Use of Fluorescence Spectroscopy and Chemometrics to Visualise Fluoroquinolones Photodegradation Major Trends: A Confirmation Study with Mass Spectrometry
Abstract
:1. Introduction
2. Results and Discussion
2.1. Photolysis of Individual Solutions
2.1.1. HPLC-UV/Vis Measurements
2.1.2. Fluorescence Spectroscopy Measurements
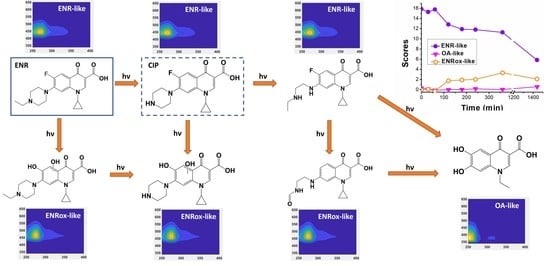
2.1.3. Mass Spectrometry Measurements: Correlating EEM-PARAFAC Components with MS Tentative Molecular Structures
2.2. Photolysis of (F)Q Mixture
3. Materials and Methods
3.1. Reagents
3.2. Irradiations
3.3. Chemical Analysis
3.4. EEM-PARAFAC Modelling
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. WHO List of Critically Important Antimicrobials for Human Medicine (WHO CIA List) 6th Rev. Available online: http://who.int/foodsafety/publications/antimicrobials-fifth/en/ (accessed on 30 January 2021).
- Petrie, B.; McAdam, E.J.; Lester, J.N.; Cartmell, E. Obtaining Process Mass Balances of Pharmaceuticals and Triclosan to Determine Their Fate during Wastewater Treatment. Sci. Total Environ. 2014, 497–498, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Van Doorslaer, X.; Dewulf, J.; Van Langenhove, H.; Demeestere, K. Fluoroquinolone Antibiotics: An Emerging Class of Environmental Micropollutants. Sci. Total Environ. 2014, 500–501, 250–269. [Google Scholar] [CrossRef] [PubMed]
- Pistocchi, A.; Dorati, C.; Grizzetti, B.; Udias, A.; Vigiak, O.; Zanni, M. Water Quality in Europe: Effects of the Urban Wastewater Treatment Directive; Publications Office of the European Union: Luxembourg, 2019. [Google Scholar]
- Ferrando-Climent, L.; Rodriguez-Mozaz, S.; Barceló, D. Incidence of Anticancer Drugs in an Aquatic Urban System: From Hospital Effluents through Urban Wastewater to Natural Environment. Environ. Pollut. 2014, 193, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Hughes, S.R.; Kay, P.; Brown, L.E. Global Synthesis and Critical Evaluation of Pharmaceutical Data Sets Collected from River Systems. Environ. Sci. Technol. 2013, 47, 661–677. [Google Scholar] [CrossRef] [PubMed]
- Snowberger, S.; Adejumo, H.; He, K.; Mangalgiri, K.P.; Hopanna, M.; Soares, A.D.; Blaney, L. Direct Photolysis of Fluoroquinolone Antibiotics at 253.7 Nm: Specific Reaction Kinetics and Formation of Equally Potent Fluoroquinolone Antibiotics. Environ. Sci. Technol. 2016, 50, 9533–9542. [Google Scholar] [CrossRef]
- Albini, A.; Monti, S. Photophysics and Photochemistry of Fluoroquinolones. Chem. Soc. Rev. 2003, 32, 238–250. [Google Scholar] [CrossRef]
- Zhang, Z.; Xie, X.; Yu, Z.; Cheng, H. Influence of Chemical Speciation on Photochemical Transformation of Three Fluoroquinolones (FQs) in Water: Kinetics, Mechanism, and Toxicity of Photolysis Products. Water Res. 2019, 148, 19–29. [Google Scholar] [CrossRef]
- Hubicka, U.; Krzek, J.; Zuromska, B.; Walczak, M.; Zylewski, M.; Pawłowski, D. Determination of Photostability and Photodegradation Products of Moxifloxacin in the Presence of Metal Ions in Solutions and Solid Phase. Kinetics and Identification of Photoproducts. Photochem. Photobiol. Sci. 2012, 11, 351–357. [Google Scholar] [CrossRef]
- Serna-Galvis, E.A.; Martínez-Mena, Y.L.; Porras, J.; Ávila-Torres, Y.; Silva-Agredo, J.; Torres-Palma, R.A. Understanding the Role of Complexation of Fluoroquinolone and Β-lactam Antibiotics with Iron (Iii) on the Photodegradation under Solar Light and Uvc Light. Water 2021, 13, 2603. [Google Scholar] [CrossRef]
- Sciscenko, I.; Garcia-Ballesteros, S.; Sabater, C.; Castillo, M.A.; Escudero-Oñate, C.; Oller, I.; Arques, A. Monitoring Photolysis and (Solar Photo)-Fenton of Enrofloxacin by a Methodology Involving EEM-PARAFAC and Bioassays: Role of PH and Water Matrix. Sci. Total Environ. 2020, 719, 137331. [Google Scholar] [CrossRef]
- Sciscenko, I.; Thị Mỹ Hắng, H.; Escudero-Oñate, C.; Oller, I.; Arques, A. Fluorescence Spectroscopy and Chemometrics: A Simple and Easy Way for the Monitoring of Fluoroquinolone Mixture Degradation. ACS Omega 2021, 6, 4663–4671. [Google Scholar] [CrossRef] [PubMed]
- Sciscenko, I.; Mora, M.; Micó, P.; Escudero-Oñate, C.; Oller, I.; Arques, A. EEM-PARAFAC as a Convenient Methodology to Study Fluorescent Emerging Pollutants Degradation: (Fluoro)Quinolones Oxidation in Different Water Matrices. Sci. Total Environ. 2022, 852, 158338. [Google Scholar] [CrossRef] [PubMed]
- Bro, R. PARAFAC. Tutorial and Applications. Chemom. Intell. Lab. Syst. 1997, 38, 149–171. [Google Scholar] [CrossRef]
- Murphy, K.R.; Stedmon, C.A.; Graeber, D.; Bro, R. Fluorescence Spectroscopy and Multi-Way Techniques. PARAFAC. Anal. Methods 2013, 5, 6557. [Google Scholar] [CrossRef] [Green Version]
- Tanioka, M.; Ebihana, T.; Uraguchi, M.; Shoji, H.; Nakamura, Y.; Ueda, R.; Ogura, S.; Wakiya, Y.; Obata, T.; Ida, T.; et al. Visualization of the Photodegradation of a Therapeutic Drug by Chemometric-Assisted Fluorescence Spectroscopy. RSC Adv. 2022, 12, 20714–20720. [Google Scholar] [CrossRef]
- Burns, R.L.; Alexander, R.; Snaychuk, L.; Edwards, J.C.; Fitzgerald, N.; Gao, P.; Quan, D.; Douvris, C.; Vaughan, T.; Bussan, D.D. A Fast, Straightforward and Inexpensive Method for the Authentication of Baijiu Spirit Samples by Fluorescence Spectroscopy. Beverages 2021, 7, 65. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, C.H. Oxidative Transformation of Fluoroquinolone Antibacterial Agents and Structurally Related Amines by Manganese Oxide. Environ. Sci. Technol. 2005, 39, 4474–4483. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Na, G.; Zhang, S.; Li, K.; Zhang, P.; Ren, H.; Yao, Z. New Insights into the Aquatic Photochemistry of Fluoroquinolone Antibiotics: Direct Photodegradation, Hydroxyl-Radical Oxidation, and Antibacterial Activity Changes. Sci. Total Environ. 2015, 527–528, 12–17. [Google Scholar] [CrossRef]
- Sturini, M.; Speltini, A.; Maraschi, F.; Pretali, L.; Ferri, E.N.; Profumo, A. Sunlight-Induced Degradation of Fluoroquinolones in Wastewater Effluent: Photoproducts Identification and Toxicity. Chemosphere 2015, 134, 313–318. [Google Scholar] [CrossRef]
- Sciscenko, I.; Arques, A.; Escudero-Oñate, C.; Roccamante, M.; Ruiz-Delgado, A.; Miralles-Cuevas, S.; Malato, S.; Oller, I. A Rational Analysis on Key Parameters Ruling Zerovalent Iron-Based Treatment Trains: Towards the Separation of Reductive from Oxidative Phases. Nanomaterials 2021, 11, 2948. [Google Scholar] [CrossRef]
- Żamojć, K.; Bylińska, I.; Wiczk, W.; Chmurzyński, L. Fluorescence Quenching Studies on the Interactions between Chosen Fluoroquinolones and Selected Stable TEMPO and PROXYL Nitroxides. Int. J. Mol. Sci. 2021, 22, 885. [Google Scholar] [CrossRef] [PubMed]
- Wammer, K.H.; Korte, A.R.; Lundeen, R.A.; Sundberg, J.E.; McNeill, K.; Arnold, W.A. Direct Photochemistry of Three Fluoroquinolone Antibacterials: Norfloxacin, Ofloxacin, and Enrofloxacin. Water Res. 2013, 47, 439–448. [Google Scholar] [CrossRef]
- An, T.; Yang, H.; Song, W.; Li, G.; Luo, H.; Cooper, W.J. Mechanistic Considerations for the Advanced Oxidation Treatment of Fluoroquinolone Pharmaceutical Compounds Using TiO2 Heterogeneous Catalysis. J. Phys. Chem. A 2010, 114, 2569–2575. [Google Scholar] [CrossRef] [PubMed]
- Dichiarante, V.; Pretali, L.; Fasani, E.; Albini, A. Photochemistry of Some Non Zwitterionic Fluoroquinolones. J. Photochem. Photobiol. A Chem. 2013, 265, 41–48. [Google Scholar] [CrossRef]
- Haddad, T.; Kümmerer, K. Characterization of Photo-Transformation Products of the Antibiotic Drug Ciprofloxacin with Liquid Chromatography-Tandem Mass Spectrometry in Combination with Accurate Mass Determination Using an LTQ-Orbitrap. Chemosphere 2014, 115, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Sciscenko, I.; Arques, A.; Varga, Z.; Bouchonnet, S.; Monfort, O.; Brigante, M.; Mailhot, G. Significant Role of Iron on the Fate and Photodegradation of Enrofloxacin. Chemosphere 2021, 270, 129791. [Google Scholar] [CrossRef]
- Sturini, M.; Speltini, A.; Maraschi, F.; Profumo, A.; Pretali, L.; Irastorza, E.A.; Fasani, E.; Albini, A. Photolytic and Photocatalytic Degradation of Fluoroquinolones in Untreated River Water under Natural Sunlight. Appl. Catal. B Environ. 2012, 119–120, 32–39. [Google Scholar] [CrossRef]
- Porras, J.; Bedoya, C.; Silva-Agredo, J.; Santamaría, A.; Fernández, J.J.; Torres-Palma, R.A. Role of Humic Substances in the Degradation Pathways and Residual Antibacterial Activity during the Photodecomposition of the Antibiotic Ciprofloxacin in Water. Water Res. 2016, 94, 1–9. [Google Scholar] [CrossRef]
- Vasconcelos, T.G.; Henriques, D.M.; König, A.; Martins, A.F.; Kümmerer, K. Photo-Degradation of the Antimicrobial Ciprofloxacin at High PH: Identification and Biodegradability Assessment of the Primary by-Products. Chemosphere 2009, 76, 487–493. [Google Scholar] [CrossRef]
- Michael, I.; Hapeshi, E.; Aceña, J.; Perez, S.; Petrović, M.; Zapata, A.; Barceló, D.; Malato, S.; Fatta-Kassinos, D. Light-Induced Catalytic Transformation of Ofloxacin by Solar Fenton in Various Water Matrices at a Pilot Plant: Mineralization and Characterization of Major Intermediate Products. Sci. Total Environ. 2013, 461–462, 39–48. [Google Scholar] [CrossRef]
- Qi, Y.; Qu, R.; Liu, J.; Chen, J.; Al-Basher, G.; Alsultan, N.; Wang, Z.; Huo, Z. Oxidation of Flumequine in Aqueous Solution by UV-Activated Peroxymonosulfate: Kinetics, Water Matrix Effects, Degradation Products and Reaction Pathways. Chemosphere 2019, 237, 124484. [Google Scholar] [CrossRef] [PubMed]
- Giraldo, A.L.; Peñuela, G.A.; Torres-Palma, R.A.; Pino, N.J.; Palominos, R.A.; Mansilla, H.D. Degradation of the Antibiotic Oxolinic Acid by Photocatalysis with TiO2 in Suspension. Water Res. 2010, 44, 5158–5167. [Google Scholar] [CrossRef] [PubMed]
- Aufartová, J.; Brabcová, I.; Torres-Padrón, M.E.; Solich, P.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J. Determination of Fluoroquinolones in Fishes Using Microwave-Assisted Extraction Combined with Ultra-High Performance Liquid Chromatography and Fluorescence Detection. J. Food Compos. Anal. 2017, 56, 140–146. [Google Scholar] [CrossRef]
- Lu, S.; Lin, C.; Lei, K.; Wang, B.; Xin, M.; Gu, X.; Cao, Y.; Liu, X.; Ouyang, W.; He, M. Occurrence, Spatiotemporal Variation, and Ecological Risk of Antibiotics in the Water of the Semi-Enclosed Urbanized Jiaozhou Bay in Eastern China. Water Res. 2020, 184, 116187. [Google Scholar] [CrossRef]
- Niu, X.Z.; Busetti, F.; Langsa, M.; Croué, J.P. Roles of Singlet Oxygen and Dissolved Organic Matter in Self-Sensitized Photo-Oxidation of Antibiotic Norfloxacin under Sunlight Irradiation. Water Res. 2016, 106, 214–222. [Google Scholar] [CrossRef]
- Yang, L.; Hur, J.; Zhuang, W. Occurrence and Behaviors of Fluorescence EEM-PARAFAC Components in Drinking Water and Wastewater Treatment Systems and Their Applications: A Review. Environ. Sci. Pollut. Res. 2015, 22, 6500–6510. [Google Scholar] [CrossRef]
- Micó, P.; García-Ballesteros, S.; Mora, M.; Vicente, R.; Amat, A.M.; Arques, A. EEMlab: A Graphical User-Friendly Interface for Fluorimetry Experiments Based on the DrEEM Toolbox. Chemom. Intell. Lab. Syst. 2019, 188, 6–13. [Google Scholar] [CrossRef]
- Sciscenko, I.; Arques, A.; Micó, P.; Mora, M.; García-Ballesteros, S. Emerging Applications of EEM-PARAFAC for Water Treatment: A Concise Review. Chem. Eng. J. Adv. 2022, 10, 100286. [Google Scholar] [CrossRef]







| Photoproduct Name | Experimental Mass from [M-H]+ (g/mol) | Molecular Formula | Tentative Molecular Structure | Reported by | Associated PARAFAC Component |
|---|---|---|---|---|---|
| E1 | 332.14050 | C17H18O3N3F |  | [7,24,28] | ENR-like |
| E2 | 334.15615 | C17H20O3N3F |  | [28,29] | ENR-like |
| E3 | 374.17105 | C19H23O5N3 |  | --- | ENRox-like |
| C1 | 346.14114 | C17H19O5N3 |  | [27,30] | ENRox-like |
| C2 | 316.13034 | C16H17O4N3 |  | [30,31] | ENRox-like |
| C3 | 250.07191 | C12H11O5N |  | --- | OA-like |
| O1 | 348.13541 | C17H18O4N3F |  | [9,32] | OFL-like |
| O2 | 364.13229 | C17H18O5N3F |  | [32] | OFL-like |
| F1 | 276.06589 | C14H10O4NF |  | [33] | --- |
| Oa1 | 278.07141 | C13H11O6N |  | [34] | --- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sciscenko, I.; García-Negueroles, P.; Amat, A.M.; Oller, I.; Escudero-Oñate, C.; Ferrando-Climent, L.; Arques, A. Use of Fluorescence Spectroscopy and Chemometrics to Visualise Fluoroquinolones Photodegradation Major Trends: A Confirmation Study with Mass Spectrometry. Molecules 2023, 28, 777. https://doi.org/10.3390/molecules28020777
Sciscenko I, García-Negueroles P, Amat AM, Oller I, Escudero-Oñate C, Ferrando-Climent L, Arques A. Use of Fluorescence Spectroscopy and Chemometrics to Visualise Fluoroquinolones Photodegradation Major Trends: A Confirmation Study with Mass Spectrometry. Molecules. 2023; 28(2):777. https://doi.org/10.3390/molecules28020777
Chicago/Turabian StyleSciscenko, Iván, Paula García-Negueroles, Ana María Amat, Isabel Oller, Carlos Escudero-Oñate, Laura Ferrando-Climent, and Antonio Arques. 2023. "Use of Fluorescence Spectroscopy and Chemometrics to Visualise Fluoroquinolones Photodegradation Major Trends: A Confirmation Study with Mass Spectrometry" Molecules 28, no. 2: 777. https://doi.org/10.3390/molecules28020777
APA StyleSciscenko, I., García-Negueroles, P., Amat, A. M., Oller, I., Escudero-Oñate, C., Ferrando-Climent, L., & Arques, A. (2023). Use of Fluorescence Spectroscopy and Chemometrics to Visualise Fluoroquinolones Photodegradation Major Trends: A Confirmation Study with Mass Spectrometry. Molecules, 28(2), 777. https://doi.org/10.3390/molecules28020777