Impact of Drying Process on the Phenolic Profile and Antioxidant Capacity of Raw and Boiled Leaves and Inflorescences of Chenopodium berlandieri ssp. berlandieri
Abstract
:1. Introduction
2. Results and Discussion
2.1. Impact of Drying Method on the Proximal Composition of C. berlandieri Leaves and Inflorescences
2.2. Impact of the Drying Process on the Phytochemical Composition and Antioxidant Capacity of Raw and Boiled Leaves and Inflorescences of C. berlandieri
2.3. Principal Component Analysis (PCA) of Oven-Drying and Lyophilization Clustering
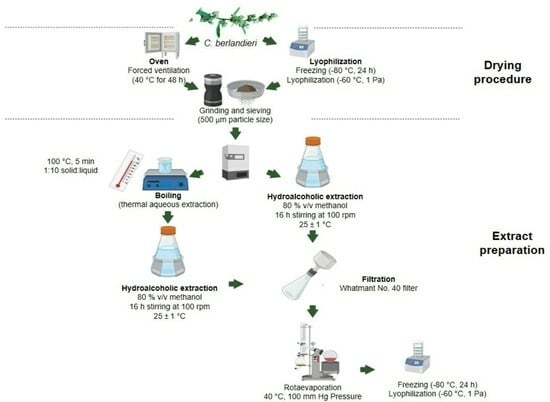
3. Materials and Methods
3.1. Chemical Reagents
3.2. Plant Material and Study Design
3.3. Drying Process
3.4. Proximate Chemical Analysis
3.5. Extraction, Identification, and Quantification of Phenolic Compounds
3.5.1. Methanolic Extraction of Raw and Boiled Samples
3.5.2. Spectrophotometric Determination of Total Phenolic Compounds (TPC) and Total Flavonoids (TF)
3.5.3. Identification and Quantification of Individual Phenolic Compounds by UPLC-DAD-ESI-QToF/MS
3.5.4. Antioxidant Capacity
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zekrumah, M.; Begua, P.; Razak, A.; Wahab, J.; Moffo, N.; Ivane, A.; Oman, M.; Elrashied, H.; Zou, X.; Zhang, D. Role of dietary polyphenols in non-communicable chronic disease prevention, and interactions in food systems: An overview. Nutrition 2023, 112, 112034. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Saenz, Y.O.; Hernández-Fuentes, A.D.; López-Palestina, C.U.; Garrido-Cauich, J.H.; Alatorre-Cruz, J.M.; Monroy-Torres, R.; Santiago-Saenz, Y.O.; Hernández-Fuentes, A.D.; López-Palestina, C.U.; Garrido-Cauich, J.H.; et al. Importancia Nutricional y Actividad Biológica de Los Compuestos Bioactivos de Quelites Consumidos En México. Rev. Chil. Nutr. 2019, 46, 593–605. [Google Scholar] [CrossRef]
- Vargas-Madriz, Á.F.; Luzardo-Ocampo, I.; Moreno-Celis, U.; Roldán-Padrón, O.; Chávez-Servín, J.L.; Vergara-Castañeda, H.A.; Martínez-Pacheco, M.; Mejía, C.; García-Gasca, T.; Kuri-García, A. Comparison of Phytochemical Composition and Untargeted Metabolomic Analysis of an Extract from Cnidoscolus Aconitifolius (Mill.) I. I. Johnst and Porophyllum Ruderale (Jacq.) Cass. and Biological Cytotoxic and Antiproliferative Activity in Vitro. Plants 2023, 12, 1987. [Google Scholar] [CrossRef]
- Pérez-Negrón, E.; Casas, A. Use, Extraction Rates and Spatial Availability of Plant Resources in the Tehuacán-Cuicatlán Valley, Mexico: The Case of Santiago Quiotepec, Oaxaca. J. Arid Environ. 2007, 70, 356–379. [Google Scholar] [CrossRef]
- Pascual-Mendoza, S.; Saynes-Vásquez, A.; Pérez-Herrera, A.; Meneses, M.E.; Coutiño-Hernández, D.; Sánchez-Medina, M.A. Nutritional Composition and Bioactive Compounds of Quelites Consumed by Indigenous Communities in the Municipality of Juquila Vijanos, Sierra Norte of Oaxaca, Mexico. Plant Foods Hum. Nutr. 2023, 78, 193–200. [Google Scholar] [CrossRef]
- Kumar, S.; Yadav, A.; Yadav, M.; Yadav, P. Effect of Climate Change on Phytochemical Diversity, Total Phenolic Content and in Vitro Antioxidant Activity of Aloe Vera (L.) Burm.F. BMC Res. Notes 2017, 10, 60. [Google Scholar] [CrossRef]
- Dadhaneeya, H.; Kesavan, R.K.; Inbaraj, B.S.; Sharma, M.; Kamma, S.; Nayak, P.K.; Sridhar, K. Impact of Different Drying Methods on the Phenolic Composition, In Vitro Antioxidant Activity, and Quality Attributes of Dragon Fruit Slices and Pulp. Foods 2023, 12, 1387. [Google Scholar] [CrossRef]
- Dong, Q.; He, D.; Ni, X.; Zhou, H.; Yang, H. Comparative Study on Phenolic Compounds, Triterpenoids, and Antioxidant Activity of Ganoderma Lucidum Affected by Different Drying Methods. J. Food Meas. Charact. 2019, 13, 3198–3205. [Google Scholar] [CrossRef]
- Garcia-Amezquita, L.E.; Tejada-Ortigoza, V.; Campanella, O.H.; Welti-Chanes, J. Influence of Drying Method on the Composition, Physicochemical Properties, and Prebiotic Potential of Dietary Fibre Concentrates from Fruit Peels. J. Food Qual. 2018, 2018, 9105237. [Google Scholar] [CrossRef]
- Santiago-Saenz, Y.O.; Hernández-Fuentes, A.D.; Monroy-Torres, R.; Cariño-Cortés, R.; Jiménez-Alvarado, R. Physicochemical, Nutritional and Antioxidant Characterization of Three Vegetables (Amaranthus hybridus L., Chenopodium berlandieri L., Portulaca oleracea L.) as Potential Sources of Phytochemicals and Bioactive Compounds. J. Food Meas. Charact. 2018, 12, 2855–2864. [Google Scholar] [CrossRef]
- Román-Cortés, N.R.; García-Mateos, M.D.; Castillo-González, A.M.; Sahagún-Castellanos, J.; Jiménez-Arellanes, M.A. Características Nutricionales y Nutracéuticas de Hortalizas de Uso Ancestral En México. Rev. Fitotec. Mex. 2018, 41, 245–253. [Google Scholar] [CrossRef]
- Oliveira-Alves, S.C.; Andrade, F.; Prazeres, I.; Silva, A.B.; Capelo, J.; Duarte, B.; Caçador, I.; Coelho, J.; Serra, A.T.; Bronze, M.R. Impact of Drying Processes on the Nutritional Composition, Volatile Profile, Phytochemical Content and Bioactivity of Salicornia Ramosissima j. Woods. Antioxidants 2021, 10, 1312. [Google Scholar] [CrossRef] [PubMed]
- Shonte, T.T.; Duodu, K.G.; de Kock, H.L. Effect of Drying Methods on Chemical Composition and Antioxidant Activity of Underutilized Stinging Nettle Leaves. Heliyon 2020, 6, e03938. [Google Scholar] [CrossRef] [PubMed]
- Radojčin, M.; Pavkov, I.; Kovačević, D.B.; Putnik, P.; Wiktor, A.; Stamenković, Z.; Kešelj, K.; Gere, A. Effect of Selected Drying Methods and Emerging Drying Intensification Technologies on the Quality of Dried Fruit: A Review. Processes 2021, 9, 132. [Google Scholar] [CrossRef]
- Li, S.; Liu, F.; Wu, M.; Li, Y.; Song, X.; Yin, J. Effects of Drying Treatments on Nutritional Compositions, Volatile Flavor Compounds, and Bioactive Substances of Broad Beans. Foods 2023, 12, 2160. [Google Scholar] [CrossRef] [PubMed]
- Raza, N.; Arshad, M.U.; Anjum, F.M.; Saeed, F.; Maan, A.A.; Bader Ul Ain, H. Impact of Drying Methods on Composition and Functional Properties of Date Powder Procured from Different Cultivars. Food Sci. Nutr. 2019, 7, 2345–2352. [Google Scholar] [CrossRef]
- Adeboye, A.O.; Bolaji, O.A.; Fasogbon, B.M.; Okunyemi, B.M. An Evaluation of the Impact of Drying on the Nutritional Composition, Functional Properties, and Sensory Characteristics of a Ready-to-Cook C. volubile Leaf Soup Powder. J. Culin. Sci. Technol. 2019, 18, 244–253. [Google Scholar] [CrossRef]
- Femenia, A.; Selvendran, R.R.; Ring, S.G.; Robertson, J.A. Effects of Heat Treatment and Dehydration on Properties of Cauliflower Fiber. J. Agric. Food Chem. 1999, 47, 728–732. [Google Scholar] [CrossRef]
- Borchani, C.; Besbes, S.; Masmoudi, M.; Bouaziz, M.A.; Blecker, C.; Attia, H. Influence of Oven-Drying Temperature on Physicochemical and Functional Properties of Date Fibre Concentrates. Food Bioprocess Technol. 2012, 5, 1541–1551. [Google Scholar] [CrossRef]
- Quispe-Fuentes, I.; Vega-Gálvez, A.; Aranda, M.; Poblete, J.; Pasten, A.; Bilbao-Sainz, C.; Wood, D.; McHugh, T.; Delporte, C. Effects of Drying Processes on Composition, Microstructure and Health Aspects from Maqui Berries. J. Food Sci. Technol. 2020, 57, 2241–2250. [Google Scholar] [CrossRef]
- Díaz-Batalla, L.; Hernández-Uribe, J.P.; Román-Gutiérrez, A.D.; Cariño-Cortés, R.; Castro-Rosas, J.; Téllez-Jurado, A.; Gómez-Aldapa, C.A. Chemical and Nutritional Characterization of Raw and Thermal-Treated Flours of Mesquite (Prosopis Laevigata) Pods and Their Residual Brans. CyTA—J. Food 2018, 16, 444–451. [Google Scholar] [CrossRef]
- De Ancos, B.; Sánchez-Moreno, C.; Zacarías, L.; Rodrigo, M.J.; Sáyago Ayerdí, S.; Blancas Benítez, F.J.; Domínguez Avila, J.A.; González-Aguilar, G.A. Effects of Two Different Drying Methods (Freeze-Drying and Hot Air-Drying) on the Phenolic and Carotenoid Profile of ‘Ataulfo’ Mango by-Products. J. Food Meas. Charact. 2018, 12, 2145–2157. [Google Scholar] [CrossRef]
- Li, H.; Deng, Z.; Liu, R.; Zhu, H.; Draves, J.; Marcone, M.; Sun, Y.; Tsao, R. Characterization of Phenolics, Betacyanins and Antioxidant Activities of the Seed, Leaf, Sprout, Flower and Stalk Extracts of Three Amaranthus Species. J. Food Compos. Anal. 2015, 37, 75–81. [Google Scholar] [CrossRef]
- Soto, K.M.; Luzardo-Ocampo, I.; López-Romero, J.M.; Mendoza, S.; Loarca-Piña, G.; Rivera-Muñoz, E.M.; Manzano-Ramírez, A. Gold Nanoparticles Synthesized with Common Mullein (Verbascum Thapsus) and Castor Bean (Ricinus Communis) Ethanolic Extracts Displayed Antiproliferative Effects and Induced Caspase 3 Activity in Human HT29 and SW480 Cancer Cells. Pharmaceutics 2022, 14, 2069. [Google Scholar] [CrossRef] [PubMed]
- Godínez-Santillán, R.I.; Chávez-Servín, J.L.; García-Gasca, T.; Guzmán-Maldonado, S.H. Caracterización Fenólica y Capacidad Antioxidante de Extractos Alcohólicos de Hojas Crudas y Hervidas de Cnidoscolus Aconitifolius (Euphorbiaceae). Acta Bot. Mex. 2019, 126, 1–15. [Google Scholar] [CrossRef]
- Fauziah, I.; Prangdimurti, E.; Palupi, N. Effect of Boiling Time on the Stability of the Phenolic Compounds in Wedang Uwuh after Gastric Digestion. IOP Conf. Ser. Earth Environ. Sci. 2023, 1200, 012014. [Google Scholar] [CrossRef]
- Balunkeswar, N.; Rui Hai, L.; Juming, T. Effect of Processing on Phenolic Antioxidants of Effect of Processing on Phenolic Antioxidants of Fruits, Vegetables, and Grains—A Review. Crit. Rev. Food Sci. Nutr. ISSN 2015, 55, 887–918. [Google Scholar] [CrossRef]
- Nurkhoeriyati, T.; Kulig, B.; Sturm, B.; Hensel, O. The Effect of Pre-Drying Treatment and Drying Conditions on Quality and Energy Consumption of Hot Air-Dried Celeriac Slices: Optimisation. Foods 2021, 10, 1758. [Google Scholar] [CrossRef]
- Kraujalis, P.; Venskutonis, P.R.; Kraujalienė, V.; Pukalskas, A. Antioxidant Properties and Preliminary Evaluation of Phytochemical Composition of Different Anatomical Parts of Amaranth. Plant Foods Hum. Nutr. 2013, 68, 322–328. [Google Scholar] [CrossRef]
- Fukalova-Fukalova, T.; García-Martínez, M.D.; Raigón, M.D. Nutritional Composition, Bioactive Compounds, and Volatiles Profile Characterization of Two Edible Undervalued Plants: Portulaca oleracea L. and Porophyllum Ruderale (Jacq.) Cass. Plants 2022, 11, 377. [Google Scholar] [CrossRef]
- Jesus, R.S.; Piana, M.; Freitas, R.B.; Brum, T.F.; Alves, C.F.S.; Belke, B.V.; Mossmann, N.J.; Cruz, R.C.; Santos, R.C.V.; Dalmolin, T.V.; et al. In Vitro Antimicrobial and Antimycobacterial Activity and HPLC–DAD Screening of Phenolics from Chenopodium ambrosioides L. Braz. J. Microbiol. 2018, 49, 296. [Google Scholar] [CrossRef]
- Arias-Rico, J.; Macías-León, F.J.; Alanís-García, E.; Cruz-Cansino, N.D.; Jaramillo-Morales, O.A.; Barrera-Gálvez, R.; Ramírez-Moreno, E. Study of Edible Plants: Effects of Boiling on Nutritional, Antioxidant, and Physicochemical Properties. Foods 2020, 9, 599. [Google Scholar] [CrossRef] [PubMed]
- Youssef, K.M.; Mokhtar, S.M. Effect of Drying Methods on the Antioxidant Capacity, Color and Phytochemicals of Portulaca oleracea L. Leaves. J. Nutr. Food Sci. 2014, 4, 1–6. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International; Horwitz, W., Latimer, G.W., Eds.; Seventeen; AOAC International: Gaithersburg, VA, USA, 2002; Volume 15, ISBN 0-935584-77-3. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The Determination of Flavonoid Contents in Mulberry and Their Scavenging Effects on Superoxide Radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Reynoso-Camacho, R.; Rodríguez-Villanueva, L.D.; Sotelo-González, A.M.; Ramos-Gómez, M.; Pérez-Ramírez, I.F. Citrus Decoction By-Product Represents a Rich Source of Carotenoid, Phytosterol, Extractable and Non-Extractable Polyphenols. Food Chem. 2021, 350, 129239. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity; Elsevier: Amsterdam, The Netherlands, 1995; Volume 28. [Google Scholar]
- Ozgen, M.; Reese, R.N.; Tulio, A.Z.; Scheerens, J.C.; Miller, A.R. Modified 2,2-Azino-Bis-3-Ethylbenzothiazoline-6-Sulfonic Acid (ABTS) Method to Measure Antioxidant Capacity of Selected Small Fruits and Comparison to Ferric Reducing Antioxidant Power (FRAP) and 2,2′-Diphenyl-1-Picrylhydrazyl (DPPH) Methods. J. Agric. Food Chem. 2006, 54, 1151–1157. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]



| Component (%) | ROL | RLL | ROI | RLI |
|---|---|---|---|---|
| Moisture | 2.91 ± 0.08 a | 2.66 ± 0.04 a | 2.59 ± 0.01 a | 2.80 ± 0.02 a |
| Protein | 23.17 ± 1.79 a | 23.97 ± 0.55 a | 23.28 ± 0.72 a | 23.00 ± 0.10 a |
| Lipids | 0.71 ± 0.02 b | 0.72 ± 0.02 b | 0.55 ± 0.54 c | 1.06 ± 0.05 a |
| Ash | 24.19 ± 0.22 a | 22.59 ± 0.04 a | 15.95 ± 0.18 b | 14.71 ± 0.33 b |
| Total carbohydrates | 29.79 ± 0.26 d | 32.45 ± 0.26 c | 41.71 ± 0.02 a | 35.67 ± 0.23 b |
| Dietary fiber | 19.23 ± 1.96 b | 17.61 ± 2.45 c | 15.92 ± 0.40 d | 22.76 ± 0.93 a |
| Sample | TPC (mg GAE/g LE) | TFC (mg CE/g LE) | DPPH (µM TE/g LE) | ABTS (µM TE/g LE) | FRAP (µM TE/g LE) |
|---|---|---|---|---|---|
| ROL | 23.29 ± 0.11 a | 6.79 ± 0.11 c | 100.32 ± 171 b | 384.15 ± 20.9 ab | 528.21 ± 15.9 b |
| RLL | 26.38 ± 0.08 a | 9.24 ± 0.08 a | 116.58 ± 0.93 a | 387.01 ± 28.3 ab | 545.49 ± 25.6 b |
| BOL | 26.09 ± 0.13 b | 8.02 ± 0.07 b | 114.68 ± 1.85 a | 424.80 ± 11.1 a | 525.12 ± 8.35 b |
| BLL | 22.06 ± 0.10 d | 7.92 ± 0.13 b | 101.52 ± 1.20 b | 341.82 ± 20.1 b | 635.00 ± 16.6 a |
| ROI | 27.69 ± 0.10 c | 11.99 ± 0.44 c | 117.76 ± 0.18 c | 554.52 ± 15.8 b | 656.60 ± 20.3 bc |
| RLI | 52.52 ± 0.15 a | 23.14 ± 0.13 a | 337.15 ± 7.75 a | 732.99 ± 61.3 a | 803.52 ± 53.3 a |
| BOI | 26.60 ± 0.11 d | 10.92 ± 0.07 d | 119.63 ± 0.21 c | 536.48 ± 36.3 b | 570.19 ± 20.6 c |
| BLI | 34.77 ± 0.18 b | 20.30 ± 0.10 b | 255.40 ± 3.39 b | 501.26 ± 38.9 b | 757.22 ± 52.8 ab |
| Samples | ||||||||
|---|---|---|---|---|---|---|---|---|
| Compounds | ROL | RLL | BOL | BLL | ROI | RIL | BOI | BLI |
| Flavanols | ||||||||
| Pro-cyanidin dimer B2 * | n. d. | n. d. | n. d. | n. d. | 599.3 ± 209.9 c | 2287.0 ± 33.4 a | 759.8 ± 148.5 c | 1939.5 ± 384.9 b |
| (+)-Catechin * | n. d. | 39.9 ± 0.6 d | 20.7 ± 10.0 d | 11.2 ± 1.4 d | 1151.4 ± 193.7 c | 3326.8 ± 41.9 a | 1204.0 ± 100.5 c | 2538.7 ± 238.2 b |
| Flavanones | ||||||||
| Naringin * | 4032.3 ± 474.0 a | 1768.5 ± 511.3 c | 4146.0 ± 215.9 a | 1480.6 ± 42.4 c | 3371.8 ± 170.9 b | 1863.5 ± 97.9 c | 3972.9 ± 86.4 a | 1868.7 ± 168.4 c |
| Naringenin hexoside | 45.8 ± 1.3 c | 42.9 ± 11.8 c | 46.0 ± 7.8 c | 31.6 ± 1.1 cd | 24.2 ± 7.8 d | 91.9 ± 9.3 a | 21.6 ± 1.8 d | 61.3 ± 1.0 b |
| Flavonols | ||||||||
| Quercetin rhamnosyl-rhamnosyl-hexoside | 1806.2 ± 122.3 g | 3766.8 ± 310.3 d | 2741.0 ± 52.7 fg | 3152.8 ± 64.3 e | 4654.4 ± 29.9 bc | 5570.0 ± 356.5 a | 5098.3 ± 35.2 ab | 4784.6 ± 6.8 b |
| Quercetin pentosyl-rutinoside | 2520.1 ± 536.9 bcd | 3350.6 ± 1162.1 abc | 2520.7 ± 636.9 bcd | 2791.4 ± 73.9 abcd | 2512.8 ± 408.2 cd | 3496.3 ± 21.7 a | 2341.5 ± 77.4 d | 3487.8 ± 5.3 a |
| Quercetin rutinoside (rutin) * | 3659.9 ± 5.8 d | 7652.8 ± 29.2 a | 4858.7 ± 905.0 c | 5695.7 ± 63.8 b | 3702.4 ± 358.2 d | 5454.6 ± 148.7 bc | 3998.5 ± 175.7 d | 5098.8 ± 70.8 b |
| Quercetin hexoside | 1827.9 ± 16.3 d | 2050.5 ± 29.1 c | 1966.5 ± 38.3 c | 1987.3 ± 46.3 c | 2334.2 ± 169.0 b | 2686.1 ± 146.2 a | 2363.2 ± 30.1 b | 2388.7 ± 63.3 b |
| Kaempferol pentosyl-hexoside | 255.5 ± 17.9 bd | 526.4 ± 51.6 a | 260.3 ± 0.7 bd | 537.50 ± 37.0 a | 241.7 ± 8.8 de | 201.8 ± 13.9 ce | 296.9 ± 9.4 b | 183.8 ± 11.5 c |
| Quercetin pentoside | 56.5 ± 5.2 d | 46.0 ± 0.3 d | 57.6 ± 0.9 d | 45.8 ± 1.7 d | 207.3 ± 22.3 c | 285.6 ± 6.7 a | 232.2 ± 3.8 b | 241.5 ± 14.3 b |
| Kaempferol hexoside-rhamnoside | 169.0 ± 36.1 bd | 246.2 ± 88.1 a | 175.8 ± 36.2 b | 193.7 ± 2.2 ab | 65.4 ± 12.3 c | 108.2 ± 0.8 cd | 74.7 ± 0.8 c | 95.8 ± 0.8 c |
| (Iso)-rhamnetin hexoside | 3052.9 ± 230.9 c | 2859.0 ± 453.6 cd | 2926.4 ± 140.9 cd | 2563.8 ± 177.3 d | 5114.9 ± 481.3 b | 5876.0 ± 197.8 a | 5292.4 ± 3.8 b | 5078.6 ± 140.1 b |
| (Iso)-rhamnetin rutinoside | 43.7 ± 3.8 bc | 59.8 ± 4.9 b | 42.5 ± 2.3 cd | 48.9 ± 1.2 bcd | 35.1 ± 9.0 d | 73.8 ± 2.8 a | 41.8 ± 0.5 cd | 56.8 ± 2.1 b |
| Quercetin * | 381.7 ± 151.6 c | 848.1 ± 213.9 c | 547.0 ± 264.0 c | 744.5 ± 163.8 c | 1872.1 ± 1235.0 b | 4099.0 ± 466.4 a | 2164.7 ± 336.0 b | 4393.1 ± 520.9 a |
| Kaempferol * | n. d. | 51.1 ± 15.0 b | 24.3 ± 0.2 c | 60.9 ± 3.8 b | 18.7 ± 0.5 c | 88.2 ± 7.9 a | n. d. | 53.1 ± 9.1 b |
| Hydroxybenzoic acids | ||||||||
| Vanillic acid * | 69.3 ± 20.6 e | 56.40 ± 0.4 e | 60.4 ± 3.7 e | 68.8 ± 2.4 e | 190.5 ± 27.6 a | 162.6 ± 21.4 c | 164.2 ± 9.8 bc | 124.9 ± 0.3 d |
| Dihydroxybenzoic acid hexoside | 2995.0 ± 618.9 b | 3842.30 ± 467.6 a | 3126.5 ± 214.8 b | 3136.9 ± 255.7 b | 4192.1 ± 448.0 a | 234.8 ± 10.3 c | 4073.9 ± 9.4 a | 3078.0 ± 21.2 b |
| 4-Hydroxy-benzoic acid * | 67.1 ± 13.0 d | n. d. | 61.0 ± 2.5 d | 62.7 ± 1.3 d | 202.3 ± 29.2 bc | 234.0 ± 9.9 a | 234.5 ± 59.3 a | 161.9 ± 7.8 c |
| 3,4-Dihydroxy-benzoic acid (protocatechuic acid) * | 80.6 ± 13.5 f | 134.30 ± 9.8 d | 107.1 ± 8.9 e | 148.0 ± 7.0 cd | 189.4 ± 30.6 c | 300.3 ± 13.9 a | 237.9 ± 23.3 b | 244.4 ± 5.6 b |
| Hydroxycinnamic acids | ||||||||
| p-Coumaric acid * | 63.9 ± 14.1 e | 84.30 ± 11.3 de | 73.2 ± 11.3 e | 64.3 ± 2.8 e | 106.3 ± 3.8 bc | 101.0 ± 8.7 cd | 126.0 ± 12.5 a | 106.3 ± 3.8 bc |
| Cinnamic acid * | 219.6 ± 37.2 a | 62.40 ± 1.5 c | 239.6 ± 24.9 a | 43.9 ± 2.8 c | 161.7 ± 18.9 b | 58.3 ± 0.3 c | 177.5 ± 11.7 b | 65.6 ± 10.9 c |
| Ferulic acid hexoside | n. d. | 27.80 ± 1.8 cd | n. d. | 21.1 ± 0.3 e | 38.4 ± 6.4 b | 31.1 ± 1.2 bc | 42.5 ± 1.4 a | 24.9 ± 1.0 de |
| Ferulic acid * | n. d. | 90.10 ± 5.7 a | 73.8 ± 3.6 a | 80.6 ± 4.5 a | 45.5 ± 12.5 b | 82.3 ± 5.7 a | 50.5 ± 9.3 b | 83.8 ± 9.6 a |
| (A) | Principal Component (PC) | Percentage | Cumulative Percentage |
| 1 | 70.924 | 70.924 | |
| 2 | 18.074 | 88.998 | |
| 3 | 5.270 | 94.269 | |
| 4 | 3.158 | 97.426 | |
| 5 | 1.260 | 98.687 | |
| 6 | 0.879 | 99.566 | |
| 7 | 0.433 | 99.999 | |
| 8 | 0.001 | 100.000 | |
| (B) | 1 | 69.529 | 69.529 |
| 2 | 20.203 | 89.732 | |
| 3 | 4.919 | 94.651 | |
| 4 | 2.947 | 97.598 | |
| 5 | 1.176 | 98.774 | |
| 6 | 0.082 | 98.856 | |
| 7 | 0.404 | 99.260 | |
| 8 | 0.001 | 99.261 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vargas-Madriz, Á.F.; Kuri-García, A.; Luzardo-Ocampo, I.; Vargas-Madriz, H.; Pérez-Ramírez, I.F.; Anaya-Loyola, M.A.; Ferriz-Martínez, R.A.; Roldán-Padrón, O.; Hernández-Sandoval, L.; Guzmán-Maldonado, S.H.; et al. Impact of Drying Process on the Phenolic Profile and Antioxidant Capacity of Raw and Boiled Leaves and Inflorescences of Chenopodium berlandieri ssp. berlandieri. Molecules 2023, 28, 7235. https://doi.org/10.3390/molecules28207235
Vargas-Madriz ÁF, Kuri-García A, Luzardo-Ocampo I, Vargas-Madriz H, Pérez-Ramírez IF, Anaya-Loyola MA, Ferriz-Martínez RA, Roldán-Padrón O, Hernández-Sandoval L, Guzmán-Maldonado SH, et al. Impact of Drying Process on the Phenolic Profile and Antioxidant Capacity of Raw and Boiled Leaves and Inflorescences of Chenopodium berlandieri ssp. berlandieri. Molecules. 2023; 28(20):7235. https://doi.org/10.3390/molecules28207235
Chicago/Turabian StyleVargas-Madriz, Ángel Félix, Aarón Kuri-García, Ivan Luzardo-Ocampo, Haidel Vargas-Madriz, Iza Fernanda Pérez-Ramírez, Miriam Aracely Anaya-Loyola, Roberto Augusto Ferriz-Martínez, Octavio Roldán-Padrón, Luis Hernández-Sandoval, Salvador Horacio Guzmán-Maldonado, and et al. 2023. "Impact of Drying Process on the Phenolic Profile and Antioxidant Capacity of Raw and Boiled Leaves and Inflorescences of Chenopodium berlandieri ssp. berlandieri" Molecules 28, no. 20: 7235. https://doi.org/10.3390/molecules28207235
APA StyleVargas-Madriz, Á. F., Kuri-García, A., Luzardo-Ocampo, I., Vargas-Madriz, H., Pérez-Ramírez, I. F., Anaya-Loyola, M. A., Ferriz-Martínez, R. A., Roldán-Padrón, O., Hernández-Sandoval, L., Guzmán-Maldonado, S. H., & Chávez-Servín, J. L. (2023). Impact of Drying Process on the Phenolic Profile and Antioxidant Capacity of Raw and Boiled Leaves and Inflorescences of Chenopodium berlandieri ssp. berlandieri. Molecules, 28(20), 7235. https://doi.org/10.3390/molecules28207235