Electron Beam Processing as a Promising Tool to Decontaminate Polymers Containing Brominated Flame Retardants
Abstract
:1. Introduction
2. Results and Discussions
2.1. Infrared Spectroscopy Studies of DBDE Degradation in PC and ABS
2.1.1. Polycarbonate-Decabromodiphenylether
2.1.2. Acrylonitrile Butadiene Styrene-Decabromodiphenylether
2.2. Detection of BFR with HR-L2MS Mass Spectrometry
2.3. Thermal Properties of PC and ABS
2.3.1. Differential Scanning Calorimetry
2.3.2. Thermogravimetric Analysis
3. Experimental Section
3.1. Materials
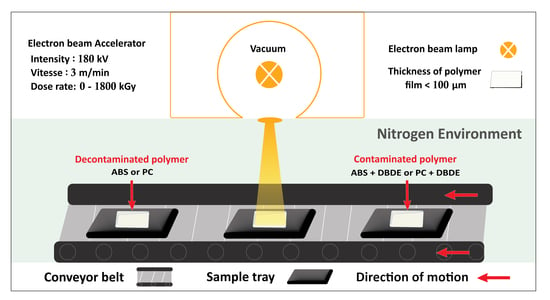
3.2. Electron-Beam Processing
3.3. Fourier Transform Infrared Spectroscopy
3.4. Differential Scanning Calorimetry
3.5. Thermogravimetric Analysis
3.6. High-Resolution Two-Step Laser Mass Spectrometry (HR-L2MS) Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wagner, F.; Peeters, J.R.; De Keyzer, J.; Janssens, K.; Duflou, J.R.; Dewulf, W. Towards a More Circular Economy for WEEE Plastics—Part A: Development of Innovative Recycling Strategies. Waste Manag. 2019, 100, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Bressanelli, G.; Saccani, N.; Pigosso, D.C.A.; Perona, M. Circular Economy in the WEEE Industry: A Systematic Literature Review and a Research Agenda. Sustain. Prod. Consum. 2020, 23, 174–188. [Google Scholar]
- Cardamone, G.F.; Ardolino, F.; Arena, U. About the Environmental Sustainability of the European Management of WEEE Plastics. Waste Manag. 2021, 126, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Harrad, S.; Drage, D.S.; Sharkey, M.; Berresheim, H. Brominated Flame Retardants and Perfluoroalkyl Substances in Landfill Leachate from Ireland. Sci. Total Environ. 2019, 695, 133810. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Kim, G.B. An Overview of Polybrominated Diphenyl Ethers (PBDEs) in the Marine Environment. Ocean Sci. J. 2015, 50, 119–142. [Google Scholar]
- Siddiqi, M.A.; Laessig, R.H.; Reed, K.D. Polybrominated Diphenyl Ethers (PBDEs): New Pollutants-Old Diseases. Clin. Med. Res. 2003, 1, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Suvorov, A.; Takser, L. Facing the Challenge of Data Transfer from Animal Models to Humans: The Case of Persistent Organohalogens. Environ. Health 2008, 7, 58. [Google Scholar] [CrossRef]
- Kim, J.S.; Klösener, J.; Flor, S.; Peters, T.M.; Ludewig, G.; Thorne, P.S.; Robertson, L.W.; Luthe, G. Toxicity Assessment of Air-Delivered Particle-Bound Polybrominated Diphenyl Ethers. Toxicology 2014, 317, 31–39. [Google Scholar] [CrossRef]
- Daso, A.P.; Fatoki, O.S.; Odendaal, J.P.; Okonkwo, J.O. A Review on Sources of Brominated Flame Retardants and Routes of Human Exposure with Emphasis on Polybrominated Diphenyl Ethers. Environ. Rev. 2010, 18, 239–254. [Google Scholar] [CrossRef]
- Trudel, D.; Scheringer, M.; Von Goetz, N.; Hungerbühler, K. Total Consumer Exposure to Polybrominated Diphenyl Ethers in North America and Europe. Environ. Sci. Technol. 2011, 45, 2391–2397. [Google Scholar] [CrossRef]
- Geyer, H.J.; Schramm, K.-W.; Darnerud, O.; Aune, M.; Feicht, E.A.; Fried, K.W.; Henkelmann, B.; Lenoir, D.; Schmid, P.; Mcdonald, T.A. Terminal Elimination Half-Lives of the Brominated Flame Retardants TBBPA, HBCD, and Lower Brominated PBDEs in Humans. In Organohalogen Compounds; Federal Environmental Agency, Gesellschaft Österreichischer Chemiker: Vienna, Austria, 2004; Volume 66, pp. 3820–3825. [Google Scholar]
- Meerts, I.A.T.M.; Letcher, R.J.; Hoving, S.; Marsh, G.; Bergman, Å.; Lemmen, J.G.; Van Der Burg, B.; Brouwer, A. In Vitro Estrogenicity of Polybrominated Diphenyl Ethers, Hydroxylated PBDEs, and Polybrominated Bisphenol A Compounds. Environ. Health Perspect. 2001, 109, 4. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Yang, X.; Chen, J.; Lyakurwa, F. QSARs on the Thyroid Hormone Effects of Polybrominated Diphenyl Ether (PBDE) Derivatives. In Comprehensive Analytical Chemistry; Elsevier B.V.: Amsterdam, The Netherlands, 2015; Volume 67, pp. 547–586. [Google Scholar] [CrossRef]
- Abdelouahab, N.; AinMelk, Y.; Takser, L. Polybrominated Diphenyl Ethers and Sperm Quality. Reprod. Toxicol. 2011, 31, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Kodavanti, P.R.S.; Coburn, C.G.; Moser, V.C.; MacPhail, R.C.; Fenton, S.E.; Stoker, T.E.; Rayner, J.L.; Kannan, K.; Birnbaum, L.S. Developmental Exposure to a Commercial PBDE Mixture, DE-71: Neurobehavioral, Hormonal, and Reproductive Effects. Toxicol. Sci. 2010, 116, 297–312. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, G.; Li, L.; Breivik, K. Global Historical Stocks and Emissions of PBDEs. Environ. Sci. Technol. 2019, 53, 6330–6340. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission regulation (EU) 2017/227-of 9 February 2017-Amending Annex XVII to Regulation (EC) No 1907/2006 of the European Parliament and of the Council Concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) as regards Bis(pentabromophenyl)ether. Off. J. Eur. Union 2017, 35, 6–9. [Google Scholar]
- Maris, E.; Botané, P.; Wavrer, P.; Froelich, D. Characterizing Plastics Originating from WEEE: A Case Study in France. Miner. Eng. 2015, 76, 28–37. [Google Scholar] [CrossRef]
- Jandric, A.; Part, F.; Fink, N.; Huber-Humer, M.; Salhofer, S.; Zafiu, C. Bromierte Flammschutzmittel in Elektroaltgeräten: Untersuchung der Brom-Konzentration nach Kunststofftypen und Gerätekategorien mittels Röntgenfluoreszenzanalyse. Osterr. Wasser Abfallwirtsch. 2020, 72, 68–76. [Google Scholar] [CrossRef]
- Wang, G.; Liu, Y.; Wang, X.; Dong, X.; Jiang, N.; Wang, H. Application of Dual Carbon-Bromine Stable Isotope Analysis to Characterize Anaerobic Micro-Degradation Mechanisms of PBDEs in Wetland Bottom-Water. Water Res. 2022, 208, 117854. [Google Scholar] [CrossRef]
- Berejka, A.J.; Cleland, M.R. Industrial Radiation Processing with Electron Beams and X-rays; Section 2.5; IAEA: Vienna, Austria, 2011; p. 25. [Google Scholar]
- Berejka, A.J. Prospects and Challenges for the Industrial use of Electron Beam Accelerators. In Proceedings of the International Topical Meeting on Nuclear Research Applications and Utilization of Accelerators, Vienna, Austria, 4–8 May 2009; IAEA: Vienna, Austria, 2009. [Google Scholar]
- Moosekian, S.R.; Jeong, S.; Marks, B.P.; Ryser, E.T. X-ray Irradiation as a Microbial Intervention Strategy for Food. Annu. Rev. Food Sci. Technol. 2012, 3, 493–510. [Google Scholar] [CrossRef]
- Parejo Calvo, W.A.; Duarte, C.L.; Machado, L.D.B.; Manzoli, J.E.; Geraldo, A.B.C.; Kodama, Y.; Silva, L.G.A.; Pino, E.S.; Somessari, E.S.R.; Silveira, C.G.; et al. Electron Beam Accelerators-Trends in Radiation Processing Technology for Industrial and Environmental Applications in Latin America and the Caribbean. Radiat. Phys. Chem. 2012, 81, 1276–1281. [Google Scholar] [CrossRef]
- Shi, W.Y.; Jiao, Z.; Gu, J.Z.; Guo, R.Y.; Wu, W.J.; Xu, G.; Hu, G.Y.; Lei, J.Q.; Wu, M.H. Degradation Characteristic of 4-Bromdiphenyl Ether in Mixed Solutions by Electron Beam Irradiation. J. Shanghai Univ. 2010, 14, 89–93. [Google Scholar] [CrossRef]
- Zhao, P.; Ye, Q.; Zheng, Y.; Whalen, J.K.; Zhang, S.; Wang, W. Radiolytic Degradation of BDE-209 in Rice-Vegetable Rotation Soils Induced by Electron Beam Irradiation. Environ. Pollut. 2021, 286, 117564. [Google Scholar] [CrossRef] [PubMed]
- Chruszcz-Lipska, K.; Trzewik, B.; Winid, B. Molecular Structure and Vibrational Spectra of 2,2′,4,4′,6-Pentabromodiphenyl Ether (BDE 100). Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 182, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Asgari, A.; Ghani, K.; Keshavarz, M.H. Investigating the Effect of Copper(II) Coordination Compound with Azodicarbonamide Ligand on the Phase-Stabilization of Ammonium Nitrate. Z. Anorg. Allg. Chem. 2018, 644, 58–64. [Google Scholar] [CrossRef]
- Zeimaran, E.; Bahraeian, S.; Ghanbari, T.; Pourshahrestani, S.; Nor, H.M. Synthesis and Characterization of Supramolecular Elastomers from Polyacids Composed of Vegetable Oils. Proc. Adv. Mater. Res. 2013, 747, 505–508. [Google Scholar] [CrossRef]
- Yousif, E.; Haddad, R. Photodegradation and Photostabilization of Polymers, especially Polystyrene: Review. SpringerPlus 2013, 2, 398. [Google Scholar] [CrossRef] [PubMed]
- Blom, H.; Yeh, R.; Wojnarowski, R.; Ling, M. Detection of Degradation of ABS Materials via DSC. J. Therm. Anal. Calorim. 2006, 83, 113–115. [Google Scholar] [CrossRef]
- Suzuki, M.; Wilkie, C.A. The Thermal Degradation of Acrylonitrile-Butadiene-Styrene Terpolymer Grafted with Methacrylic Acid. Polym. Degrad. Stab. 1995, 47, 217–221. [Google Scholar] [CrossRef]
- Tiganis, B.E.; Burn, L.S.; Davis, P.; Hill, A.J. Thermal Degradation of Acrylonitrile-Butadiene-Styrene (ABS) Blends. Polym. Degrad. Stab. 2002, 76, 425–434. [Google Scholar] [CrossRef]
- Yang, S.; Castilleja, J.R.; Barrera, E.V.; Lozano, K. Thermal Analysis of an Acrylonitrile-Butadiene-Styrene/SWNT Composite. Polym. Degrad. Stab. 2004, 83, 383–388. [Google Scholar] [CrossRef]
- Shapi, M.M. TG and DSC studies of some thermal properties and stability aspects of poly(acrylonitrile butadiene styrene), polysterene and poly(acrylonitrile styrene) plastics. Thermochim. Acta 1991, 175, 25–34. [Google Scholar] [CrossRef]
- Duca, D.; Rahman, M.; Carpentier, Y.; Pirim, C.; Boies, A.; Focsa, C. Chemical characterization of size-selected nanoparticles emitted by a gasoline direct injection engine: Impact of a catalytic stripper. Fuel 2021, 294, 120317. [Google Scholar] [CrossRef]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benmammar, R.K.; Mundlapati, V.R.; Bouberka, Z.; Barrera, A.; Staelens, J.-N.; Tahon, J.-F.; Ziskind, M.; Carpentier, Y.; Focsa, C.; Supiot, P.; et al. Electron Beam Processing as a Promising Tool to Decontaminate Polymers Containing Brominated Flame Retardants. Molecules 2023, 28, 7753. https://doi.org/10.3390/molecules28237753
Benmammar RK, Mundlapati VR, Bouberka Z, Barrera A, Staelens J-N, Tahon J-F, Ziskind M, Carpentier Y, Focsa C, Supiot P, et al. Electron Beam Processing as a Promising Tool to Decontaminate Polymers Containing Brominated Flame Retardants. Molecules. 2023; 28(23):7753. https://doi.org/10.3390/molecules28237753
Chicago/Turabian StyleBenmammar, Rachida Khadidja, Venkateswara Rao Mundlapati, Zohra Bouberka, Ana Barrera, Jean-Noël Staelens, Jean-François Tahon, Michael Ziskind, Yvain Carpentier, Cristian Focsa, Philippe Supiot, and et al. 2023. "Electron Beam Processing as a Promising Tool to Decontaminate Polymers Containing Brominated Flame Retardants" Molecules 28, no. 23: 7753. https://doi.org/10.3390/molecules28237753
APA StyleBenmammar, R. K., Mundlapati, V. R., Bouberka, Z., Barrera, A., Staelens, J. -N., Tahon, J. -F., Ziskind, M., Carpentier, Y., Focsa, C., Supiot, P., Foissac, C., & Maschke, U. (2023). Electron Beam Processing as a Promising Tool to Decontaminate Polymers Containing Brominated Flame Retardants. Molecules, 28(23), 7753. https://doi.org/10.3390/molecules28237753