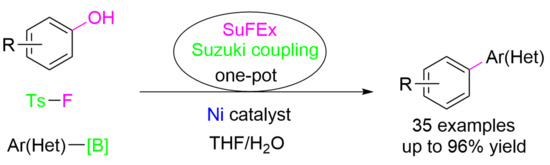
Nickel-Catalyzed Suzuki Coupling of Phenols Enabled by SuFEx of Tosyl Fluoride
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. General Procedure for Nickel/Phosphine Catalyzed Suzuki Coupling of Phenols with Aryl Boronic Acids Enabled by SuFEx of TsF
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Zhuang, Y.; Wachall, B.G.; Hartmann, R.W. Novel imidazolyl and triazolyl substituted biphenyl compounds: Synthesis and evaluation as nonsteroidal inhibitors of human 17α-hydroxylase-C17, 20-lyase. Bioorgan. Med. Chem. 2000, 8, 1245–1252. [Google Scholar] [CrossRef] [PubMed]
- Kerdesky, F.A.J.; Leanna, M.R.; Zhang, J.; Li, W.K.; Lallaman, J.E.; Ji, J.G.; Morton, H.E. An efficient multikilogram synthesis of ABT-963: A selective COX-2 inhibitor. Org. Process Res. Dev. 2006, 10, 512–517. [Google Scholar] [CrossRef]
- Song, K.C.; Kim, H.; Lee, K.M.; Lee, Y.S.; Do, Y.; Lee, M.H. Ratiometric fluorescence sensing of fluoride ions by triarylborane-phenanthroimidazole conjugates. Sens. Actuators B Chem. 2013, 176, 850–857. [Google Scholar] [CrossRef]
- Torborg, C.; Beller, M. Recent applications of palladium-catalyzed coupling reactions in the pharmaceutical, agrochemical, and fine chemical industries. Adv. Synth. Catal. 2009, 351, 3027–3043. [Google Scholar] [CrossRef]
- Percec, V.; Bae, J.-Y.; Hill, D.H. Aryl mesylates in metal catalyzed homocoupling and cross-coupling reactions. 2. Suzuki-type nickel-catalyzed cross-coupling of aryl arenesulfonates and aryl mesylates with arylboronic acids. J. Organomet. Chem. 1995, 60, 1060–1065. [Google Scholar] [CrossRef]
- Wolfe, J.P.; Tomori, H.; Sadighi, J.P.; Yin, J.J.; Buchwald, S.L. Simple, efficient catalyst system for the palladium-catalyzed amination of aryl chlorides, bromides, and triflates. J. Organomet. Chem. 2000, 65, 1158–1174. [Google Scholar] [CrossRef]
- Zim, D.; Lando, V.R.; Dupont, J.; Monteiro, A.L. NiCl2(PCy3)2: A simple and efficient catalyst precursor for the Suzuki cross-coupling of aryl tosylates and arylboronic acids. Org. Lett. 2001, 3, 3049–3051. [Google Scholar] [CrossRef]
- Huang, X.H.; Anderson, K.W.; Zim, D.; Jiang, L.; Klapars, A.; Buchwald, S.L. Expanding Pd-catalyzed C-N bond-forming processes: The first amidation of aryl sulfonates, aqueous amination, and complementarity with Cu-catalyzed reactions. J. Am. Chem. Soc. 2003, 125, 6653–6655. [Google Scholar] [CrossRef]
- Nguyen, H.N.; Huang, X.H.; Buchwald, S.L. The first general palladium catalyst for the Suzuki-Miyaura and carbonyl enolate coupling of aryl arenesulfonates. J. Am. Chem. Soc. 2003, 125, 11818–11819. [Google Scholar] [CrossRef]
- Tang, Z.-Y.; Hu, Q.-S. Room-temperature Ni(0)-catalyzed cross-coupling reactions of aryl arenesulfonates with arylboronic acids. J. Am. Chem. Soc. 2004, 126, 3058–3059. [Google Scholar] [CrossRef]
- Munday, R.H.; Martinelli, J.R.; Buchwald, S.L. Palladium-catalyzed carbonylation of aryl tosylates and mesylates. J. Am. Chem. Soc. 2008, 130, 2754–2755. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, J.-I.; Inamoto, K.; Hiroya, K.; Doi, T. N-heterocyclic carbene derived nickel-pincer complexes: Efficient and applicable catalysts for Suzuki-Miyaura coupling reactions of aryl/alkenyl tosylates and mesylates. Eur. J. Org. Chem. 2009, 14, 2251–2261. [Google Scholar] [CrossRef]
- Molander, G.A.; Beaumard, F. Nickel-catalyzed C-O activation of phenol derivatives with potassium heteroaryltrifluoroborates. Org. Lett. 2010, 12, 4022–4025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, H.; Li, Y.; Zhou, Y.-G.; Han, F.-S.; Lin, Y.-J. Highly efficient Suzuki-Miyaura coupling of aryl tosylates and mesylates catalyzed by stable, cost-effective [1,3- bis(diphenylphosphino)propane] nickel(II) chloride [Ni(dppp)Cl2] with only 1 mol% loading. Adv. Synth. Catal. 2011, 353, 309–314. [Google Scholar] [CrossRef]
- Li, X.M.; Zhang, T.T.; Hu, R.; Zhang, H.; Ren, C.Y.; Yuan, Z.L. A one-pot protocol for the fluorosulfonation and Suzuki coupling of phenols and bromophenols, streamlined access to biaryls and terphenyls. Org. Biomol. Chem. 2020, 18, 4748–4753. [Google Scholar] [CrossRef] [PubMed]
- Quasdorf, K.W.; Riener, M.; Petrova, K.V.; Garg, N.K. Suzuki-Miyaura coupling of aryl carbamates, carbonates, and sulfamates. J. Am. Chem. Soc. 2009, 131, 17748–17749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antoft-Finch, A.; Blackburn, T.; Snieckus, V. N, N-diethyl O-carbamate: Directed metalation group and orthogonal Suzuki-Miyaura cross-coupling partner. J. Am. Chem. Soc. 2009, 131, 17750–17752. [Google Scholar] [CrossRef]
- Xu, L.; Li, B.-J.; Wu, Z.-H.; Lu, X.-Y.; Guan, B.-T.; Wang, B.-Q.; Zhao, K.-Q.; Shi, Z.-J. Nickel-catalyzed efficient and practical Suzuki-Miyaura coupling of alkenyl and aryl carbamates with aryl boroxines. Org. Lett. 2010, 12, 884–887. [Google Scholar] [CrossRef]
- Mesganaw, T.; Garg, N.K. Ni-and Fe-catalyzed cross-coupling reactions of phenol derivatives. Org. Process Res. Dev. 2013, 17, 29–39. [Google Scholar] [CrossRef]
- Luo, Y.; Wu, J. Palladium-catalyzed direct arylation of 4-hydroxycoumarins with arylboronic acids via C-OH bond activation. Tetrahedron Lett. 2009, 50, 2103–2105. [Google Scholar] [CrossRef]
- Chen, L.S.; Lang, H.Y.; Fang, L.; Zhu, M.Y.; Liu, J.Q.; Yu, J.J.; Wang, L.M. Nickel-catalyzed one-pot Suzuki-Miyaura cross-coupling of phenols and arylboronic acids mediated by N, N-ditosylaniline. Eur. J. Org. Chem. 2014, 23, 4953–4957. [Google Scholar] [CrossRef]
- Ikawa, T.; Saito, K.; Akai, S. Palladium-catalyzed one-pot cross-coupling of phenols using nonafluorobutanesulfonyl fluoride. Synlett 2012, 23, 2241–2246. [Google Scholar] [CrossRef]
- Quasdorf, K.W.; Tian, X.; Garg, N.K. Cross-coupling reactions of aryl pivalates with boronic acids. J. Am. Chem. Soc. 2008, 130, 14422–14423. [Google Scholar] [CrossRef] [PubMed]
- Madankar, K.; Mokhtari, J.; Mirjafary, Z. Dichloroimidazolidinedione-activated one-pot Suzuki-Miyaura cross-coupling of phenols. Appl. Organomet. Chem. 2020, 34, 5383–5389. [Google Scholar] [CrossRef]
- Yu, D.-G.; Li, B.-J.; Zheng, S.-F.; Guan, B.-T.; Wang, B.-Q.; Shi, Z.-J. Direct application of phenolic salts to nickel-catalyzed cross-coupling reactions with aryl Grignard reagents. Angew. Chem. Int. Ed. 2010, 49, 4566–4570. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-J.; Huang, J.; Gao, L.-X.; Han, F.-S. Nickel-catalyzed cross-coupling of phenols and arylboronic acids through an in situ phenol activation mediated by PyBroP. Chem. Eur. J. 2011, 17, 4038–4042. [Google Scholar] [CrossRef]
- Rosen, B.M.; Quasdorf, K.W.; Wilson, D.A.; Zhang, N.; Resmerita, A.-M.; Garg, N.K.; Percec, V. Nickel-catalyzed cross-couplings involving carbon-oxygen bonds. Chem. Rev. 2011, 111, 1346–1416. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.-L.; Li, Y.; Li, S.-M.; Zhou, Y.-G.; Sun, F.-Y.; Gao, L.-X.; Han, F.-S. A highly practical and reliable nickel catalyst for Suzuki-Miyaura coupling of aryl halides. Adv. Synth. Catal. 2011, 353, 1543–1550. [Google Scholar] [CrossRef]
- Lipshutz, B.H.; Sclafani, J.A.; Blomgren, P.A. Biaryls via Suzuki cross-couplings catalyzed by nickel on charcoal. Tetrahedron 2000, 56, 2139–2144. [Google Scholar] [CrossRef]
- Fan, X.-H.; Yang, L.-M. Room-temperature nickel-catalysed Suzuki-Miyaura reactions of aryl sulfonates/halides with arylboronic acids. Eur. J. Org. Chem. 2011, 8, 1467–1471. [Google Scholar] [CrossRef]
- Chen, Q.; Fan, X.-H.; Zhang, L.-P.; Yang, L.-M. Nickel-catalyzed cross-coupling of carboxylic anhydrides with arylboronic acids. RSC Adv. 2014, 4, 53885–53890. [Google Scholar] [CrossRef]
- Chen, Q.; Fan, X.-H.; Zhang, L.-P.; Yang, L.-M. Ni(II) source as a pre-catalyst for the cross-coupling of benzylic pivalates with arylboronic acids: Facile access to tri- and diarylmethanes. RSC Adv. 2015, 5, 15338–15340. [Google Scholar] [CrossRef]
- Jezorek, R.L.; Zhang, N.; Leowanawat, P.; Bunner, M.H.; Gutsche, N.; Pesti, A.K.R.; Olsen, J.T.; Percec, V. Air-stable nickel precatalysts for fast and quantitative cross-coupling of aryl sulfamates with aryl neopentylglycolboronates at room temperature. Org. Lett. 2014, 16, 6326–6329. [Google Scholar] [CrossRef]
- Malineni, J.; Jezorek, R.L.; Zhang, N.; Percec, V. NiIICl(1-Naphthyl)(PCy3)2, an air-stable σ-NiII precatalyst for quantitative cross-coupling of aryl C-O electrophiles with aryl neopentylglycolboronates. Synthesis 2016, 48, 2808–2815. [Google Scholar]
- Malineni, J.; Jezorek, R.L.; Zhang, N.; Percec, V. An indefinitely air-stable σ-NiII precatalyst for quantitative cross-coupling of unreactive aryl halides and mesylates with aryl neopentylglycolboronates. Synthesis 2016, 48, 2795–2807. [Google Scholar]
- Li, B.-Y.; Voets, L.; Van Lommel, R.; Hoppenbrouwers, F.; Alonso, M.; Verhelst, S.H.L.; De Borggraeve, W.M.; Demaerel, J. SuFEx-enabled, chemoselective synthesis of triflates, triflamides and triflimidates. Chem. Sci. 2022, 13, 2270–2279. [Google Scholar] [CrossRef] [PubMed]
- Fattaha, T.A.; Saeeda, A.; Albericio, F. Recent advances towards sulfur (VI) fluoride exchange (SuFEx) click chemistry. J. Fluor. Chem. 2018, 213, 87–112. [Google Scholar] [CrossRef]
- Barrow, A.S.; Smedley, C.J.; Zheng, Q.; Li, S.; Dong, J.; Moses, J.E. The growing applications of SuFEx click chemistry. Chem. Soc. Rev. 2019, 48, 4731–4758. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.J.; Krasnova, L.; Finn, M.G.; Sharpless, K.B. Sulfur(VI) fluoride exchange (SuFEx): Another good reaction for click chemistry. Angew. Chem. Int. Ed. 2014, 53, 9430–9448. [Google Scholar] [CrossRef]
- Hanley, P.S.; Ober, M.S.; Krasovskiy, A.L.; Whiteker, G.T.; Kruper, W.J. Nickel- and palladium-catalyzed coupling of aryl fluorosulfonates with aryl boronic acids enabled by sulfuryl fluoride. ACS Catal. 2015, 5, 5041–5046. [Google Scholar] [CrossRef]
- Ke, H.; Chen, X.; Zou, G. N-Heterocyclic carbene-assisted, bis(phosphine)nickel catalyzed cross-couplings of diarylborinic acids with aryl chlorides, tosylates, and sulfamates. J. Organomet. Chem. 2014, 79, 7132–7140. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Zeng, J.S.; Zou, G. Nickel-catalyzed cross-coupling of O, N-chelated diarylborinates with aryl chlorides and mesylates. New J. Chem. 2019, 43, 1589–1596. [Google Scholar] [CrossRef]
- Guo, D.S.; Shi, W.J.; Zou, G. Suzuki coupling of activated aryltriazenes for practical synthesis of biaryls from anilines. Adv. Synth. Catal. 2022, 364, 2438–2442. [Google Scholar] [CrossRef]
- Wang, F.Z.; Wang, C.; Sun, G.P.; Zou, G. Highly efficient palladium-catalyzed cross-coupling of diarylborinic acids with arenediazoniums for practical diaryl synthesis. Tetrahedron Lett. 2020, 61, 151491. [Google Scholar] [CrossRef]
- Fan, X.-H.; Yang, L.-M. NiII-(σ-Aryl) complex catalyzed Suzuki reaction of aryl tosylates with arylboronic acids. Eur. J. Org. Chem. 2010, 13, 2457–2460. [Google Scholar] [CrossRef]
- Duczynski, J.; Sobolev, A.N.; Moggach, S.A.; Dorta, R.; Stewart, S.G. The synthesis and catalytic activity of new mixed NHC-phosphite nickel(0) complexes. Organometallics 2020, 39, 105–115. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; Chen, G.-Q.; Shao, L.X. N-heterocyclic carbene-palladium(II)-1-methylimidazole complex-catalyzed Suzuki-Miyaura coupling of aryl sulfonates with arylboronic acids. J. Organomet. Chem. 2012, 77, 6608–6614. [Google Scholar] [CrossRef]
- Chen, X.F.; Ke, H.H.; Zou, G. Nickel-catalyzed cross-coupling of diarylborinic acids with aryl chlorides. ACS Catal. 2014, 4, 379–385. [Google Scholar] [CrossRef]
- Entz, E.D.; Russell, J.E.A.; Hooker, L.V.; Neufeldt, S.R. Small phosphine ligands enable selective oxidative addition of Ar-O over Ar-Cl bonds at Nickel(0). J. Am. Chem. Soc. 2020, 142, 15454–15463. [Google Scholar] [CrossRef] [PubMed]
- Standley, E.A.; Smith, S.J.; Müller, P.; Jamison, T.F. A broadly applicable strategy for entry into homogeneous nickel(0) catalysts from air-stable nickel(II) complexes. Organometallics 2014, 33, 2012–2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morvillo, A.; Turco, A. Reactions of organic halides and cyanides with bis(tricyclohexylphosphine) nickel(0). J. Organomet. Chem. 1981, 208, 103–113. [Google Scholar] [CrossRef]
- Zhang. G. Efficient Protocol for the Phosphine-Free Suzuki-miyaura reaction catalyzed by palladium on carbon at room temperature. Synthesis 2005, 4, 537–542. [Google Scholar]
- Xu, T.F.; Lu, P.; Wohlrab, S.; Chen, W.X.; Springer, A.; Wu, X.F.; Lu, W.Y. In situ grown palladium nanoparticles on polyester fabric as easy-separable and recyclable catalyst for suzuki-miyaura reaction. Cat. Commun. 2021, 157, 106328–106333. [Google Scholar] [CrossRef]
- Schmidt, A.; Rahimia, A. A versatile catalyst system for suzuki-miyaura syntheses of sterically hindered biaryls employing a cyclobutene-1,2-bis(imidazolium)salt. Chem. Commun. 2010, 46, 2995–2997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.L.; Wu, F.S.; Wan, Z.J.; Wang, Y.C.; He, X.; Guo, B.; You, H.Z.; Chen, F.E. A palladium polyaniline complex: a simple and efficient catalyst for batch and flow suzuki-miyaura cross-couplings. Chem. Commun. 2022, 58, 10845–10848. [Google Scholar] [CrossRef]
- Woolard, K.J.; Sandala, J.L.; Melander, R.J.; Gunn, J. S. Melander, C. Development of small molecules that work cooperatively with ciprofloxacin to clear salmonella biofilms in a chronic gallbladder carriage model. Eur. J. Med. Chem. 2022, 232, 114203–114211. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Long, S.Y.; Jiao, H. B.; Liu, Z.Y.; Zhang, P.; Lei, A.W.; Gong, W.; Pei, X.L. Cellulose derived Pd nano-catalyst for efficient catalysis. RSC Adv. 2022, 12, 18676–18684. [Google Scholar] [CrossRef]
- Watson, M.B.; Rath, N.P.; Mirica, L.M. Oxidative C-C bond formation reactivity of organometallic Ni(II), Ni(III), and Ni(IV) complexes. J. Am. Chem. Soc. 2017, 139, 35–38. [Google Scholar] [CrossRef]
- Ohgi, A.; Semba, K.; Hiyama, T.; Nakao, Y. Silicon-based cross-coupling of aryl tosylates by cooperative palladium/copper catalysis. Chem. Lett. 2016, 45, 973–975. [Google Scholar] [CrossRef]
- Rühl, P.; Rosato, A.S.; Urban, N.; Gerndt, S.; Tang, R. Abrahamian, C.; Leser, C.; Sheng, J.S.; Jha, A.; Vollmer, G.; Schaefer, M.; Bracher, F.; Grimm, C. Estradiol analogs attenuate autophagy, cell migration and invasion by direct and selective inhibition of trpml1, independent of estrogen receptors. Sci. Rep. 2021, 11, 8313–8326. [Google Scholar] [CrossRef]
- Song, C.; Dong, X.; Wang, Z.J.; Liu, K.; Chiang, C.W.; Lei, A. Visible-light induced [4+2] annulation of thiophenes and alkynes to construct benzene rings. Angew. Chem. Int. Ed. 2019, 58, 12206–12210. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, H.; Sato, K.; Hoshi, T.; Suzuki, T. A highly sustainable and active catalyst for suzuki-miyaura reaction: palladium-supported ionic liquid catalyst (SILC) coated with polymer. Synlett 2011, 17, 2545–2550. [Google Scholar] [CrossRef]


 | |||||
|---|---|---|---|---|---|
| Entry | Cat. (mol%) | Ligand | Base (equiv.) | Sol. (vol/vol) | Yield(%) b |
| 1 | NiCl2(PR3)2 (5) c | / | K3PO4·3H2O (5) | THF/H2O (4/1) | trace |
| 2 | cat-1 (5) | / | K3PO4·3H2O (5) | THF/H2O (4/1) | 26 |
| 3 | cat-2 (5) | / | K3PO4·3H2O (5) | THF/H2O (4/1) | 59 |
| 4 | cat-3 (5) | / | K3PO4·3H2O (5) | THF/H2O (4/1) | 56 |
| 5 | cat-4 (5) | / | K3PO4·3H2O (5) | THF/H2O (4/1) | 20 |
| 6 | cat-5 (5) | / | K3PO4·3H2O (5) | THF/H2O (4/1) | 17 |
| 7 | cat-6 (5) | / | K3PO4·3H2O (5) | THF/H2O (4/1) | 46 |
| 8 | cat-7 (5) | / | K3PO4·3H2O (5) | THF/H2O (4/1) | 30 |
| 9 | cat-8 (5) | / | K3PO4·3H2O (5) | THF/H2O (4/1) | 55 |
| 10 | cat-9 (5) | / | K3PO4·3H2O (5) | THF/H2O (4/1) | 87 |
| 11 | cat-9 (5) | PCy3(10) | K3PO4·3H2O (5) | THF/H2O (4/1) | 96 |
| 12 | cat-9 (3) | PCy3(6) | K3PO4·3H2O (5) | THF/H2O (4/1) | 93 d |
| 13 | cat-9 (1) | PCy3(2) | K3PO4·3H2O (5) | THF/H2O (4/1) | 32 d |
| 14 | cat-9 (3) | PCy3(6) | K3PO4·3H2O (5) | THF | 83 |
| 15 | cat-9 (3) | PCy3(6) | K3PO4 (5) | THF | 12 |
| 16 | cat-9 (3) | PCy3(6) | K3PO4·3H2O (5) | THF/H2O (2/1) | 78 d |
| 17 | cat-9 (3) | PCy3(6) | K3PO4·3H2O (5) | THF/H2O (6/1) | 93 d |
| 18 | cat-9 (3) | PCy3(6) | K3PO4·3H2O (5) | THF/H2O (8/1) | 90 d |
| 19 | cat-9 (3) | PCy3(6) | K3PO4·3H2O (5) | Diox/H2O (4/1) | 87 |
| 20 | cat-9 (3) | PCy3(6) | K3PO4·3H2O (5) | DME/H2O (4/1) | 15 |
| 21 | cat-9 (3) | PCy3(6) | K3PO4·3H2O (5) | Tol/H2O (4/1) | 78 |
| 22 | cat-9 (3) | PCy3(6) | K3PO4·3H2O (5) | MeCN/H2O(4/1) | 90 |
| 23 | cat-9 (3) | PCy3(6) | K3PO4·3H2O (5) | DMF/H2O (4/1) | 66 |
| 24 | cat-9 (3) | PCy3(6) | K3PO4·3H2O (5) | DMA/H2O (4/1) | 77 |
| 25 | cat-9 (3) | PCy3(6) | K3PO4·3H2O (5) | DMSO/H2O(4/1) | 75 |
| 26 | cat-9 (3) | PCy3(6) | K2CO3 (5) | THF/H2O (4/1) | 20 |
| 27 | cat-9 (3) | PCy3(6) | NaOH (5) | THF/H2O (4/1) | 18 |
| 28 | cat-9 (3) | PCy3(6) | AcOK (5) | THF/H2O (4/1) | 47 |
| 29 | cat-9 (3) | PCy3(6) | KOH (5) | THF/H2O (4/1) | 30 |
| 30 | cat-9 (3) | PCy3(6) | NaF/KF (5) | THF/H2O (4/1) | trace |
| 31 | cat-9 (3) | PCy3(6) | K3PO4·3H2O (4) | THF/H2O (4/1) | 82 |
| Nickel/Phosphine Catalyzed Suzuki Coupling of Phenols with Arylboronic Acids Enabled by SuFEx of TsF |
|---|
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Zhang, S.; Xü, M.; Zou, G. Nickel-Catalyzed Suzuki Coupling of Phenols Enabled by SuFEx of Tosyl Fluoride. Molecules 2023, 28, 636. https://doi.org/10.3390/molecules28020636
Wang H, Zhang S, Xü M, Zou G. Nickel-Catalyzed Suzuki Coupling of Phenols Enabled by SuFEx of Tosyl Fluoride. Molecules. 2023; 28(2):636. https://doi.org/10.3390/molecules28020636
Chicago/Turabian StyleWang, Huimin, Shuqin Zhang, Minling Xü, and Gang Zou. 2023. "Nickel-Catalyzed Suzuki Coupling of Phenols Enabled by SuFEx of Tosyl Fluoride" Molecules 28, no. 2: 636. https://doi.org/10.3390/molecules28020636
APA StyleWang, H., Zhang, S., Xü, M., & Zou, G. (2023). Nickel-Catalyzed Suzuki Coupling of Phenols Enabled by SuFEx of Tosyl Fluoride. Molecules, 28(2), 636. https://doi.org/10.3390/molecules28020636