Enhancement of Electricity Production in Microbial Fuel Cells Using a Biosurfactant-Producing Co-Culture
Abstract
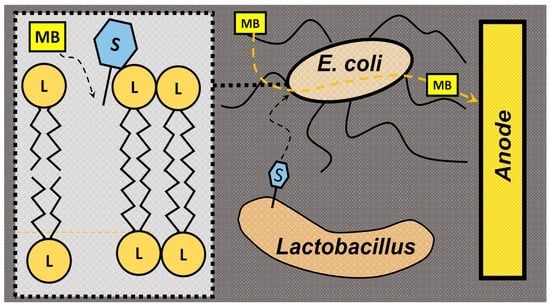
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Reactor Design and Operation
3.2. Experimental Design and Statistical Analysis
3.3. Analytical Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, X.; Abu-Reesh, I.M.; He, Z. Development of Bioelectrochemical Systems to Promote Sustainable Agriculture. Agriculture 2015, 5, 367–388. [Google Scholar] [CrossRef]
- Ghangrekar, M.M.; Chatterjee, P. A Systematic Review on Bioelectrochemical Systems Research. Curr. Pollut. Rep. 2017, 3, 281–288. [Google Scholar] [CrossRef]
- Santoro, C.; Arbizzani, C.; Erable, B.; Ieropoulos, I. Microbial Fuel Cells: From Fundamentals to Applications—A Review. J. Power Sources 2017, 356, 225–244. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, A.; Hafeez, A.; Abbas, S.Z.; ul Haq, I.; Mukhtar, H.; Rafatullah, M. A State of the Art Review on Electron Transfer Mechanisms, Characteristics, Applications and Recent Advancements in Microbial Fuel Cells Technology. Green Chem. Lett. Rev. 2020, 13, 365–381. [Google Scholar] [CrossRef]
- Ng, I.-S.; Hsueh, C.-C.; Chen, B.-Y. Electron Transport Phenomena of Electroactive Bacteria in Microbial Fuel Cells: A Review of Proteus Hauseri. Bioresour. Bioprocess. 2017, 4, 53. [Google Scholar] [CrossRef]
- Li, T.; Yang, X.-L.; Chen, Q.-L.; Song, H.-L.; He, Z.; Yang, Y.-L. Enhanced Performance of Microbial Fuel Cells with Electron Mediators from Anthraquinone/Polyphenol-Abundant Herbal Plants. ACS Sustain. Chem. Eng. 2020, 8, 11263–11275. [Google Scholar] [CrossRef]
- Martinez, C.M.; Alvarez, L.H. Application of Redox Mediators in Bioelectrochemical Systems. Biotechnol. Adv. 2018, 36, 1412–1423. [Google Scholar] [CrossRef] [PubMed]
- Kaczorek, E.; Pacholak, A.; Zdarta, A.; Smułek, W. The Impact of Biosurfactants on Microbial Cell Properties Leading to Hydrocarbon Bioavailability Increase. Colloids Interfaces 2018, 2, 35. [Google Scholar] [CrossRef]
- Wen, Q.; Kong, F.; Ma, F.; Ren, Y.; Pan, Z. Improved Performance of Air-Cathode Microbial Fuel Cell through Additional Tween 80. J. Power Sources 2011, 196, 899–904. [Google Scholar] [CrossRef]
- Liu, J.; Qiao, Y.; Lu, Z.S.; Song, H.; Li, C.M. Enhance Electron Transfer and Performance of Microbial Fuel Cells by Perforating the Cell Membrane. Electrochem. Commun. 2012, 15, 50–53. [Google Scholar] [CrossRef]
- Shen, H.B.; Yong, X.Y.; Chen, Y.L.; Liao, Z.H.; Si, R.W.; Zhou, J.; Wang, S.Y.; Yong, Y.C.; OuYang, P.K.; Zheng, T. Enhanced Bioelectricity Generation by Improving Pyocyanin Production and Membrane Permeability through Sophorolipid Addition in Pseudomonas Aeruginosa-Inoculated Microbial Fuel Cells. Bioresour. Technol. 2014, 167, 490–494. [Google Scholar] [CrossRef]
- Pasternak, G.; Askitosari, T.D.; Rosenbaum, M.A. Biosurfactants and Synthetic Surfactants in Bioelectrochemical Systems: A Mini-Review. Front. Microbiol. 2020, 11, 358. [Google Scholar] [CrossRef] [PubMed]
- Merrettig-Bruns, U.; Jelen, E. Anaerobic Biodegradation of Detergent Surfactants. Materials 2009, 2, 181–206. [Google Scholar] [CrossRef]
- Wu, Q.; Zhao, L.; Song, R.; Ma, A. Research Progress of Surfactant Biodegradation. IOP Conf. Ser. Earth Environ. Sci. 2019, 227, 052023. [Google Scholar] [CrossRef]
- Nielsen, C.K.; Kjems, J.; Mygind, T.; Snabe, T.; Meyer, R.L. Effects of Tween 80 on Growth and Biofilm Formation in Laboratory Media. Front. Microbiol. 2016, 7, 1878. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Xu, Y.-S.; Yong, X.-Y.; Li, B.; Yin, D.; Cheng, Q.-W.; Yuan, H.-R.; Yong, Y.-C. Endogenously Enhanced Biosurfactant Production Promotes Electricity Generation from Microbial Fuel Cells. Bioresour. Technol. 2015, 197, 416–421. [Google Scholar] [CrossRef]
- Qu, Y.; Feng, Y.; Wang, X.; Logan, B.E. Use of a Coculture To Enable Current Production by Geobacter Sulfurreducens. Appl. Environ. Microbiol. 2012, 78, 3484–3487. [Google Scholar] [CrossRef]
- Schmitz, S.; Rosenbaum, M.A. Boosting Mediated Electron Transfer in Bioelectrochemical Systems with Tailored Defined Microbial Cocultures. Biotechnol. Bioeng. 2018, 115, 2183–2193. [Google Scholar] [CrossRef] [PubMed]
- Aiyer, K.S. Synergistic Effects in a Microbial Fuel Cell between Co-Cultures and a Photosynthetic Alga Chlorella Vulgaris Improve Performance. Heliyon 2021, 7, e05935. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Ethiraj, B.; Cheng, C.K.; Yousuf, A.; Khan, M.M.R. An Insight of Synergy between Pseudomonas Aeruginosa and Klebsiella Variicola in a Microbial Fuel Cell. ACS Sustain. Chem. Eng. 2018, 6, 4130–4137. [Google Scholar] [CrossRef]
- Song, Y.-C.; Kim, D.-S.; Woo, J.-H.; Subha, B.; Jang, S.-H.; Sivakumar, S. Effect of Surface Modification of Anode with Surfactant on the Performance of Microbial Fuel Cell. Int. J. Energy Res. 2015, 39, 860–868. [Google Scholar] [CrossRef]
- Ren, L.; Tokash, J.C.; Regan, J.M.; Logan, B.E. Current Generation in Microbial Electrolysis Cells with Addition of Amorphous Ferric Hydroxide, Tween 80, or DNA. Int. J. Hydrogen Energy 2012, 37, 16943–16950. [Google Scholar] [CrossRef]
- Mohanty, S.S.; Koul, Y.; Varjani, S.; Pandey, A.; Ngo, H.H.; Chang, J.-S.; Wong, J.W.C.; Bui, X.-T. A Critical Review on Various Feedstocks as Sustainable Substrates for Biosurfactants Production: A Way towards Cleaner Production. Microb. Cell Factories 2021, 20, 120. [Google Scholar] [CrossRef] [PubMed]
- Montoya Vallejo, C.; Flórez Restrepo, M.A.; Guzmán Duque, F.L.; Quintero Díaz, J.C. Production, Characterization and Kinetic Model of Biosurfactant Produced by Lactic Acid Bacteria. Electron. J. Biotechnol. 2021, 53, 14–22. [Google Scholar] [CrossRef]
- Ačai, P.; Valík, L.; Medved’ová, A.; Rosskopf, F. Modelling and Predicting the Simultaneous Growth of Escherichia Coli and Lactic Acid Bacteria in Milk. Food Sci. Technol. Int. 2016, 22, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.; Jung, D.; Wang, X.; Seo, D.J.; Lee, M.H.; Lee, B.-H.; Choi, C. Combined Effect of Lactic Acid Bacteria and Citric Acid on Escherichia Coli O157:H7 and Salmonella Typhimurium. Food Sci. Biotechnol. 2013, 22, 1171–1174. [Google Scholar] [CrossRef]
- Coroller, L.; Guerrot, V.; Huchet, V.; Le Marc, Y.; Mafart, P.; Sohier, D.; Thuault, D. Modelling the Influence of Single Acid and Mixture on Bacterial Growth. Int. J. Food Microbiol. 2005, 100, 167–178. [Google Scholar] [CrossRef]
- Mizuno, K.; Mizuno, M.; Yamauchi, M.; Takemura, A.J.; Medrano Romero, V.; Morikawa, K. Adjacent-Possible Ecological Niche: Growth of Lactobacillus Species Co-Cultured with Escherichia Coli in a Synthetic Minimal Medium|Scientific Reports. Sci. Rep. 2017, 7, 12880. [Google Scholar] [CrossRef]
- Ganzorig, B.; Zayabaatar, E.; Pham, M.T.; Marito, S.; Huang, C.-M.; Lee, Y.-H. Lactobacillus Plantarum Generate Electricity through Flavin Mononucleotide-Mediated Extracellular Electron Transfer to Upregulate Epithelial Type I Collagen Expression and Thereby Promote Microbial Adhesion to Intestine. Biomedicines 2023, 11, 677. [Google Scholar] [CrossRef]
- Vilas Boas, J.; Oliveira, V.B.; Marcon, L.R.C.; Simões, M.; Pinto, A.M.F.R. Optimization of a Single Chamber Microbial Fuel Cell Using Lactobacillus Pentosus: Influence of Design and Operating Parameters. Sci. Total Environ. 2019, 648, 263–270. [Google Scholar] [CrossRef]
- Roy, H.; Rahman, T.U.; Tasnim, N.; Arju, J.; Rafid, M.M.; Islam, M.R.; Pervez, M.N.; Cai, Y.; Naddeo, V.; Islam, M.S. Microbial Fuel Cell Construction Features and Application for Sustainable Wastewater Treatment. Membranes 2023, 13, 490. [Google Scholar] [CrossRef] [PubMed]
- Sugnaux, M.; Mermoud, S.; da Costa, A.F.; Happe, M.; Fischer, F. Probing Electron Transfer with Escherichia Coli: A Method to Examine Exoelectronics in Microbial Fuel Cell Type Systems. Bioresour. Technol. 2013, 148, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Del Campo, A.G.; Canizares, P.; Lobato, J.; Rodrigo, M.F.J. Fernandez Morales Effects of External Resistance on Microbial Fuel Cell’s Performance. In Environment, Energy and Climate Change II. The Handbook of Environmental Chemistry; Lefebvre, G., Jiménez, E., Cabañas, B., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 41–53. ISBN 1433-6863r978-3-642-03970-6. [Google Scholar]
- Bide, Y.; Fashapoyeh, M.A.; Shokrollahzadeh, S. Structural Investigation and Application of Tween 80-Choline Chloride Self-Assemblies as Osmotic Agent for Water Desalination. Sci. Rep. 2021, 11, 17068. [Google Scholar] [CrossRef]
- Dominguez, R.B.; Orozco, M.A.; Chávez, G.; Márquez-Lucero, A. The Evaluation of a Low-Cost Colorimeter for Glucose Detection in Salivary Samples. Sensors 2017, 17, 2495. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.; Sekhar, A.C.; Upreti, R.; Mujawar, M.M.; Pasha, S.S. Optimization of Single Plate-Serial Dilution Spotting (SP-SDS) with Sample Anchoring as an Assured Method for Bacterial and Yeast Cfu Enumeration and Single Colony Isolation from Diverse Samples. Biotechnol. Rep. 2015, 8, 45–55. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montoya-Vallejo, C.; Gil Posada, J.O.; Quintero-Díaz, J.C. Enhancement of Electricity Production in Microbial Fuel Cells Using a Biosurfactant-Producing Co-Culture. Molecules 2023, 28, 7833. https://doi.org/10.3390/molecules28237833
Montoya-Vallejo C, Gil Posada JO, Quintero-Díaz JC. Enhancement of Electricity Production in Microbial Fuel Cells Using a Biosurfactant-Producing Co-Culture. Molecules. 2023; 28(23):7833. https://doi.org/10.3390/molecules28237833
Chicago/Turabian StyleMontoya-Vallejo, Carolina, Jorge Omar Gil Posada, and Juan Carlos Quintero-Díaz. 2023. "Enhancement of Electricity Production in Microbial Fuel Cells Using a Biosurfactant-Producing Co-Culture" Molecules 28, no. 23: 7833. https://doi.org/10.3390/molecules28237833
APA StyleMontoya-Vallejo, C., Gil Posada, J. O., & Quintero-Díaz, J. C. (2023). Enhancement of Electricity Production in Microbial Fuel Cells Using a Biosurfactant-Producing Co-Culture. Molecules, 28(23), 7833. https://doi.org/10.3390/molecules28237833