileged Exchange Channels. ChemistryOpen 2019, 8, 497–507. [Google Scholar] [CrossRef]
- Jabłoński, M. Counterintuitive bond paths: An intriguing case of the C(NO2)3− ion. Chem. Phys. Lett. 2020, 759, 137946. [Google Scholar] [CrossRef]
- Jabłoński, M. The physical nature of the ultrashort spike–ring interaction in iron maiden molecules. J. Comput. Chem. 2022, 43, 1206–1220. [Google Scholar] [CrossRef]
- Jabłoński, M. The Ultrashort Spike–Ring Interaction in Substituted Iron Maiden Molecules. Molecules 2023, 28, 2244. [Google Scholar]
- Cioslowski, J.; Mixon, S.T. Universality among topological properties of electron density associated with the hydrogen–hydrogen nonbonding interactions. Can. J. Chem. 1992, 70, 443–449. [Google Scholar] [CrossRef]
- Matta, C.F.; Hernández-Trujillo, J.; Tang, T.-H.; Bader, R.F.W. Hydrogen-Hydrogen Bonding: A Stabilizing Interaction in Molecules and Crystals. Chem. Eur. J. 2003, 9, 1940–1951. [Google Scholar] [CrossRef]
- Poater, J.; Solà, M.; Bickelhaupt, F.M. Hydrogen–Hydrogen Bonding in Planar Biphenyl, Predicted by Atoms-in-Molecules Theory, Does Not Exist. Chem. Eur. J. 2006, 12, 2889–2895. [Google Scholar]
- Grimme, S.; Mück-Lichtenfeld, C.; Erker, G.; Kehr, G.; Wang, H.; Beckers, H.; Willner, H. When Do Interacting Atoms Form a Chemical Bond? Spectroscopic Measurements and Theoretical Analyses of Dideuteriophenanthrene. Angew. Chem. Int. Ed. 2009, 48, 2592–2595. [Google Scholar] [CrossRef]
- Dillen, J. Congested Molecules. Where is the Steric Repulsion? An Analysis of the Electron Density by the Method of Interacting Quantum Atoms. Int. J. Quantum Chem. 2013, 113, 2143–2153. [Google Scholar]
- Keyvani, Z.A.; Shahbazian, S.; Zahedi, M. To What Extent are “Atoms in Molecules” Structures of Hydrocarbons Reproducible from the Promolecule Electron Densities? Chem. Eur. J. 2016, 22, 5003–5009. [Google Scholar]
- Bader, R.F.W. Bond Paths Are Not Chemical Bonds. J. Phys. Chem. A 2009, 113, 10391–10396. [Google Scholar] [CrossRef] [PubMed]
- Sekine, Y.; Brown, M.; Boekelheide, V. [2.2.2.2.2.2](l,2,3,4,5,6)Cyclophane: Superphane. J. Am. Chem. Soc. 1979, 101, 3126–3127. [Google Scholar] [CrossRef]
- Iwamura, H.; Katoh, M.; Kihara, H. How Strained Is the “Flat” Benzene Ring in Superphane ? Tetrahedron Lett. 1980, 21, 1757–1760. [Google Scholar] [CrossRef]
- Hanson, A.W.; Cameron, T.S. Crystal structures of, and strain in, some adjacent-bridged [2n]cyclophanes. J. Chem. Res. Synop. 1980, 10, 336–337. [Google Scholar]
- Sekine, Y.; Boekelheide, V. A Study of the Synthesis and Properties of [26](1,2,3,4,5,6)Cyclophane (Superphane). J. Am. Chem. Soc. 1981, 103, 1777–1785. [Google Scholar] [CrossRef]
- Gleiter, R.; Kratz, D. “Super” Phanes. Acc. Chem. Res. 1993, 26, 311–318. [Google Scholar] [CrossRef]
- Gleiter, R.; Hopf, H. (Eds.) Modern Cyclophane Chemistry; Wiley-VCH: Weinheim, Germany, 2004. [Google Scholar]
- Jabłoński, M. Does the Presence of a Bond Path Really Mean Interatomic Stabilization? The Case of the Ng@Superphane (Ng = He, Ne, Ar, and Kr) Endohedral Complexes. Symmetry 2021, 13, 2241. [Google Scholar] [CrossRef]
- Jabłoński, M. Determining Repulsion in Cyclophane Cages. Molecules 2022, 27, 3969. [Google Scholar] [CrossRef]
- Linder, H.J. Atomisierungsenergien Gespannter Kohlenwasserstoffe–II: Cyclophane. Tetrahedron 1976, 32, 753–757. [Google Scholar]
- Schirch, P.F.T.; Boekelheide, V. [2.2.2.2.2](1,2,3,4,5)Cyclophane. J. Am. Chem. Soc. 1979, 101, 3125–3126. [Google Scholar] [CrossRef]
- Kleinschroth, J.; Hopf, H. The Chemical Behavior of Multibridged [2n]Cyclophanes. Angew Chem. Int. Ed. Engl. 1982, 21, 469–480. [Google Scholar] [CrossRef]
- Spanget-Larsen, J. Electronic states of the [2n]cyclophanes. Theoret. Chim. Acta 1983, 64, 187–203. [Google Scholar] [CrossRef]
- Czuchajowski, L.; Wisor, A.K. Electronic Effects In Multibridged Cyclophanes As Viewed by the Indices of Excitation. J. Electron Spectrosc. Relat. Phenom. 1987, 43, 163–181. [Google Scholar] [CrossRef]
- Caramori, G.F.; Galembeck, S.E.; Laali, K.K. A Computational Study of [2.2]Cyclophanes. J. Org. Chem. 2005, 70, 3242–3250. [Google Scholar] [CrossRef] [PubMed]
- Caramori, G.F.; Galembeck, S.E. Computational Study about Through-Bond and Through-Space Interactions in [2.2]Cyclophanes. J. Phys. Chem. A 2007, 111, 1705–1712. [Google Scholar] [CrossRef] [PubMed]
- Caramori, G.F.; Galembeck, S.E. A Computational Study of Tetrafluoro-[2.2]Cyclophanes. J. Phys. Chem. A 2008, 112, 11784–11800. [Google Scholar] [CrossRef] [PubMed]
- Dodziuk, H.; Szymański, S.; Jaźwiński, J.; Ostrowski, M.; Demissie, T.B.; Ruud, K.; Kuś, P.; Hopf, H.; Lin, S.-T. Structure and NMR Spectra of Some [2.2]Paracyclophanes. The Dilemma of [2.2]Paracyclophane Symmetry. J. Phys. Chem. A 2011, 115, 10638–10649. [Google Scholar] [CrossRef] [PubMed]
- Dodziuk, H.; Vetokhina, V.; Hopf, H.; Luboradzki, R.; Gaweł, P.; Waluk, J. Electronic states of cyclophanes with small bridges. J. Chem. Soc. 2012, 136, 074201. [Google Scholar] [CrossRef]
- Fujitsuka, M.; Miyazaki, T.; Lu, C.; Shinmyozu, T.; Majima, T. Multistep Electron Transfer Systems Containing [2.2]- or [3.3]Paracyclophane. J. Phys. Chem. A 2016, 120, 1184–1189. [Google Scholar] [CrossRef]
- Matsuiwa, K.; Hayashi, S.; Nakanishi, W. Dynamic and Static Behavior of Intramolecular π–π Interactions in [2.2]- and [3.3]Cyclophanes, Elucidated by QTAIM Dual Functional Analysis with QC Calculations. ChemistrySelect 2017, 2, 1774–1782. [Google Scholar] [CrossRef]
- Majerz, I.; Dziembowska, T. What Is the Main Feature Distinguishing the Through-Space Interactions in Cyclophanes from Their Aliphatic Analogues? ACS Omega 2020, 5, 22314–22324. [Google Scholar] [CrossRef] [PubMed]
- Meno, T.; Sako, K.; Suenaga, M.; Mouri, M.; Shinmyozu, T.; Takemura, H. Conformational analysis of [3.3.3](1,3,5)cyclophane systems. Can. J. Chem. 1990, 68, 440–445. [Google Scholar] [CrossRef]
- Shinmyozu, T.; Hirakida, M.; Kusumoto, S.; Tomonou, M.; Inzau, T.; Rudziński, J.M. Synthesis of 35(1,2,3,4,5)Cyclophane. Chem. Lett. 1994, 669–672. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Miyoshi, N.; Shinmyozu, T. Synthesis of a “Molecular Pinwheel”: [3.3.3.3.3.3](1,2,3,4,5,6)Cyclophane. Angew. Chem. Int. Ed. Engl. 1996, 35, 549–550. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Miyoshi, N.; Hirakida, M.; Kusumoto, S.; Kawase, H.; Rudzinski, J.M.; Shinmyozu, T. Syntheses, Structures, and Transannular π–π Interactions of Multibridged [3n]Cyclophanes. J. Am. Chem. Soc. 1996, 118, 12267–12275. [Google Scholar] [CrossRef]
- Hori, K.; Sentou, W.; Shinmyozu, T. Ab lnitio Molecular Orbital Study on Inversion Mechanism of Trimethylene Bridges of [33](1,3,5)- and [36](1,2,3,4,5,6) Cyclophanes. Tetrahedron Lett. 1997, 38, 8955–8958. [Google Scholar] [CrossRef]
- Bettinger, H.F.; Schleyer, P.v.R.; Schaefer, H.F., III. [36](1,2,3,4,5,6)Cyclophane–A Molecular Pinwheel and Its Correlated Inversion: NMR and Energetic Considerations. J. Am. Chem. Soc. 1998, 120, 1074–1075. [Google Scholar] [CrossRef]
- Yasutake, M.; Sakamoto, Y.; Onaka, S.; Sako, K.; Tatemitsu, H.; Shinmyozu, T. Crystal structural properties of a pinwheel compound: [36](1,2,3,4,5,6)cyclophane. Tetrahedron Lett. 2000, 41, 7933–7938. [Google Scholar] [CrossRef]
- Yasutake, M.; Koga, T.; Sakamoto, Y.; Komatsu, S.; Zhou, M.; Sako, K.; Tatemitsu, H.; Onaka, S.; Aso, Y.; Inoue, S.; et al. An Alternative Synthetic Route of [35](1,2,3,4,5)Cyclophane, and Structural Properties of Multibridged [3n]Cyclophanes and Their Charge-Transfer Complexes in the Solid State. J. Am. Chem. Soc. 2002, 124, 10136–10145. [Google Scholar] [CrossRef]
- Nogita, R.; Matohara, K.; Yamaji, M.; Oda, T.; Sakamoto, Y.; Kumagai, T.; Lim, C.; Yasutake, M.; Shimo, T.; Jefford, C.W.; et al. Photochemical Study of [33](1,3,5)Cyclophane and Emission Spectral Properties of [3n]Cyclophanes (n = 2–6). J. Am. Chem. Soc. 2004, 126, 13732–13741. [Google Scholar] [CrossRef]
- Fujitsuka, M.; Tojo, S.; Shinmyozu, T.; Majima, T. Intramolecular dimer radical anions of [3n] cyclophanes: Transannular distance dependent stabilization energy. Chem. Commun. 2009, 1553–1555. [Google Scholar] [CrossRef] [PubMed]
- Shibahara, M.; Watanabe, M.; Miyazaki, T.; Goto, K.; Matsumoto, T.; Shinmyozu, T. Synthesis of Dibromo[3.3]paracyclophanes. Synthesis 2016, 48, 1197–1201. [Google Scholar] [CrossRef]
- Zhang, X.-X.; Li, J.; Niu, Y.-Y. A Review of Crystalline Multibridged Cyclophane Cages: Synthesis, Their Conformational Behavior, and Properties. Molecules 2022, 27, 7083. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, P.R.; Chernish, L.V.; Gunchenko, P.A.; Tikhonchuk, E.Y.; Hausmann, H.; Serafin, M.; Schlecht, S.; Dahl, J.E.P.; Carlson, R.M.K.; Fokin, A.A. Overcoming lability of extremely long alkane carbon–carbon bonds through dispersion forces. Nature 2011, 477, 308–311. [Google Scholar] [CrossRef]
- Fokin, A.A.; Chernish, L.V.; Gunchenko, P.A.; Tikhonchuk, E.Y.; Hausmann, H.; Serafin, M.; Dahl, J.E.P.; Carlson, R.M.K.; Schreiner, P.R. Stable Alkanes Containing Very Long Carbon–Carbon Bonds. J. Am. Chem. Soc. 2012, 134, 13641–13650. [Google Scholar] [CrossRef]
- Ishigaki, Y.; Shimajiri, T.; Takeda, T.; Katoono, R.; Suzuki, T. Longest C–C Single Bond among Neutral Hydrocarbons with a Bond Length beyond 1.8 Å. Chem 2018, 4, 795–806. [Google Scholar] [CrossRef]
- Brown, D.A.; Clegg, W.; Colquhoun, H.M.; Daniels, J.A.; Stephenson, I.R.; Wade, K. A pentuply-bridging carbonyl group: Crystal and molecular structure of a salt of the 1-oxo-2-phenyl-1,2-dicarbadodecaborate(12) anion, [LH]+[O(Ph)C2B10H10]− (L = 1,8-N,N,N’,N’-tetramethylnaphthalenediamine). J. Chem. Soc. Chem. Commun. 1987, 889–891. [Google Scholar] [CrossRef]
- Llop, J.; Viñas, C.; Teixidor, F.; Victori, L.; Kivekäs, R.; Sillanpää, R. Redox Potential Modulation in Mixed Sandwich Pyrrolyl/Dicarbollide Complexes. Inorg. Chem. 2002, 41, 3347–3352. [Google Scholar] [CrossRef]
- Li, J.; Pang, R.; Li, Z.; Lai, G.; Xiao, X.-Q.; Müller, T. Exceptionally Long C–C Single Bonds in Diamino-o-carborane as Induced by Negative Hyperconjugation. Angew. Chem. Int. Ed. 2019, 58, 1397–1401. [Google Scholar] [CrossRef]
- Novoa, J.J.; Lafuente, P.; Del Sesto, R.E.; Miller, J.S. Exceptionally Long (≥2.9 Å) C–C Bonds between [TCNE]− Ions: Two-Electron, Four-Center π★-π★ C–C Bonding in π-[TCNE]22−. Angew. Chem. Int. Ed. 2001, 40, 2540–2545. [Google Scholar] [CrossRef]
- Novoa, J.J.; Stephens, P.W.; Weerasekare, M.; Shum, W.W.; Miller, J.S. The Tetracyanopyrazinide Dimer Dianion, [TCNP]22−. 2-Electron 8-Center Bonding. J. Am. Chem. Soc. 2009, 131, 9070–9075. [Google Scholar] [CrossRef]
- Jabłoński, M. The first theoretical proof of the existence of a hydride-carbene bond. Chem. Phys. Lett. 2018, 710, 78–83. [Google Scholar] [CrossRef]
- Johnson, E.R.; Keinan, S.; Mori-Sánchez, P.; Contreras-García, J.; Cohen, A.J.; Yang, W. Revealing Noncovalent Interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef]
- Contreras-García, J.; Johnson, E.R.; Keinan, S.; Chaudret, R.; Piquemal, J.-P.; Beratan, D.N.; Yang, W. NCIPLOT: A Program for Plotting Noncovalent Interaction Regions. J. Chem. Theory Comput. 2011, 7, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Jabłoński, M. Theoretical Study of N-Heterocyclic-Carbene–ZnX2 (X = H, Me, Et) Complexes. Materials 2021, 14, 6147. [Google Scholar] [CrossRef] [PubMed]
- Chai, J.-D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef] [PubMed]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef]
- Parr, R.G.; Yang, W. Density-Functional Theory of Atoms and Molecules; Oxford University Press: New York, NY, USA, 1989. [Google Scholar]
- Jensen, F. Introduction to Computational Chemistry; John Wiley & Sons Ltd.: Chichester, UK, 2007. [Google Scholar]
- Pritchard, B.P.; Altarawy, D.; Didier, B.; Gibson, T.D.; Windus, T.L. New Basis Set Exchange: An Open, Up-to-Date Resource for the Molecular Sciences Community. J. Chem. Inf. Model. 2019, 59, 4814–4820. [Google Scholar] [CrossRef]
- Mardirossian, N.; Head-Gordon, M. Thirty years of density functional theory in computational chemistry: An overview and extensive assessment of 200 density functionals. Mol. Phys. 2017, 115, 2315–2372. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2019. [Google Scholar]
- Dennington, R.; Keith, T.; Millam, J. (Eds.) GaussView, Version 6; Semichem Inc.: Shawnee Mission, KS, USA, 2019. [Google Scholar]
- Jabłoński, M. A Critical Overview of Current Theoretical Methods of Estimating the Energy of Intramolecular Interactions. Molecules 2020, 25, 5512. [Google Scholar] [CrossRef]
- Keith, T.A. AIMAll, Version 15.05.18; TK Gristmill Software: Overland Park, KS, USA, 2015. [Google Scholar]















| CP | Label | Symm. | ||||||
|---|---|---|---|---|---|---|---|---|
| [2](1,2,3,4,5,6) | 1 | C | 2.654 | 1.406 | 1.520 | 1.592 | 110.3 | n/a |
| [3](1,2,3,4,5,6) | D | 2.954 | 1.400 | 1.519 | 1.482 | 120.4 | 129.5 | |
| [3](1,2,3,4,5,6) | D | 2.919 | 1.403 | 1.515 | 1.567 | 120.4 | 124.7 | |
| [3](1,2,3,4,5,6) | 2 | C | 2.961 | 1.403 | 1.522 | 1.551 | 117.2 | 120.8 |
| [3](1,2,3,4,5) | 3a | C | 2.958–3.204 | 1.385–1.409 | 1.517–1.521 | 1.544–1.549 | 114.0–117.5 | 119.0–120.9 |
| [3](1,2,3,4,5) | 3b | C | 2.952–3.281 | 1.384–1.411 | 1.517–1.522 | 1.542–1.551 | 113.7–117.6 | 117.3–121.0 |
| [3](1,2,3,4,5) | 3c | C | 2.950–3.279 | 1.385–1.411 | 1.517–1.524 | 1.542–1.550 | 113.9–117.2 | 118.8–121.2 |
| [3](1,2,3,4,5) | 3d | C | 2.942–3.136 | 1.387–1.408 | 1.518–1.519 | 1.544–1.573 | 117.2–121.1 | 118.7–124.0 |
| [3](1,2,3,5) | 4a | C | 2.973–3.228 | 1.384–1.408 | 1.512–1.520 | 1.541–1.549 | 114.1–117.4 | 116.8–120.3 |
| [3](1,2,3,5) | 4b | C | 2.940–3.156 | 1.385–1.407 | 1.513–1.518 | 1.543–1.570 | 114.2–121.5 | 117.5–123.5 |
| [3](1,2,4,5) | 5a | D | 3.075–3.247 | 1.392–1.400 | 1.517 | 1.541 | 114.2 | 116.9 |
| [3](1,2,4,5) | 5b | D | 2.990–3.120 | 1.392–1.401 | 1.516–1.519 | 1.542–1.559 | 118.0–119.4 | 120.7 |
| [3](1,2,3) | 6a | C | 3.118–3.168 | 1.386–1.397 | 1.514 | 1.543 | 114.5 | 117.4 |
| [3](1,2,3) | 6b | C | 3.104–3.212 | 1.390–1.393 | 1.514 | 1.542–1.544 | 114.4–114.5 | 117.1–117.6 |
| System | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| [2]SP | n/a | n/a | n/a | 2.654 | 1.406 | 1.520 | 1.592 | 110.3 | n/a |
| He@[2]SP | 80.7 | 8.5 | 10.5 | 2.819 | 1.417 | 1.528 | 1.601 | 113.3 | n/a |
| Ne@[2]SP | 202.1 | 59.5 | 29.4 | 3.130 | 1.431 | 1.545 | 1.630 | 118.8 | n/a |
| Ar@[2]SP | 438.4 | 187.6 | 42.8 | 3.568 | 1.447 | 1.575 | 1.707 | 126.1 | n/a |
| Kr@[2]SP | 551.5 | 241.7 | 43.8 | 3.701–3.706 | 1.434–1.482 | 1.587 | 1.751–1.754 | 127.9 | n/a |
| [3]SP | n/a | n/a | n/a | 2.961 | 1.403 | 1.522 | 1.551 | 117.2 | 120.8 |
| He@[3]SP | 51.3 | 7.7 | 15.1 | 3.194 | 1.410 | 1.523 | 1.559 | 118.6 | 123.9 |
| Ne@[3]SP | 120.7 | 43.7 | 36.2 | 3.560 | 1.416 | 1.528 | 1.574 | 120.8 | 128.8 |
| Ar@[3]SP | 255.2 | 129.1 | 50.6 | 3.954–4.168 | 1.417–1.426 | 1.534–1.545 | 1.593–1.618 | 123.6–124.5 | 133.4–137.2 |
| Kr@[3]SP | 315.7 | 166.9 | 52.9 | 4.228–4.249 | 1.424–1.425 | 1.544–1.545 | 1.618–1.622 | 125.2–125.3 | 137.5–137.9 |
| Complex | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 3a | n/a | n/a | n/a | 2.958–3.204 | 1.385–1.409 | 1.517–1.521 | 1.544–1.549 | 114.0–117.5 | 119.0–120.9 |
| He@3a | 44.2 | 9.6 | 21.8 | 3.137–3.739 | 1.392–1.412 | 1.520–1.524 | 1.552–1.563 | 115.0–119.1 | 122.3–124.4 |
| 4a | n/a | n/a | n/a | 2.973–3.228 | 1.384–1.408 | 1.512–1.520 | 1.541–1.549 | 114.1–117.4 | 116.8–120.3 |
| He@4a | 39.1 | 9.3 | 23.8 | 3.177–3.709 | 1.388–1.412 | 1.517–1.522 | 1.542–1.562 | 115.1–118.9 | 119.0–123.7 |
| 5a | n/a | n/a | n/a | 3.075–3.247 | 1.392–1.400 | 1.517 | 1.541 | 114.2 | 116.9 |
| He@5a | 38.2 | 8.7 | 22.8 | 3.391–3.653 | 1.396–1.403 | 1.518 | 1.550 | 115.4 | 119.8 |
| Ar@5a | 170.1 | 86.0 | 50.5 | 4.237–4.698 | 1.407–1.410 | 1.536 | 1.591 | 119.4 | 127.5 |
| Kr@5a | 215.6 | 106.5 | 49.4 | 4.368–4.868 | 1.411–1.414 | 1.544 | 1.605 | 121.0 | 129.4 |
| 6a | n/a | n/a | n/a | 3.118–3.168 | 1.386–1.397 | 1.514 | 1.543 | 114.5 | 117.4 |
| He@6a | 37.7 | 8.9 | 23.5 | 3.453–3.540 | 1.391–1.400 | 1.517 | 1.554 | 115.7–115.8 | 120.6 |
| Ne@6a | 79.8 | 36.3 | 45.5 | 3.839–4.039 | 1.394–1.404 | 1.524–1.527 | 1.569–1.575 | 116.7–116.8 | 123.6–124.5 |
| Ar@6a | 162.2 | 86.6 | 53.4 | 4.376–4.563 | 1.401–1.405 | 1.544–1.545 | 1.606–1.607 | 118.6–118.8 | 127.0–127.2 |
| Kr@6a | 204.2 | 107.1 | 52.5 | 4.526–4.722 | 1.406 | 1.555 | 1.624 | 120.7–120.8 | 129.1 |
| 6b | n/a | n/a | n/a | 3.104–3.212 | 1.390–1.393 | 1.514 | 1.542–1.544 | 114.4–114.5 | 117.1–117.6 |
| He@6b | 37.5 | 8.9 | 23.8 | 3.406–3.642 | 1.394–1.397 | 1.516–1.518 | 1.551–1.557 | 115.6–115.7 | 119.7–121.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jabłoński, M. Bader’s Topological Bond Path Does Not Necessarily Indicate Stabilizing Interaction—Proof Studies Based on the Ng@[3n]cyclophane Endohedral Complexes. Molecules 2023, 28, 6353. https://doi.org/10.3390/molecules28176353
Jabłoński M. Bader’s Topological Bond Path Does Not Necessarily Indicate Stabilizing Interaction—Proof Studies Based on the Ng@[3n]cyclophane Endohedral Complexes. Molecules. 2023; 28(17):6353. https://doi.org/10.3390/molecules28176353
Chicago/Turabian StyleJabłoński, Mirosław. 2023. "Bader’s Topological Bond Path Does Not Necessarily Indicate Stabilizing Interaction—Proof Studies Based on the Ng@[3n]cyclophane Endohedral Complexes" Molecules 28, no. 17: 6353. https://doi.org/10.3390/molecules28176353
APA StyleJabłoński, M. (2023). Bader’s Topological Bond Path Does Not Necessarily Indicate Stabilizing Interaction—Proof Studies Based on the Ng@[3n]cyclophane Endohedral Complexes. Molecules, 28(17), 6353. https://doi.org/10.3390/molecules28176353