Knotwood and Branchwood Polyphenolic Extractives of Silver Fir, Spruce and Douglas Fir and Their Antioxidant, Antifungal and Antibacterial Properties
Abstract
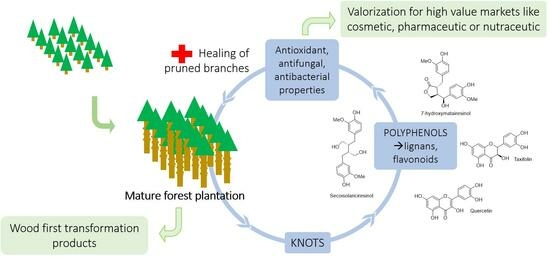
:1. Introduction
2. Results and Discussion
2.1. Yield of Extracts and Chromatographic Analyses
2.2. Radical Scavenging Activities of the Different Ethanolic Extracts and of Pure Chemicals
2.3. Antibacterial Activities of Ethanol Extracts
2.4. Antifungal Activities of Ethanol Extracts
3. Materials and Methods
3.1. Material
- Knotwood: Knots of three economically significant softwoods Abies alba (Silver fir), Picea abies (spruce) and Pseudotsuga menziesii (Douglas fir) were sampled from Savoie Pan company (Tournon, France) and Poirot Construction Bois company (La Bresse, France). Knots were first air-dried before milling at 1100 rpm in a Fritsch Pulverisette 9 (Fritsch, Idar Oberstein, Germany) until no knotwood remained. Knotwood sawdust was oven-dried at 103 °C until constant weight before solvent extraction.
- Branchwood: Three branches, each from the three softwood species described above, were sampled within the framework of the ExtraFor_Est project (Brennan et al., 2020). The branches were chosen at a height of 1.3 m for each tree. These branches were dried and sliced at different distances from the trunk (0 (near the trunk), 25, 50, 100 and 250 cm) to obtain one 2 cm wide disk for each species. The debarked disks were air-dried and ground at 1100 rpm using a Fritsch Pulverisette 9 (Fritsch, Idar Oberstein, Germany). The sawdust was oven-dried at 103 °C to a constant mass.
3.2. Extraction
3.3. Chromatographic Analysis
3.4. Total Flavonoids Content
3.5. Pure Compounds
3.6. Radical Scavenging Activity
3.7. Antibacterial Activity
3.8. Antifungal Activity
3.9. Statistics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Vek, V.; Keržič, E.; Poljanšek, I.; Eklund, P.; Humar, M.; Oven, P. Wood Extractives of Silver Fir and Their Antioxidant and Antifungal Properties. Molecules 2021, 26, 6412. [Google Scholar] [CrossRef]
- Holmbom, B.; Eckerman, C.; Eklund, P.; Hemming, J.; Nisula, L.; Reunanen, M.; Sjöholm, R.; Sundberg, A.; Sundberg, K.; Willför, S. Knots in trees-a new rich source of lignans. Phytochem. Rev. 2003, 2, 331–340. [Google Scholar] [CrossRef]
- Pietarinen, S.P.; Willför, S.M.; Ahotupa, M.O.; Hemming, J.E.; Holmbom, B.R. Knotwood and bark extracts: Strong antioxidants from waste materials. J. Wood Sci. 2006, 52, 436–444. [Google Scholar] [CrossRef]
- Kebbi-Benkeder, Z.; Colin, F.; Dumarçay, S.; Gérardin, P. Quantification and characterization of knotwood extractives of 12 European softwood and hardwood species. Ann. For. Sci. 2014, 72, 277–284. [Google Scholar] [CrossRef]
- Brennan, M.; Hentges, D.; Cosgun, S.; Dumarcay, S.; Colin, F.; Gérardin, C.; Gérardin, P. Intraspecific variability of quantity and chemical composition of ethanolic knotwood extracts along the stems of three industrially important softwood species: Abies alba, Picea abies and Pseudotsuga menziesii. Holzforschung 2021, 75, 168–179. [Google Scholar] [CrossRef]
- Willför, S.; Hemming, J.; Reunanen, M.; Eckerman, C.; Holmbom, B. Lignans and Lipophilic Extractives in Norway Spruce Knots and Stemwood. Holzforschung 2003, 57, 27–36. [Google Scholar] [CrossRef]
- Piispanen, R.; Willför, S.; Saranpää, P.; Holmbom, B. Variation of lignans in Norway spruce (Picea abies [L.] Karst.) knotwood: Within-stem variation and the effect of fertilisation at two experimental sites in Finland. Trees 2008, 22, 317–328. [Google Scholar] [CrossRef]
- Lundquist, K.; Langer, V.; Li, S.; Stomberg, R. Lignin stereochemistry and its biosynthetic implications. In Proceedings of the 12th International Symposium on Wood and Pulping Chemistry, Madison, WI, USA, 9–12 June 2003; pp. 239–244. [Google Scholar]
- MacRae, W.; Towers, G. Biological activities of lignans. Phytochemistry 1984, 23, 1207–1220. [Google Scholar] [CrossRef]
- Smeds, A.I.; Eklund, P.C.; Willför, S.M. Chemical characterization of high-molar-mass fractions in a Norway spruce knotwood ethanol extract. Phytochemistry 2016, 130, 207–217. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Eklund, P.C.; Långvik, O.K.; Wärnå, J.P.; Salmi, T.O.; Willför, S.M.; Sjöholm, R.E. Chemical studies on antioxidant mechanisms and free radical scavenging properties of lignans. Org. Biomol. Chem. 2005, 3, 3336–3347. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.M.; Santos, M.R.; Caroço, G.; Rocha, R.; Justino, G.; Mira, L. Structure-antioxidant Activity Relationships of Flavonoids: A Re-examination. Free. Radic. Res. 2002, 36, 1219–1227. [Google Scholar] [CrossRef]
- Yamauchi, S.; Sugahara, T.; Matsugi, J.; Someya, T.; Masuda, T.; Kishida, T.; Akiyama, K.; Maruyama, M. Effect of the Benzylic Structure of Lignan on Antioxidant Activity. Biosci. Biotechnol. Biochem. 2007, 71, 2283–2290. [Google Scholar] [CrossRef]
- Ahamad, S.T.; Lakshmi, T.; Rajeshkumar, S.; Roy, A.; Gurunadhan, D.; Geetha, R. Antibacterial Activity of Taxifolin Isolated from Acacia Catechu Leaf Extract-An Invitro Study. Indian J. Public Health Res. Dev. 2019, 10, 3540–3544. [Google Scholar] [CrossRef]
- Farhadi, F.; Khameneh, B.; Iranshahi, M.; Iranshahy, M. Antibacterial activity of flavonoids and their structure–activity relationship: An update review. Phytother. Res. 2019, 33, 13–40. [Google Scholar] [CrossRef]
- Shevelev, A.B.; La Porta, N.; Isakova, E.P.; Martens, S.; Biryukova, Y.K.; Belous, A.S.; Sivokhin, D.A.; Trubnikova, E.V.; Zylkova, M.V.; Belyakova, A.V.; et al. In Vivo Antimicrobial and Wound-Healing Activity of Resveratrol, Dihydroquercetin, and Dihydromyricetin against Staphylococcus aureus, Pseudomonas aeruginosa, and Candida albicans. Pathogens 2020, 9, 296. [Google Scholar] [CrossRef]
- Al-Ani, W.M.; Aziz, F.M. Antimicrobial Activity of Hydroxymatairesinol (HMR) Lignan. Iraqi J. Pharm. Sci. 2013, 22, 30–34. [Google Scholar] [CrossRef]
- Venäläinen, M.; Harju, A.M.; Terziev, N.; Laakso, T.; Saranpää, P. Decay Resistance, Extractive Content, and Water Sorption Capacity of Siberian Larch (Larix sibirica Lebed.) Heartwood Timber. Holzforschung 2006, 60, 99–103. [Google Scholar] [CrossRef]
- Nezu, I.; Ishiguri, F.; Suzuki, H.; Takahashi, Y.; Takashima, Y.; Hiraoka, Y.; Iki, T.; Miyashita, H.; Matsushita, M.; Habu, N.; et al. Inheritance of wood color, decay resistance, and polyphenol content of heartwood in full-sib families of Japanese larch (Larix kaempferi (Lamb.) Carr.). Holzforschung 2022, 76, 348–355. [Google Scholar] [CrossRef]
- Špetík, M.; Balík, J.; Híc, P.; Hakalová, E.; Štůsková, K.; Frejlichová, L.; Tříska, J.; Eichmeier, A. Lignans extract from knotwood of Norway spruce—A possible new weapon against GTDs. J. Fungi 2022, 8, 357. [Google Scholar] [CrossRef]
- Trujillo-Moya, C.; Ganthaler, A.; Stöggl, W.; Arc, E.; Kranner, I.; Schueler, S.; Ertl, R.; Espinosa-Ruiz, A.; Martínez-Godoy, M.; George, J.-P.; et al. Advances in understanding Norway spruce natural resistance to needle bladder rust infection: Transcriptional and secondary metabolites profiling. BMC Genom. 2022, 23, 435. [Google Scholar] [CrossRef] [PubMed]
- Mäkinen, H.; Hein, S. Effect of wide spacing on increment and branch properties of young Norway spruce. Eur. J. For. Res. 2006, 125, 239–248. [Google Scholar] [CrossRef]
- Schütz, J.-P.; Ammann, P.L.; Zingg, A. Optimising the yield of Douglas-fir with an appropriate thinning regime. Eur. J. For. Res. 2015, 134, 469–480. [Google Scholar] [CrossRef]
- Holmbom, T.; Reunanen, M.; Fardim, P. Composition of callus resin of Norway spruce, Scots pine, European larch and Douglas fir. Holzforschung 2008, 62, 417–422. [Google Scholar] [CrossRef]
- Davis, W.B. Determination of Flavanones in Citrus Fruits. Anal. Chem. 1947, 19, 476–478. [Google Scholar] [CrossRef]








| Species | Knotwood (%) | Branchwood (%) | ||||
|---|---|---|---|---|---|---|
| 0 cm | 25 cm | 50 cm | 100 cm | 250 cm | ||
| P. abies | 22.69 ± 1.48 | 14.46 ± 2,10 | 3.82 ± 0.13 | 2.41 ± 0.02 | 1.59 ± 0.52 | 2.34 ± 1.59 |
| A. alba | 21.64 ± 0.67 | 21.59 ± 0.77 | 11.20 ± 2.42 | 8.01 ± 1.13 | 4.11 ± 0.50 | 1.82 ± 1.01 |
| P. menziesii | 11.81 ± 1.99 | 3.80 ± 0.96 | 1.98 ± 0.46 | 2.19 ± 0.71 | 1.85 ± 0.39 | 2.27 ± 0.97 |
| Compound | Retention Time (min) | Annotation | [M-H]+ | [M-H]− | λ Max | Spuce a | Fir a | Douglas Fir a |
|---|---|---|---|---|---|---|---|---|
| 1 | 8.61 | Conidendric acid | 261; 341; 399 | 375; 421 | 199; 282 | - | ++ | - |
| 2 | 8.12 | Taxifolin | 305 | 303; 607 | 199; 288 | - | - | ++ |
| 3 | 9.57 | Hydroxymatairesinol | 327; 397 | 342; 373; 419 | 201; 280 | +++ | ++ | ++ |
| 4 | 9.92 | Secoisolariciresinol | 327; 345; 363; 385 | 361; 407 | 196; 280 | +++ | +++ | +++ |
| 5 | 10.38 | Secoisolariciresinol sesquilignan | 493; 581 | 557 | 200; 279 | ++ | ++ | ++ |
| 6 | 11.72 | Arctigenin | 359; 399 | 375; 421 | 199; 219; 280 | ++ | ++ | ++ |
| 7 | 11.72 | Quercetin | 303 | 301 | 199; 219; 280 | - | + | + |
| 8 | 11.96 | oxomatairesinol | 373; 395; 511 | 371; 551 | 201; 281 | + | + | - |
| 9 | 12.40 | α-Conidendrin | 357; 398 | 355; 721 | 203; 220; 280 | ++ | + | ++ |
| 10 | 12.57 | dimere HMR | 587; 770 | 745 | 204; 220; 282 | + | + | + |
| 11 | 12.83 | Dimere lariciresinol-secoisolariciresinol | 359; 744 | 357; 719 | 202; 220; 281 | ++ | - | - |
| 12 | 12.83 | Matairesinol | 359 | 357 | 202; 220; 281 | + | ++ | ++ |
| 13 | 14.039 | Non identified | 373; 413 | 361; 373; 403 | 221 | + | + | - |
| 14 | 15.17 | Pinocembrin | 257; 305 | 255 | 200; 214; 278 | - | ++ | ++ |
| 15 | 15.56 | Resin acid | 219 | 227; 383; 407 | 223 | ++ | ++ | ++ |
| 16 | 15.96 | Resin acid | 207; 235; 357; 398 | 269; 333; 391 | 222 | ++ | ++ | ++ |
| 17 | 16.29 | Todomatuic acid | 255; 401; 554 | 253; 321; 389; 529 | 222 | ++ | ++ | ++ |
| 18 | 17.77 | Dehydrojuvabion | 265 | 248; 355; 401; 643 | 224 | ++ | ++ | ++ |
| Tested Chemical | IC50 (µg/mL) |
|---|---|
| Spruce ethanolic extract | 54.38 ± 0.01 |
| Fir ethanolic extract | 45.81 ± 0.01 |
| Douglas fir ethanolic extract | 23.96 ± 0.02 |
| Hydroxymatairesinol | 29.43 ± 0.04 |
| Taxifolin | 10.06 ± 1.08 |
| Quercetin | 1.59 ± 0.02 |
| Secoisolariciresinol | 9.39 ± 0.01 |
| Gallic acid | 2.04 ± 0.03 |
| Catechin | 4.23 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gérardin, P.; Hentges, D.; Gérardin, P.; Vinchelin, P.; Dumarçay, S.; Audoin, C.; Gérardin-Charbonnier, C. Knotwood and Branchwood Polyphenolic Extractives of Silver Fir, Spruce and Douglas Fir and Their Antioxidant, Antifungal and Antibacterial Properties. Molecules 2023, 28, 6391. https://doi.org/10.3390/molecules28176391
Gérardin P, Hentges D, Gérardin P, Vinchelin P, Dumarçay S, Audoin C, Gérardin-Charbonnier C. Knotwood and Branchwood Polyphenolic Extractives of Silver Fir, Spruce and Douglas Fir and Their Antioxidant, Antifungal and Antibacterial Properties. Molecules. 2023; 28(17):6391. https://doi.org/10.3390/molecules28176391
Chicago/Turabian StyleGérardin, Pauline, David Hentges, Philippe Gérardin, Pierre Vinchelin, Stéphane Dumarçay, Coralie Audoin, and Christine Gérardin-Charbonnier. 2023. "Knotwood and Branchwood Polyphenolic Extractives of Silver Fir, Spruce and Douglas Fir and Their Antioxidant, Antifungal and Antibacterial Properties" Molecules 28, no. 17: 6391. https://doi.org/10.3390/molecules28176391
APA StyleGérardin, P., Hentges, D., Gérardin, P., Vinchelin, P., Dumarçay, S., Audoin, C., & Gérardin-Charbonnier, C. (2023). Knotwood and Branchwood Polyphenolic Extractives of Silver Fir, Spruce and Douglas Fir and Their Antioxidant, Antifungal and Antibacterial Properties. Molecules, 28(17), 6391. https://doi.org/10.3390/molecules28176391