Synthesis and Antiparasitic Activity of New Trithiolato-Bridged Dinuclear Ruthenium(II)-arene-carbohydrate Conjugates
Abstract
:1. Introduction
2. Results and Discussions
2.1. Synthesis
2.1.1. Synthesis of the Dinuclear Ruthenium(II)-arene Intermediates 2–9
2.1.2. Synthesis of the Azide and Alkyne Functionalized Carbohydrate Intermediates 10–18
2.1.3. Synthesis of the Carbohydrate Functionalized Trithiolato-Bridged Dinuclear Ruthenium(II)-arene Complexes 19–26
2.1.4. Stability of the Compounds
2.2. Assessment of the In Vitro Activity against T. Gondii β-gal and Human Foreskin Fibroblast Host Cells
2.2.1. Primary Screening
2.2.2. IC50 Values against T. gondii β-gal Tachyzoites and HFF Toxicity at 2.5 µM
3. Materials and Methods
3.1. Chemistry
3.2. Biological Evaluation
3.2.1. Cell and Parasite Culture
3.2.2. In Vitro Activity Assessment against T. Gondii Tachyzoites and Human Foreskin Fibroblasts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Muhammad, N.; Guo, Z. Metal-based anticancer chemotherapeutic agents. Curr. Opin. Chem. Biol. 2014, 19, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Farrell, N. Metal complexes as drugs and chemotherapeutic agents. In Comprehensive Coordination Chemistry II; Elsevier: Amsterdam, The Netherlands, 2004; Volume 9, pp. 809–840. [Google Scholar]
- Ndagi, U.; Mhlongo, N.; Soliman, M.E. Metal complexes in cancer therapy–an update from drug design perspective. Drug Des. Devel. Ther. 2017, 11, 599–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makovec, T. Cisplatin and beyond: Molecular mechanisms of action and drug resistance development in cancer chemotherapy. Radiol. Oncol. 2019, 53, 148–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galluzzi, L.; Senovilla, L.; Vitale, I.; Michels, J.; Martins, I.; Kepp, O.; Castedo, M.; Kroemer, G. Molecular mechanisms of cisplatin resistance. Oncogene 2012, 31, 1869–1883. [Google Scholar] [CrossRef] [Green Version]
- Karges, J.; Stokes, R.W.; Cohen, S.M. Metal complexes for therapeutic applications. Trends Chem. 2021, 3, 523–534. [Google Scholar] [CrossRef]
- Ong, Y.C.; Gasser, G. Organometallic compounds in drug discovery: Past, present and future. Drug Discov. Today Technol. 2020, 37, 117–124. [Google Scholar] [CrossRef]
- de Oliveira Silva, D. Ruthenium compounds targeting cancer therapy. In Frontiers in Anti-Cancer Drug Discovery; Atta-ur-Rahman, I., Choudhary, M., Eds.; Bentham Science: Sharjah, United Arab Emirates, 2014; Volume 4, pp. 88–156. [Google Scholar]
- Frei, A.; Zuegg, J.; Elliott, A.G.; Baker, M.; Braese, S.; Brown, C.; Chen, F.; Dowson, C.G.; Du, G. Metal complexes, an untapped source of antibiotic potential? Antibiotics 2020, 9, 90. [Google Scholar] [CrossRef] [Green Version]
- Frei, A.; Zuegg, J.; Elliott, A.G.; Baker, M.; Braese, S.; Brown, C.; Chen, F.; Dowson, C.G.; Dujardin, G.; Jung, N.; et al. Metal complexes as a promising source for new antibiotics. Chem. Sci. 2020, 11, 2627–2639. [Google Scholar] [CrossRef] [Green Version]
- Gambino, D.; Otero, L. Design of prospective antiparasitic metal-based compounds including selected organometallic cores. Inorg. Chim. Acta 2018, 472, 58–75. [Google Scholar] [CrossRef]
- Gambino, D.; Otero, Á.L. Metal compounds in the development of antiparasitic agents: Rational design from basic chemistry to the clinic. Met. Ions Life Sci. 2019, 19, 331–358. [Google Scholar] [CrossRef]
- Mbaba, M.; Golding, T.M.; Smith, G.S. Recent advances in the biological investigation of organometallic platinum-group metal (Ir, Ru, Rh, Os, Pd, Pt) complexes as antimalarial agents. Molecules 2020, 25, 5276. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Delgado, R.A.; Anzellotti, A. Metal complexes as chemotherapeutic agents against tropical diseases: Trypanosomiasis, malaria and leishmaniasis. Mini Rev. Med. Chem. 2004, 4, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Navarro, M.; Gabbiani, C.; Messori, L.; Gambino, D. Metal-based drugs for malaria, trypanosomiasis and leishmaniasis: Recent achievements and perspectives. Drug Discov. Today 2010, 15, 1070–1078. [Google Scholar] [CrossRef] [PubMed]
- Ong, Y.C.; Roy, S.; Andrews, P.C.; Gasser, G. Metal compounds against neglected tropical diseases. Chem. Rev. 2019, 119, 730–796. [Google Scholar] [CrossRef] [PubMed]
- Bergamo, A.; Gaiddon, C.; Schellens, J.H.M.; Beijnen, J.H.; Sava, G. Approaching tumour therapy beyond platinum drugs: Status of the art and perspectives of ruthenium drug candidates. J. Inorg. Biochem. 2012, 106, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Alessio, E.; Messori, L. NAMI-A and KP1019/1339, two iconic ruthenium anticancer drug candidates face-to-face: A case story in medicinal inorganic chemistry. Molecules 2019, 24, 1995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiss, A.; Berndsen, R.H.; Dubois, M.; Müller, C.; Schibli, R.; Griffioen, A.W.; Dyson, P.J.; Nowak-Sliwinska, P. In vivo anti-tumor activity of the organometallic ruthenium(II)-arene complex [Ru(η6-p-cymene)Cl2(pta)] (RAPTA-C) in human ovarian and colorectal carcinomas. Chem. Sci. 2014, 5, 4742–4748. [Google Scholar] [CrossRef] [Green Version]
- Aird, R.E.; Cummings, J.; Ritchie, A.A.; Muir, M.; Morris, R.E.; Chen, H.; Sadler, P.J.; Jodrell, D.I. In vitro and in vivo activity and cross resistance profiles of novel ruthenium(II) organometallic arene complexes in human ovarian cancer. Br. J. Cancer 2002, 86, 1652–1657. [Google Scholar] [CrossRef] [Green Version]
- Su, W.; Tang, Z.; Li, P. Development of arene ruthenium antitumor complexes. Mini Rev. Med. Chem. 2016, 16, 787–795. [Google Scholar] [CrossRef]
- Su, W.; Li, Y.; Li, P. Design of Ru-arene complexes for antitumor drugs. Mini Rev. Med. Chem. 2018, 18, 184–193. [Google Scholar] [CrossRef]
- Golbaghi, G.; Castonguay, A. Rationally designed ruthenium complexes for breast cancer therapy. Molecules 2020, 25, 265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biersack, B. Anticancer activity and modes of action of (arene) ruthenium(II) complexes coordinated to C-, N-, and O-ligands. Mini Rev. Med. Chem. 2016, 16, 804–814. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Wu, Q.; Ding, Y.; Mei, W. Arene ruthenium(II) complexes: The promising chemotherapeutic agent in inhibiting the proliferation, migration and invasion. Mini-Rev. Med. Chem. 2016, 16, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Laurent, Q.; Batchelor, L.K.; Dyson, P.J. Applying a Trojan horse strategy to ruthenium complexes in the pursuit of novel antibacterial agents. Organometallics 2018, 37, 915–923. [Google Scholar] [CrossRef]
- Furrer, J.; Süss-Fink, G. Thiolato-bridged dinuclear arene ruthenium complexes and their potential as anticancer drugs. Coord. Chem. Rev. 2016, 309, 36–50. [Google Scholar] [CrossRef]
- Basto, A.P.; Müller, J.; Rubbiani, R.; Stibal, D.; Giannini, F.; Süss-Fink, G.; Balmer, V.; Hermphill, A.; Gasser, G.; Furrer, J. Characterization of the activities of dinuclear thiolato-bridged arene ruthenium complexes against Toxoplasma gondii. Antimicrob. Agents Chemother. 2017, 61, e01031-17. [Google Scholar] [CrossRef] [Green Version]
- Basto, A.P.; Anghel, N.; Rubbiani, R.; Müller, J.; Stibal, D.; Giannini, F.; Süss-Fink, G.; Balmer, V.; Gasser, G.; Furrer, J.; et al. Targeting of the mitochondrion by dinuclear thiolato-bridged arene ruthenium complexes in cancer cells and in the apicomplexan parasite Neospora caninum. Metallomics 2019, 11, 462–474. [Google Scholar] [CrossRef] [Green Version]
- Jelk, J.; Balmer, V.; Stibal, D.; Giannini, F.; Süss-Fink, G.; Bütikofer, P.; Furrer, J.; Hemphill, A. Anti-parasitic dinuclear thiolato-bridged arene ruthenium complexes alter the mitochondrial ultrastructure and membrane potential in Trypanosoma brucei bloodstream forms. Exp. Parasitol. 2019, 205, 107753. [Google Scholar] [CrossRef]
- Hill, D.; Dubey, J.P. Toxoplasma gondii: Transmission, diagnosis and prevention. Clin. Microbiol. Infect. 2002, 8, 634–640. [Google Scholar] [CrossRef] [Green Version]
- Tenter, A.M.; Heckeroth, A.R.; Weiss, L.M. Toxoplasma gondii: From animals to humans. Int. J. Parasitol. 2000, 30, 1217–1258. [Google Scholar] [CrossRef]
- Konstantinovic, N.; Guegan, H.; Stäjner, T.; Belaz, S.; Robert-Gangneux, F. Treatment of toxoplasmosis: Current options and future perspectives. Food Waterborne Parasitol. 2019, 15, e00036. [Google Scholar] [CrossRef]
- Alday, P.H.; Doggett, J.S. Drugs in development for toxoplasmosis: Advances, challenges, and current status. Drug Des. Dev. Ther. 2017, 11, 273–293. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Wu, T.; Zhai, S.Q.; Li, C.H. Recent progress on anti-Toxoplasma drugs discovery: Design, synthesis and screening. Eur. J. Med. Chem. 2019, 183, 111711. [Google Scholar] [CrossRef] [PubMed]
- Antczak, M.; Dzitko, K.; Dlugonska, H. Human toxoplasmosis-Searching for novel chemotherapeutics. Biomed. Pharmacother. 2016, 82, 677–684. [Google Scholar] [CrossRef]
- Coppens, I. Exploitation of auxotrophies and metabolic defects in Toxoplasma as therapeutic approaches. Int. J. Parasitol. 2014, 44, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Blume, M.; Seeber, F. Metabolic interactions between Toxoplasma gondii and its host. F1000Research 2018, 7, 1719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kenny, R.G.; Marmion, C.J. Toward multi-targeted platinum and ruthenium drugs—A new paradigm in cancer drug treatment regimens? Chem. Rev. 2019, 119, 1058–1137. [Google Scholar] [CrossRef]
- Bononi, G.; Iacopini, D.; Cicio, G.; Di Pietro, S.; Granchi, C.; Di Bussolo, V.; Minutolo, F. Glycoconjugated metal complexes as cancer diagnostic and therapeutic agents. ChemMedChem 2021, 16, 30–64. [Google Scholar] [CrossRef]
- Pettenuzzo, A.; Pigot, R.; Ronconi, L. Metal-based glycoconjugates and their potential in targeted anticancer chemotherapy. Metallodrugs 2015, 1, 36–61. [Google Scholar] [CrossRef] [Green Version]
- Byrne, J.P.; Musembi, P.; Albrecht, M. Carbohydrate-functionalized N-heterocyclic carbene Ru(II) complexes: Synthesis, characterization and catalytic transfer hydrogenation activity. Dalton Trans. 2019, 48, 11838–11847. [Google Scholar] [CrossRef]
- Pretorius, R.; Olguín, J.; Albrecht, M. Carbohydrate-functionalized 1,2,3-triazolylidene complexes for application in base-free alcohol and amine oxidation. Inorg. Chem. 2017, 56, 12410–12420. [Google Scholar] [CrossRef] [PubMed]
- Cucciolito, M.E.; D’Amora, A.; De Feo, G.; Ferraro, G.; Giorgio, A.; Petruk, G.; Monti, D.M.; Merlino, A.; Ruffo, F. Five-coordinate platinum(II) compounds containing sugar ligands: Synthesis, characterization, cytotoxic activity, and interaction with biological macromolecules. Inorg. Chem. 2018, 57, 3133–3143. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, A.; Cucciolito, M.E.; Esposito, R.; Ferraro, G.; Monti, D.M.; Merlino, A.; Ruffo, F. Five-coordinate platinum(II) compounds as potential anticancer agents. Eur. J. Inorg. Chem. 2020, 2020, 918–929. [Google Scholar] [CrossRef]
- Annunziata, A.; Liberti, D.; Bedini, E.; Cucciolito, M.E.; Loreto, D.; Monti, D.M.; Merlino, A.; Ruffo, F. Square-planar vs. trigonal bipyramidal geometry in Pt(II) complexes containing triazole-based glucose ligands as potential anticancer agents. Int. J. Mol. Sci. 2021, 22, 8704. [Google Scholar] [CrossRef]
- Patra, M.; Johnstone, T.C.; Suntharalingam, K.; Lippard, S.J. A potent glucose-platinum conjugate exploits glucose transporters and preferentially accumulates in cancer cells. Angew. Chem. Int. Ed. Engl. 2016, 55, 2550–2554. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Liu, S.; Shi, Y.; Huang, Z.; Mi, Y.; Mi, Q.; Yang, J.; Gao, Q. Mechanistic and biological characteristics of different sugar conjugated 2-methyl malonatoplatinum(II) complexes as new tumor targeting agents. Eur. J. Med. Chem. 2017, 125, 372–384. [Google Scholar] [CrossRef]
- Wu, M.; Li, H.; Liu, R.; Gao, X.; Zhang, M.; Liu, P.; Fu, Z.; Yang, J.; Zhang-Negrerie, D.; Gao, Q. Galactose conjugated platinum(II) complex targeting the Warburg effect for treatment of non-small cell lung cancer and colon cancer. Eur. J. Med. Chem. 2016, 110, 32–42. [Google Scholar] [CrossRef]
- Quan, L.; Lin, Z.; Lin, Y.; Wei, Y.; Lei, L.; Li, Y.; Tan, G.; Xiao, M.; Wu, T. Glucose-modification of cisplatin to facilitate cellular uptake, mitigate toxicity to normal cells, and improve anti-cancer effect in cancer cells. J. Mol. Struct. 2020, 1203, 127361. [Google Scholar] [CrossRef]
- Wang, H.; Yang, X.; Zhao, C.; Wang, P.G.; Wang, X. Glucose-conjugated platinum(IV) complexes as tumor-targeting agents: Design, synthesis and biological evaluation. Bioorg. Med. Chem. 2019, 27, 1639–1645. [Google Scholar] [CrossRef]
- Patra, M.; Awuah, S.G.; Lippard, S.J. Chemical approach to positional isomers of glucose–platinum conjugates reveals specific cancer targeting through glucose-transporter-mediated uptake in vitro and in vivo. J. Am. Chem. Soc. 2016, 138, 12541–12551. [Google Scholar] [CrossRef]
- Ma, J.; Liu, H.; Xi, Z.; Hou, J.; Li, Y.; Niu, J.; Liu, T.; Bi, S.; Wang, X.; Wang, C.; et al. Protected and de-protected platinum(IV) glycoconjugates with GLUT1 and OCT2-mediated selective cancer targeting: Demonstrated enhanced transporter-mediated cytotoxic properties in vitro and in vivo. Front. Chem. 2018, 6, 386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, J.; Wang, Q.; Yang, X.; Hao, W.; Huang, Z.; Zhang, J.; Wang, X.; Wang, P.G. Glycosylated platinum(IV) prodrugs demonstrated significant therapeutic efficacy in cancer cells and minimized side-effects. Dalton Trans. 2016, 45, 11830–11838. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Yang, X.; Hao, W.; Huang, Z.; Wang, X.; Wang, P.G. Mono-functionalized glycosylated platinum(IV) complexes possessed both pH and redox dual-responsive properties: Exhibited enhanced safety and preferentially accumulated in cancer cells in vitro and in vivo. Eur. J. Med. Chem. 2017, 128, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Calvaresi, E.C.; Hergenrother, P.J. Glucose conjugation for the specific targeting and treatment of cancer. Chem. Sci. 2013, 4, 2319–2333. [Google Scholar] [CrossRef] [Green Version]
- Medina, R.A.; Owen, G.I. Glucose transporters: Expression, regulation and cancer. Biol. Res. 2002, 35, 9–26. [Google Scholar] [CrossRef]
- Macheda, M.L.; Rogers, S.; Best, J.D. Molecular and cellular regulation of glucose transporter (GLUT) proteins in cancer. J. Cell. Physiol. 2005, 202, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Szablewski, L. Expression of glucose transporters in cancers. Biochim. Biophys. Acta 2013, 1835, 164–169. [Google Scholar] [CrossRef]
- Fernandes, A.C. Synthesis, biological activity and medicinal applications of ruthenium complexes containing carbohydrate ligands. Curr. Med. Chem. 2019, 26, 6412–6437. [Google Scholar] [CrossRef]
- Iacopini, D.; Biancalana, L.; Di Pietro, S.; Marchetti, F.; Di Bussolo, V. Stereoselective synthesis of new glycoconjugated ruthenium(II)-arene complexes as potential anticancer agents. In Proceedings of the EuroCarb XX, Leiden, The Netherlands, 30 June–4 July 2019. [Google Scholar]
- D’Amora, A.; Cucciolito, M.E.; Iannitti, R.; Morelli, G.; Palumbo, R.; Ruffo, F.; Tesauro, D. Pyridine ruthenium(III) complexes entrapped in liposomes with enhanced cytotoxic properties in PC-3 prostate cancer cells. J. Drug Deliv. Sci. Technol. 2019, 51, 552–558. [Google Scholar] [CrossRef]
- Valente, A.; Garcia, M.H.; Marques, F.; Miao, Y.; Rousseau, C.; Zinck, P. First polymer “ruthenium-cyclopentadienyl” complex as potential anticancer agent. J. Inorg. Biochem. 2013, 127, 79–81. [Google Scholar] [CrossRef]
- Lamač, M.; Horáček, M.; Červenková Šťastná, L.; Karban, J.; Sommerová, L.; Skoupilová, H.; Hrstka, R.; Pinkas, J. Harmless glucose-modified ruthenium complexes suppressing cell migration of highly invasive cancer cell lines. Appl. Organometal. Chem. 2019, 34, e5318. [Google Scholar] [CrossRef]
- Hanif, M.; Meier, S.M.; Nazarov, A.A.; Risse, J.; Legin, A.; Casini, A.; Jakupec, M.A.; Keppler, B.K.; Hartinger, C.G. Influence of the π-coordinated arene on the anticancer activity of ruthenium(II)carbohydrate organometallic complexes. Front. Chem. 2013, 1, 27. [Google Scholar] [CrossRef] [Green Version]
- Berger, I.; Hanif, M.; Nazarov, A.A.; Hartinger, C.G.; John, R.O.; Kuznetsov, M.L.; Groessl, M.; Schmitt, F.; Zava, O.; Biba, F.; et al. In vitro anticancer activity and biologically relevant metabolization of organometallic ruthenium com plexes with carbohydrate-based ligands. Chem. Eur. J. 2008, 14, 9046–9057. [Google Scholar] [CrossRef] [PubMed]
- Böge, M.; Fowelin, C.; Bednarski, P.; Heck, J. Diaminohexopyranosides as ligands in half-sandwich ruthenium(II), rhodium(III), and iridium(III) complexes. Organometallics 2015, 34, 1507–1521. [Google Scholar] [CrossRef]
- Florindo, P.; Marques, I.J.; Nunes, C.D.; Fernandes, A.C. Synthesis, characterization and cytotoxicity of cyclopentadienyl ruthenium(II) complexes containing carbohydrate-derived ligands. J. Organomet. Chem. 2014, 760, 240–247. [Google Scholar] [CrossRef]
- Florindo, P.R.; Pereira, D.M.; Borralho, P.M.; Rodrigues, C.M.P.; Piedade, M.F.M.; Fernandes, A.C. Cyclopentadienyl–ruthenium(II) and iron(II) organometallic compounds with carbohydrate derivative ligands as good colorectal anticancer agents. J. Med. Chem. 2015, 58, 4339–4347. [Google Scholar] [CrossRef]
- Hamala, V.; Martišová, A.; Červenková Šťastná, L.; Karban, J.; Dančo, A.; Šimarek, A.; Lamač, M.; Horáček, M.; Kolářová, T.; Hrstka, R.; et al. Ruthenium tetrazene complexes bearing glucose moieties on their periphery: Synthesis, characterization, and in vitro cytotoxicity. Appl. Organomet. Chem. 2020, 34, e5896. [Google Scholar] [CrossRef]
- Kilpin, K.J.; Crot, S.; Riedel, T.; Kitchen, J.A.; Dyson, P.J. Ruthenium(II) and osmium(II) 1,2,3-triazolylidene organometallics: A preliminary investigation into the biological activity of ‘click’ carbene complexes. Dalton Trans. 2014, 43, 1443–1448. [Google Scholar] [CrossRef] [Green Version]
- Florindo, P.R.; Pereira, D.M.; Borralho, P.M.; Costa, P.J.; Piedade, M.F.M.; Rodrigues, C.M.P.; Fernandes, A.C. New [(η5-C5H5)Ru(N–N)(PPh3)][PF6] compounds: Colon anticancer activity and GLUT-mediated cellular uptake of carbohydrate-appended complexes. Dalton Trans. 2016, 45, 11926–11930. [Google Scholar] [CrossRef]
- Kacsir, I.; Sipos, A.; Ujlaki, G.; Buglyó, P.; Somsák, L.; Bai, P.; Bokor, É. Ruthenium half-sandwich type complexes with bidentate monosaccharide ligands show antineoplastic activity in ovarian cancer cell models through reactive oxygen species production. Int. J. Mol. Sci. 2021, 22, 10454. [Google Scholar] [CrossRef]
- Hartinger, C.G.; Nazarov, A.A.; Ashraf, S.M.; Dyson, P.J.; Keppler, B.K. Carbohydrate-metal complexes and their potential as anticancer agents. Curr. Med. Chem. 2008, 15, 2574–2591. [Google Scholar] [CrossRef]
- Wang, X.; Guo, Z. Targeting and delivery of platinum-based anticancer drugs. Chem. Soc. Rev. 2013, 42, 202–224. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Yang, J.; Seeberger, P.H.; Yin, J. Glycoconjugates for glucose transporter-mediated cancer-specific targeting and treatment. Carbohydr. Res. 2020, 498, 108195. [Google Scholar] [CrossRef] [PubMed]
- Franconetti, A.; López, Ó.; Fernandez-Bolanos, J.G. Carbohydrates: Potential sweet tools against cancer. Curr. Med. Chem. 2020, 27, 1206–1242. [Google Scholar] [CrossRef]
- Roux, C.; Biot, C. Ferrocene-based antimalarials. Future Med. Chem. 2012, 4, 783–797. [Google Scholar] [CrossRef]
- Ferreira, C.L.; Ewart, C.B.; Barta, C.A.; Little, S.; Yardley, V.; Martins, C.; Polishchuk, E.; Smith, P.J.; Moss, J.R.; Merkel, M.; et al. Synthesis, structure, and biological activity of ferrocenyl carbohydrate conjugates. Inorg. Chem. 2006, 45, 8414–8422. [Google Scholar] [CrossRef]
- Reddy, A.; Sangenito, L.S.; Guedes, A.A.; Branquinha, M.H.; Kavanagh, K.; McGinley, J.; Dos Santos, A.L.S.; Velasco-Torrijos, T. Glycosylated metal chelators as anti-parasitic agents with tunable selectivity. Dalton Trans. 2017, 46, 5297–5307. [Google Scholar] [CrossRef] [Green Version]
- Trivedi, R.; Deepthi, S.B.; Giribabu, L.; Sridhar, B.; Sujitha, P.; Kumar, C.G.; Ramakrishna, K.V.S. Synthesis, crystal structure, electronic spectroscopy, electrochemistry and biological studies of ferrocene-carbohydrate conjugates. Eur. J. Inorg. Chem. 2012, 2012, 2267–2277. [Google Scholar] [CrossRef]
- Panaka, S.; Trivedi, R.; Jaipal, K.; Giribabu, L.; Sujitha, P.; Kumar, C.G.; Sridhar, B. Ferrocenyl chalcogeno (sugar) triazole conjugates: Synthesis, characterization and anticancer properties. J. Organomet. Chem. 2016, 813, 125–130. [Google Scholar] [CrossRef]
- Coppens, I.; Asai, T.; Tomavo, S. Chapter 8-Biochemistry and Metabolism of Toxoplasma gondii: Carbohydrates, Lipids and Nucleotides. In Toxoplasma Gondii. In the Model Apicomplexan-Perspectives and Methods, 2nd ed.; Weiss, L.M., Kim, K., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 257–295. [Google Scholar] [CrossRef]
- Fleige, T.; Fischer, K.; Ferguson, D.J.; Gross, U.; Bohne, W. Carbohydrate metabolism in the Toxoplasma gondii apicoplast: Localization of three glycolytic isoenzymes, the single pyruvate dehydrogenase complex, and a plastid phosphate translocator. Eukaryot. Cell 2007, 6, 984–996. [Google Scholar] [CrossRef] [Green Version]
- Coppin, A.; Dzierszinski, F.; Legrand, S.; Mortuaire, M.; Ferguson, D.; Tomavo, S. Developmentally regulated biosynthesis of carbohydrate and storage polysaccharide during differentiation and tissue cyst formation in Toxoplasma gondii. Biochimie 2003, 85, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Blume, M.; Rodriguez-Contreras, D.; Landfear, S.; Fleige, T.; Soldati-Favre, D.; Lucius, R.; Gupta, N. Host-derived glucose and its transporter in the obligate intracellular pathogen Toxoplasma gondii are dispensable by glutaminolysis. Proc. Natl. Acad. Sci. USA 2009, 106, 12998–13003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giannini, F.; Furrer, J.; Süss-Fink, G.; Clavel, C.M.; Dyson, P.J. Synthesis, characterization and in vitro anticancer activity of highly cytotoxic trithiolato diruthenium complexes of the type [(η6-p-MeC6H4iPr)2Ru2(μ2-SR1)2(μ2-SR2)]+ containing different thiolato bridges. J. Organomet. Chem. 2013, 744, 41–48. [Google Scholar] [CrossRef]
- Păunescu, E.; Boubaker, G.; Desiatkina, O.; Anghel, N.; Amdouni, Y.; Hemphill, A.; Furrer, J. The quest of the best—A SAR study of trithiolato-bridged dinuclear ruthenium(II)-arene compounds presenting antiparasitic properties. Eur. J. Med. Chem. 2021, 222, 113610. [Google Scholar] [CrossRef] [PubMed]
- Desiatkina, O.; Paunescu, E.; Mosching, M.; Anghel, N.; Boubaker, G.; Amdouni, Y.; Hemphill, A.; Furrer, J. Coumarin-tagged dinuclear trithiolato-bridged ruthenium(II)arene complexes: Photophysical properties and antiparasitic activity. ChemBioChem 2020, 21, 2818–2835. [Google Scholar] [CrossRef]
- Giannini, F.; Bartoloni, M.; Paul, L.E.H.; Süss-Fink, G.; Reymond, J.L.; Furrer, J. Cytotoxic peptide conjugates of dinuclear areneruthenium trithiolato complexes. MedChemComm 2015, 6, 347–350. [Google Scholar] [CrossRef]
- Stíbal, D.; Therrien, B.; Süss-Fink, G.; Nowak-Sliwinska, P.; Dyson, P.J.; Čermáková, E.; Řezáčová, M.; Tomšík, P. Chlorambucil conjugates of dinuclear p-cymene ruthenium trithiolato complexes: Synthesis, characterization and cytotoxicity study in vitro and in vivo. J. Biol. Inorg. Chem. 2016, 21, 443–452. [Google Scholar] [CrossRef]
- Desiatkina, O.; Johns, S.K.; Anghel, N.; Boubaker, G.; Hemphill, A.; Furrer, J.; Păunescu, E. Synthesis and antiparasitic activity of new conjugates-organic drugs tethered to trithiolato-bridged dinuclear ruthenium(II)-arene complexes. Inorganics 2021, 9, 59. [Google Scholar] [CrossRef]
- Desiatkina, O.; Mösching, M.; Anghel, N.; Boubaker, G.; Amdouni, Y.; Hemphill, A.; Furrer, J.; Păunescu, E. New nucleic base-tethered trithiolato-bridged dinuclear ruthenium(II)-arene compounds: Synthesis and antiparasitic activity. Molecules 2022, 27, 8173. [Google Scholar] [CrossRef]
- Studer, V.; Anghel, N.; Desiatkina, O.; Felder, T.; Boubaker, G.; Amdouni, Y.; Ramseier, J.; Hungerbuhler, M.; Kempf, C.; Heverhagen, J.T.; et al. Conjugates containing two and three trithiolato-bridged dinuclear ruthenium(II)-arene units as in vitro antiparasitic and anticancer agents. Pharmaceuticals 2020, 13, 471. [Google Scholar] [CrossRef]
- Renfrew, A.K.; Juillerat-Jeanneret, L.; Dyson, P.J. Adding diversity to ruthenium(II)–arene anticancer (RAPTA) compounds via click chemistry: The influence of hydrophobic chains. J. Organomet. Chem. 2011, 696, 772–779. [Google Scholar] [CrossRef]
- Mandal, S.; Das, R.; Gupta, P.; Mukhopadhyay, B. Synthesis of a sugar-functionalized iridium complex and its application as a fluorescent lectin sensor. Tetrahedron Lett. 2012, 53, 3915–3918. [Google Scholar] [CrossRef]
- Schmollinger, D.; Kraft, J.; Ewald, C.; Ziegler, T. Synthesis of ruthenium and palladium complexes from glycosylated 2,20-bipyridine and terpyridine ligands. Tetrahedron Lett. 2017, 58, 3643–3645. [Google Scholar] [CrossRef]
- Mede, T.; Jager, M.; Schubert, U.S. “Chemistry-on-the-complex”: Functional Ru(II) polypyridyl-type sensitizers as divergent building blocks. Chem. Soc. Rev. 2018, 47, 7577–7627. [Google Scholar] [CrossRef] [PubMed]
- Cisnetti, F.; Gibard, C.; Gautier, A. Post-functionalization of metal–NHC complexes: A useful toolbox for bioorganometallic chemistry (and beyond)? J. Organomet. Chem. 2015, 782, 22–30. [Google Scholar] [CrossRef]
- van Hilst, Q.V.C.; Lagesse, N.R.; Preston, D.; Crowley, J.D. Functional metal complexes from CuAAC “click” bidentate and tridentate pyridyl-1,2,3-triazole ligands. Dalton Trans. 2018, 47, 997–1002. [Google Scholar] [CrossRef]
- Casas-Solvas, J.M.; Ortiz-Salmeron, E.; Gimenez-Martinez, J.J.; Garcia-Fuentes, L.; Capitan-Vallvey, L.F.; Santoyo-Gonzalez, F.; Vargas-Berenguel, A. Ferrocene-carbohydrate conjugates as electrochemical probes for molecular recognition studies. Chem. Eur. J. 2009, 15, 710–725. [Google Scholar] [CrossRef]
- Casas-Solvas, J.M.; Vargas-Berenguel, A.; Capitan-Vallvey, L.F.; Santoyo-Gonzalez, F. Convenient methods for the synthesis of ferrocene-carbohydrate conjugates. Org. Lett. 2004, 6, 3687–3690. [Google Scholar] [CrossRef]
- Meldal, M.; Tornoe, C.W. Cu-catalyzed azide-alkyne cycloaddition. Chem. Rev. 2008, 108, 2952–3015. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click chemistry: Diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. Engl. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Singh, M.S.; Chowdhury, S.; Koley, S. Advances of azide-alkyne cycloaddition-click chemistry over the recent decade. Tetrahedron 2016, 72, 5257–5283. [Google Scholar] [CrossRef]
- Ibao, A.F.; Gras, M.; Therrien, B.; Süss-Fink, G.; Zava, O.; Dyson, P.J. Thiolato-bridged arene-ruthenium complexes: Synthesis, molecular structure, reactivity, and anticancer activity of the dinuclear complexes [(arene)2Ru2(SR)2Cl2]. Eur. J. Inorg. Chem. 2012, 2012, 1531–1535. [Google Scholar] [CrossRef]
- Soli, E.D.; DeShong, P. Advances in glycosyl azide preparation via hypervalent silicates. J. Org. Chem. 1999, 64, 9724–9726. [Google Scholar] [CrossRef]
- Paterson, S.M.; Clark, J.; Stubbs, K.A.; Chirila, T.V.; Baker, M.V. Carbohydrate-based crosslinking agents: Potential use in hydrogels. J. Polym. Sci. A Polym. Chem. 2011, 49, 4312–4315. [Google Scholar] [CrossRef]
- Mereyala, H.B.; Gurrala, S.R. A highly diastereoselective, practical synthesis of allyl, propargyl 2,3,4,6-tetra-O-acetyl-β-d-gluco, β-d-galactopyranosides and allyl, propargyl heptaacetyl-β-d-lactosides. Carbohydr. Res. 1998, 307, 351–354. [Google Scholar] [CrossRef]
- Roy, B.; Dutta, S.; Choudhary, A.; Basak, A.; Dasgupta, S. Design, synthesis and RNase A inhibition activity of catechin and epicatechin and nucleobase chimeric molecules. Bioorg. Med. Chem. Lett. 2008, 18, 5411–5414. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, M.; Gao, F.; Wei, W.; Qian, Y.; Liu, H.K.; Zhao, J. Imaging of a clickable anticancer iridium catalyst. J. Inorg. Biochem. 2018, 180, 179–185. [Google Scholar] [CrossRef]
- Zabarska, N.; Stumper, A.; Rau, S. CuAAC click reactions for the design of multifunctional luminescent ruthenium complexes. Dalton Trans. 2016, 45, 2338–2351. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, L.Y.; Li, S.; Wang, F.X.; Li, J.; Qian, Y.; Su, Z.; Mao, Z.W.; Sadler, P.J.; Liu, H.K. Rigid dinuclear ruthenium-arene complexes showing strong DNA interactions. J. Inorg. Biochem. 2018, 189, 30–39. [Google Scholar] [CrossRef]
- Zhao, J.; Li, S.; Wang, X.; Xu, G.; Gou, S. Dinuclear organoruthenium complexes exhibiting antiproliferative activity through DNA damage and a Reactive-Oxygen-Species-mediated endoplasmic reticulum stress pathway. Inorg. Chem. 2019, 58, 2208–2217. [Google Scholar] [CrossRef]
- Murray, B.S.; Menin, L.; Scopelliti, R.; Dyson, P.J. Conformational control of anticancer activity: The application of arene-linked dinuclear ruthenium(II) organometallics. Chem. Sci. 2014, 5, 2536–2545. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Parkinson, J.A.; Novakova, O.; Bella, J.; Wang, F.; Dawson, A.; Gould, R.; Parsons, S.; Brabec, V.; Sadler, P.J. Induced-fit recognition of DNA by organometallic complexes with dynamic stereogenic centers. Proc. Natl. Acad. Sci. USA 2003, 100, 14623–14628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.-Y.; Qian, Y.; Wang, F.-X.; Habtemariam, A.; Mao, Z.-W.; Sadler, P.J.; Liu, H.-K. Ruthenium(II)–arene metallacycles: Crystal structures, interaction with DNA, and cytotoxicity. Eur. J. Inorg. Chem. 2017, 2017, 1792–1799. [Google Scholar] [CrossRef] [Green Version]
- Pettinari, C.; Pettinari, R.; Xhaferai, N.; Giambastiani, G.; Rossin, A.; Bonfili, L.; Eleuteri, A.M.; Cuccioloni, M. Binuclear 3,3′,5,5′-tetramethyl-1H,H-4,4′-bipyrazole Ruthenium(II) complexes: Synthesis, characterization and biological studies. Inorg. Chim. Acta 2020, 513, 119902. [Google Scholar] [CrossRef]
- Giannini, F.; Furrer, J.; Ibao, A.F.; Suss-Fink, G.; Therrien, B.; Zava, O.; Baquie, M.; Dyson, P.J.; Stepnicka, P. Highly cytotoxic trithiophenolatodiruthenium complexes of the type [(η6-p-MeC6H4Pri)2Ru2(SC6H4-p-X)3]+: Synthesis, molecular structure, electrochemistry, cytotoxicity, and glutathione oxidation potential. J. Biol. Inorg. Chem. 2012, 17, 951–960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giannini, F.; Suss-Fink, G.; Furrer, J. Efficient oxidation of cysteine and glutathione catalyzed by a dinuclear areneruthenium trithiolato anticancer complex. Inorg. Chem. 2011, 50, 10552–10554. [Google Scholar] [CrossRef] [Green Version]
- Desiatkina, O.; Boubaker, G.; Anghel, N.; Amdouni, Y.; Hemphill, A.; Furrer, J.; Paunescu, E. Synthesis, photophysical properties and biological evaluation of new conjugates BODIPY–dinuclear trithiolato-bridged ruthenium(II)-arene complexes. ChemBioChem 2022, 23, e202200536. [Google Scholar] [CrossRef]
- Barna, F.; Debache, K.; Vock, C.A.; Kuster, T.; Hemphill, A. In vitro effects of novel ruthenium complexes in Neospora caninum and Toxoplasma gondii tachyzoites. Antimicrob. Agents Chemother. 2013, 57, 5747–5754. [Google Scholar] [CrossRef] [Green Version]
- McFadden, D.C.; Seeber, F.; Boothroyd, J.C. Use of Toxoplasma gondii expressing beta-galactosidase for colorimetric assessment of drug activity in vitro. Antimicrob. Agents Chemother. 1997, 41, 1849–1853. [Google Scholar] [CrossRef]
- Muller, J.; Aguado-Manser, A.; Manser, V.; Balmer, V.; Winzer, P.; Ritler, D.; Hostettler, I.; Arranz-Solis, D.; Ortega-Mora, L.; Hemphill, A. Buparvaquone is active against Neospora caninum in vitro and in experimentally infected mice. Int. J. Parasitol. Drugs Drug. Resist. 2015, 5, 16–25. [Google Scholar] [CrossRef] [Green Version]
- Fulmer, G.R.; Miller, A.J.M.; Sherden, N.H.; Gottlieb, H.E.; Nudelman, A.; Stoltz, B.M.; Bercaw, J.E.; Goldberg, K.I. NMR chemical shifts of trace impurities: Common laboratory solvents, organics, and gases in deuterated solvents relevant to the organometallic chemist. Organometallics 2010, 29, 2176–2179. [Google Scholar] [CrossRef] [Green Version]
- Ogawa, T.; Nakabayashi, S.; Shibata, S. Synthetic studies on nephritogenic glycosides. Synthesis of N-(β-l-Aspartyl)-α-d-glucopyranosylamine. Agric. Biol. Chem. 1983, 47, 281–285. [Google Scholar] [CrossRef]












 | |||||
|---|---|---|---|---|---|
| Compound | R | HFF Viability (%) | T. Gondii β-gal Growth (%) | ||
| 0.1 µM | 1 µM | 0.1 µM | 1 µM | ||
| Diruthenium intermediates | |||||
| 2 a,* |  | 76 ± 6 | 46 ± 6 | 66 ± 14 | 2 ± 0 |
| 3 a,* |  | 74 ± 2 | 48 ± 1 | 57 ± 1 | 2 ± 0 |
| 4 a |  | 91 ± 4 | 73 ± 1 | 114 ± 2 | 110 ± 2 |
| 5 a,* |  | 80 ± 1 | 69 ± 6 | 2 ± 0 | 1 ± 0 |
| Alkyne and azide functionalized diruthenium compounds | |||||
| 6 * |  | 101 ± 1 | 100 ± 1 | 4 ± 0 | 0 ± 0 |
| 7a,* |  | 101 ± 0 | 96 ± 0 | 21 ± 2 | 0 ± 0 |
| 8 a |  | 71 ± 2 | 46 ± 6 | 52 ± 13 | 3 ± 1 |
| 9 a,* |  | 96 ± 1 | 64 ± 1 | 9 ± 1 | 1 ± 1 |
| Azido glucose derivative | |||||
| 10 |  | 98 ± 1 | 99 ± 0 | 101 ± 1 | 99 ± 0 |
| Diruthenium—glucose conjugates | |||||
| 19 * |  | 102 ± 1 | 97 ± 1 | 9 ± 1 | 0 ± 0 |
| 20 * |  | 98 ± 1 | 99 ± 0 | 100 ± 1 | 1 ± 2 |
| 21 * |  | 100 ± 1 | 101 ± 0 | 87 ± 1 | 1 ± 0 |
| 24 * |  | 99 ± 1 | 98 ± 0 | 66 ± 2 | 3 ± 0 |
| 25 |  | 98 ± 1 | 97 ± 1 | 75 ± 2 | 27 ± 0 |
| Diruthenium—galactose conjugates | |||||
| 22 * |  | 100 ± 0 | 101 ± 0 | 73 ± 1 | 2 ± 0 |
| 23 * |  | 99 ± 1 | 101 ± 1 | 85 ± 1 | 1 ± 0 |
| 26 * |  | 98 ± 1 | 99 ± 1 | 89 ± 1 | 0 ± 0 |
 | ||||||
|---|---|---|---|---|---|---|
| Compound | R | IC50 T. Gondii β-gal (µM) | [LS; LI] b | SE c | HFF Viability at 2.5 µM (%) d | SD e |
| Pyrimethamine a | 0.326 | [0.396; 0.288] | 0.051 | 99 | 6 | |
| Diruthenium intermediates | ||||||
| 2 a |  | 0.117 | [0.139; 0.098] | 0.051 | 56 | 6 |
| 3 a |  | 0.153 | [0.185; 0.127] | 0.049 | 51 | 5 |
| 5 a |  | 0.038 | [0.023; 0.060] | 0.110 | 4 | 2 |
| Alkyne and azide functionalized diruthenium compounds | ||||||
| 6 |  | 0.023 | [0.030; 0.018] | 0.503 | 7 | 1 |
| 7 a |  | 0.038 | [0.050; 0.029] | 0.063 | 34 | 1 |
| 9 a |  | 0.048 | [0.058; 0.040] | 0.139 | 11 | 1 |
| Diruthenium-glucose conjugates | ||||||
| 19 |  | 0.018 | [0.031; 0.011] | 0.387 | 29 | 3 |
| 20 |  | 0.110 | [0.151; 0.080] | 0.416 | 77 | 1 |
| 21 |  | 0.087 | [0.135; 0.056] | 0.274 | 0 | 0 |
| 24 |  | 0.328 | [0.437; 0.247] | 0.339 | 66 | 2 |
| Diruthenium-galactose conjugates | ||||||
| 22 |  | 0.298 | [0.364; 0.244] | 0.066 | 73 | 1 |
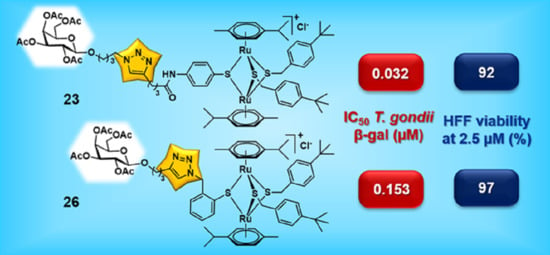
| 23 |  | 0.032 | [0.044; 0.023] | 0.278 | 92 | 2 |
| 26 |  | 0.153 | [0.178; 0.132] | 0.476 | 97 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holzer, I.; Desiatkina, O.; Anghel, N.; Johns, S.K.; Boubaker, G.; Hemphill, A.; Furrer, J.; Păunescu, E. Synthesis and Antiparasitic Activity of New Trithiolato-Bridged Dinuclear Ruthenium(II)-arene-carbohydrate Conjugates. Molecules 2023, 28, 902. https://doi.org/10.3390/molecules28020902
Holzer I, Desiatkina O, Anghel N, Johns SK, Boubaker G, Hemphill A, Furrer J, Păunescu E. Synthesis and Antiparasitic Activity of New Trithiolato-Bridged Dinuclear Ruthenium(II)-arene-carbohydrate Conjugates. Molecules. 2023; 28(2):902. https://doi.org/10.3390/molecules28020902
Chicago/Turabian StyleHolzer, Isabelle, Oksana Desiatkina, Nicoleta Anghel, Serena K. Johns, Ghalia Boubaker, Andrew Hemphill, Julien Furrer, and Emilia Păunescu. 2023. "Synthesis and Antiparasitic Activity of New Trithiolato-Bridged Dinuclear Ruthenium(II)-arene-carbohydrate Conjugates" Molecules 28, no. 2: 902. https://doi.org/10.3390/molecules28020902
APA StyleHolzer, I., Desiatkina, O., Anghel, N., Johns, S. K., Boubaker, G., Hemphill, A., Furrer, J., & Păunescu, E. (2023). Synthesis and Antiparasitic Activity of New Trithiolato-Bridged Dinuclear Ruthenium(II)-arene-carbohydrate Conjugates. Molecules, 28(2), 902. https://doi.org/10.3390/molecules28020902



.jpg)