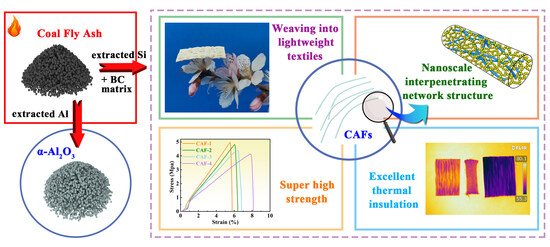
Recycling Coal Fly Ash for Super-Thermal-Insulating Aerogel Fiber Preparation with Simultaneous Al2O3 Extraction
Abstract
:1. Introduction
2. Results and Discussion
2.1. Morphologies and Structure of CAFs
2.2. Hydrophobization of the Surfaces of CAFs
2.3. Mechanical Properties
2.4. Thermal Insulation Performance of CAFs
2.5. α-Al2O3 Powder
3. Experimental Section
3.1. Materials
3.2. Preparation of the SiO2 Sol
3.3. Preparation of Bacterial Cellulose Matrix
3.4. Preparation of Silica–Bacterial Cellulose Composite Wet Gel Fibers
3.5. Hydrophobic Modification and Atmospheric Drying of Wet Gel Fibers to Obtain CAFs
3.6. Preparation of α-Al2O3 from the Extraction Raffinate of SiO2
3.7. Characterizations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zeng, L.; Sun, H.; Peng, T.; Zheng, W. Preparation of Porous Glass-Ceramics from Coal Fly Ash and Asbestos Tailings by High-Temperature Pore-Forming. Waste Manag. 2020, 106, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Fan, M.; Mclaughlin, J.F.; Shen, X.; Tan, G. A Novel Low-Cost Method of Silica Aerogel Fabrication Using Fly Ash and Trona Ore with Ambient Pressure Drying Technique. Powder Technol. 2018, 323, 310–322. [Google Scholar] [CrossRef]
- Rubio, B.; Izquierdo, M.T. Coal Fly Ash Based Carbons for SO2 Removal from Flue Gases. Waste Manag. 2010, 30, 1341–1347. [Google Scholar] [CrossRef]
- Shen, M.; Jiang, X.; Zhang, M.; Guo, M. Synthesis of SiO2–Al2O3 Composite Aerogel from Fly Ash: A Low-Cost and Facile Approach. J. Sol-Gel Sci. Technol. 2020, 93, 281–290. [Google Scholar] [CrossRef]
- Mushtaq, F.; Zahid, M.; Bhatti, I.A.; Nasir, S.; Hussain, T. Possible Applications of Coal Fly Ash in Wastewater Treatment. J. Environ. Manag. 2019, 240, 27–46. [Google Scholar] [CrossRef] [PubMed]
- Abbas, S.; Saleem, M.A.; Kazmi, S.M.S.; Munir, M.J. Production of Sustainable Clay Bricks Using Waste Fly Ash: Mechanical and Durability Properties. J. Build. Eng. 2017, 14, 7–14. [Google Scholar] [CrossRef]
- Telesca, A.; Marroccoli, M.; Calabrese, D.; Valenti, G.L.; Montagnaro, F. Flue Gas Desulfurization Gypsum and Coal Fly Ash as Basic Components of Prefabricated Building Materials. Waste Manag. 2013, 33, 628–633. [Google Scholar] [CrossRef]
- Valeev, D.; Bobylev, P.; Osokin, N.; Zolotova, I.; Rodionov, I.; Salazar-Concha, C.; Verichev, K. A Review of the Alumina Production from Coal Fly Ash, with a Focus in Russia. J. Clean. Prod. 2022, 363, 132360. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, L.; Yang, Y.; Pang, B.; Xu, W.; Duan, G.; Jiang, S.; Zhang, K. Recent Progress on Nanocellulose Aerogels: Preparation, Modification, Composite Fabrication, Applications. Adv. Mater. 2021, 33, 2005569. [Google Scholar] [CrossRef]
- Gupta, P.; Singh, B.; Agrawal, A.K.; Maji, P.K. Low Density and High Strength Nanofibrillated Cellulose Aerogel for Thermal Insulation Application. Mater. Des. 2018, 158, 224–236. [Google Scholar] [CrossRef]
- Soleimani Dorcheh, A.; Abbasi, M.H. Silica Aerogel; Synthesis, Properties and Characterization. J. Mater. Process. Technol. 2008, 199, 10–26. [Google Scholar] [CrossRef]
- Su, L.; Wang, H.; Niu, M.; Dai, S.; Cai, Z.; Yang, B.; Huyan, H.; Pan, X. Anisotropic and Hierarchical SiC@SiO2 Nanowire Aerogel with Exceptional Stiffness and Stability for Thermal Superinsulation. Sci. Adv. 2020, 6, eaay6689. [Google Scholar] [CrossRef] [PubMed]
- Randall, J.P.; Meador, M.A.B.; Jana, S.C. Tailoring Mechanical Properties of Aerogels for Aerospace Applications. ACS Appl. Mater. Interfaces 2011, 3, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ni, X.; Li, C.; You, B.; Sun, G. Co-Gel Strategy for Preparing Hierarchically Porous Silica/Polyimide Nanocomposite Aerogel with Thermal Insulation and Flame Retardancy. J. Mater. Chem. A 2020, 8, 9701–9712. [Google Scholar] [CrossRef]
- He, F.; Qi, Z.; Zhen, W.; Wu, J.; Huang, Y.; Xiong, X.; Zhang, R. Thermal Conductivity of Silica Aerogel Thermal Insulation Coatings. Int. J. Thermophys. 2019, 40, 92. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, X.; Wang, J.; Liu, Z.; Zhang, K.; Ji, X.; You, Y.; Zhang, X. Reaction-Spun Transparent Silica Aerogel Fibers. ACS Nano 2020, 14, 11919–11928. [Google Scholar] [CrossRef] [PubMed]
- Gurav, J.L.; Jung, I.-K.; Park, H.-H.; Kang, E.S.; Nadargi, D.Y. Silica Aerogel: Synthesis and Applications. J. Nanomater. 2010, 2010, 23. [Google Scholar] [CrossRef]
- Zhu, J.; Guo, S.; Li, X. Facile Preparation of a SiO2–Al2O3 Aerogel Using Coal Gangue as a Raw Material via an Ambient Pressure Drying Method and Its Application in Organic Solvent Adsorption. RSC Adv. 2015, 5, 103656–103661. [Google Scholar] [CrossRef]
- Hu, W.; Li, M.; Chen, W.; Zhang, N.; Li, B.; Wang, M.; Zhao, Z. Preparation of Hydrophobic Silica Aerogel with Kaolin Dried at Ambient Pressure. Colloids Surf. Physicochem. Eng. Asp. 2016, 501, 83–91. [Google Scholar] [CrossRef]
- Duong, H.M.; Ling, N.R.B.; Thai, Q.B.; Le, D.K.; Nguyen, P.T.T.; Goh, X.Y.; Phan-Thien, N. A Novel Aerogel from Thermal Power Plant Waste for Thermal and Acoustic Insulation Applications. Waste Manag. 2021, 124, 1–7. [Google Scholar] [CrossRef]
- He, H.; Liu, J.; Wang, Y.; Zhao, Y.; Qin, Y.; Zhu, Z.; Yu, Z.; Wang, J. An Ultralight Self-Powered Fire Alarm e-Textile Based on Conductive Aerogel Fiber with Repeatable Temperature Monitoring Performance Used in Firefighting Clothing. ACS Nano 2022, 16, 2953–2967. [Google Scholar] [CrossRef]
- Li, M.; Gan, F.; Dong, J.; Fang, Y.; Zhao, X.; Zhang, Q. Facile Preparation of Continuous and Porous Polyimide Aerogel Fibers for Multifunctional Applications. ACS Appl. Mater. Interfaces 2021, 13, 10416–10427. [Google Scholar] [CrossRef]
- Cui, Y.; Gong, H.; Wang, Y.; Li, D.; Bai, H. A Thermally Insulating Textile Inspired by Polar Bear Hair. Adv. Mater. 2018, 30, 1706807. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Zhang, X.; Hu, G.; Huang, T.; Yu, H.; Yu, B.; Zhu, M. In Situ Crosslinking of Mechanically Robust Waterproof and Moisture Permeable Cellulose Diacetate Nanofiber Aerogels for Warm Clothing. Chem. Eng. J. 2022, 444, 136528. [Google Scholar] [CrossRef]
- Wu, H.; Zhao, L.; Zhang, S.; Si, Y.; Yu, J.; Ding, B. Ultralight and Mechanically Robust Fibrous Sponges Tailored by Semi-Interpenetrating Polymer Networks for Warmth Retention. ACS Appl. Mater. Interfaces 2021, 13, 18165–18174. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Zuo, D.; Gan, H.; Yi, C. Comparative Study on the Effects of Laser Bleaching and Conventional Bleaching on the Physical Properties of Indigo Kapok/Cotton Denim Fabrics. Appl. Sci. 2019, 9, 4662. [Google Scholar] [CrossRef]
- Sun, C.; Fan, J.; Wu, H.; Wu, Y.; Wan, X. Cold Protective Clothing with Reflective Nano-Fibrous Interlayers for Improved Comfort. Int. J. Cloth. Sci. Technol. 2013, 25, 380–388. [Google Scholar] [CrossRef]
- Li, Q.; Yuan, Z.; Zhang, C.; Hu, S.; Chen, Z.; Wu, Y.; Chen, P.; Qi, H.; Ye, D. Tough, Highly Oriented, Super Thermal Insulating Regenerated All-Cellulose Sponge-Aerogel Fibers Integrating a Graded Aligned Nanostructure. Nano Lett. 2022, 22, 3516–3524. [Google Scholar] [CrossRef]
- Hu, R.; Liu, Y.; Shin, S.; Huang, S.; Ren, X.; Shu, W.; Cheng, J.; Tao, G.; Xu, W.; Chen, R.; et al. Emerging Materials and Strategies for Personal Thermal Management. Adv. Energy Mater. 2020, 10, 1903921. [Google Scholar] [CrossRef]
- Tao, P.; Shang, W.; Song, C.; Shen, Q.; Zhang, F.; Luo, Z.; Yi, N.; Zhang, D.; Deng, T. Bioinspired Engineering of Thermal Materials. Adv. Mater. 2015, 27, 428–463. [Google Scholar] [CrossRef]
- Oh, K.W.; Kim, D.K.; Kim, S.H. Ultra-Porous Flexible PET/Aerogel Blanket for Sound Absorption and Thermal Insulation. Fibers Polym. 2009, 10, 731–737. [Google Scholar] [CrossRef]
- Altay, P.; Atakan, R.; Özcan, G. Silica Aerogel Application to Polyester Fabric for Outdoor Clothing. Fibers Polym. 2021, 22, 1025–1032. [Google Scholar] [CrossRef]
- Rahman Bhuiyan, M.A.; Wang, L.; Shaid, A.; Shanks, R.A.; Ding, J. Polyurethane-Aerogel Incorporated Coating on Cotton Fabric for Chemical Protection. Prog. Org. Coat. 2019, 131, 100–110. [Google Scholar] [CrossRef]
- Jahid, M.A.; Hu, J.; Thakur, S. Mechanically Robust, Responsive Composite Membrane for a Thermoregulating Textile. ACS Omega 2020, 5, 3899–3907. [Google Scholar] [CrossRef]
- Xiong, X.; Yang, T.; Mishra, R.; Kanai, H.; Militky, J. Thermal and Compression Characteristics of Aerogel-Encapsulated Textiles. J. Ind. Text. 2018, 47, 1998–2013. [Google Scholar] [CrossRef]
- Meng, S.; Zhang, J.; Chen, W.; Wang, X.; Zhu, M. Construction of Continuous Hollow Silica Aerogel Fibers with Hierarchical Pores and Excellent Adsorption Performance. Microporous Mesoporous Mater. 2019, 273, 294–296. [Google Scholar] [CrossRef]
- Lazzari, L.K.; Perondi, D.; Zampieri, V.B.; Zattera, A.J.; Santana, R.M.C. Cellulose/Biochar Aerogels with Excellent Mechanical and Thermal Insulation Properties. Cellulose 2019, 26, 9071–9083. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Y.; Li, J.; Sun, Y.; Li, H.; Qiu, W.; Luo, Z.; Zhao, T. Tough Macroporous Phenolic Resin/Bacterial Cellulose Composite with Double-Network Structure Fabricated by Ambient Pressure Drying. Cellulose 2020, 27, 5029–5039. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, M.; Li, M.; Liu, L.; Liu, L.; Yu, J. Cellulose Nanofibril (CNF) Based Aerogels Prepared by a Facile Process and the Investigation of Thermal Insulation Performance. Cellulose 2020, 27, 6217–6233. [Google Scholar] [CrossRef]
- Maleki, H.; Durães, L.; Portugal, A. An Overview on Silica Aerogels Synthesis and Different Mechanical Reinforcing Strategies. J. Non-Cryst. Solids 2014, 385, 55–74. [Google Scholar] [CrossRef]
- Linhares, T.; Pessoa De Amorim, M.T.; Durães, L. Silica Aerogel Composites with Embedded Fibres: A Review on Their Preparation, Properties and Applications. J. Mater. Chem. A 2019, 7, 22768–22802. [Google Scholar] [CrossRef]
- Zimmermann, M.V.G.; Zattera, A.J. Silica Aerogel Reinforced with Cellulose Nanofibers. J. Porous Mater. 2021, 28, 1325–1333. [Google Scholar] [CrossRef]
- Song, Q.; Miao, C.; Sai, H.; Gu, J.; Wang, M.; Jiang, P.; Wang, Y.; Fu, R.; Wang, Y. Silica-Bacterial Cellulose Composite Aerogel Fibers with Excellent Mechanical Properties from Sodium Silicate Precursor. Gels 2021, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Liu, S.; Feng, J.; Kimura, S.; Wada, M.; Kuga, S.; Zhang, L. Cellulose-Silica Nanocomposite Aerogels by In Situ Formation of Silica in Cellulose Gel. Angew. Chem. Int. Ed. 2012, 51, 2076–2079. [Google Scholar] [CrossRef]
- Sai, H.; Fu, R.; Xiang, J.; Guan, Y.; Zhang, F. Fabrication of Elastic Silica-Bacterial Cellulose Composite Aerogels with Nanoscale Interpenetrating Network by Ultrafast Evaporative Drying. Compos. Sci. Technol. 2018, 155, 72–80. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Feng, Q.; Chen, K.; Ma, D.; Lin, H.; Liu, Z.; Qin, S.; Luo, Y. Synthesis of High Specific Surface Area Silica Aerogel from Rice Husk Ash via Ambient Pressure Drying. Colloids Surf. Physicochem. Eng. Asp. 2018, 539, 399–406. [Google Scholar] [CrossRef]
- Dou, B.; Li, J.; Wang, Y.; Wang, H.; Ma, C.; Hao, Z. Adsorption and Desorption Performance of Benzene over Hierarchically Structured Carbon–Silica Aerogel Composites. J. Hazard. Mater. 2011, 196, 194–200. [Google Scholar] [CrossRef]
- Hwang, S.-W.; Jung, H.-H.; Hyun, S.-H.; Ahn, Y.-S. Effective Preparation of Crack-Free Silica Aerogels via Ambient Drying. J. Sol-Gel Sci. Technol. 2007, 41, 139–146. [Google Scholar] [CrossRef]
- Mulyadi, A.; Zhang, Z.; Deng, Y. Fluorine-Free Oil Absorbents Made from Cellulose Nanofibril Aerogels. ACS Appl. Mater. Interfaces 2016, 8, 2732–2740. [Google Scholar] [CrossRef] [PubMed]
- Hayase, G.; Kanamori, K.; Abe, K.; Yano, H.; Maeno, A.; Kaji, H.; Nakanishi, K. Polymethylsilsesquioxane–Cellulose Nanofiber Biocomposite Aerogels with High Thermal Insulation, Bendability, and Superhydrophobicity. ACS Appl. Mater. Interfaces 2014, 6, 9466–9471. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, P.; Li, D. Study on Low-Cost Preparation of Glass–Ceramic from Municipal Solid Waste Incineration (MSWI) Fly Ash and Lead–Zinc Tailings. Constr. Build. Mater. 2022, 356, 129231. [Google Scholar] [CrossRef]
- Sun, J.; Wu, Z.; An, B.; Ma, C.; Xu, L.; Zhang, Z.; Luo, S.; Li, W.; Liu, S. Thermal-Insulating, Flame-Retardant and Mechanically Resistant Aerogel Based on Bio-Inspired Tubular Cellulose. Compos. Part B Eng. 2021, 220, 108997. [Google Scholar] [CrossRef]
- Abdul Halim, Z.A.; Awang, N.; Yajid, M.A.M.; Ahmad, N.; Hamdan, H. A Comparison between the Effects of Hydrophobic and Hydrophilic Silica Aerogel Fillers on Tensile and Thermal Properties of Unsaturated Polyester Composites. Polym. Bull. 2022, 79, 6173–6191. [Google Scholar] [CrossRef]
- Zeng, W.; Gao, L.; Gui, L.; Guo, J. Sintering Kinetics of α-Al2O3 Powder. Ceram. Int. 1999, 25, 723–726. [Google Scholar] [CrossRef]
- Han, L.; Ren, W.; Wang, B.; He, X.; Ma, L.; Huo, Q.; Wang, J.; Bao, W.; Chang, L. Extraction of SiO2 and Al2O3 from Coal Gangue Activated by Supercritical Water. Fuel 2019, 253, 1184–1192. [Google Scholar] [CrossRef]
- Sai, H.; Fu, R.; Xing, L.; Xiang, J.; Li, Z.; Li, F.; Zhang, T. Surface Modification of Bacterial Cellulose Aerogels’ Web-like Skeleton for Oil/Water Separation. ACS Appl. Mater. Interfaces 2015, 7, 7373–7381. [Google Scholar] [CrossRef]
- Heath, L.; Thielemans, W. Cellulose Nanowhisker Aerogels. Green Chem. 2010, 12, 1448. [Google Scholar] [CrossRef]






| Samples | SiO2 in Samples [% w/w] | Bulk Density [g/cm3] | SBET [m2/g] | Pore Size [nm] | Porosity a [%] |
|---|---|---|---|---|---|
| CAF-1 | 57 | 0.1307 | 588.75 | 13.99 | 91.8 |
| CAF-2 | 49 | 0.1238 | 564.31 | 12.64 | 90.1 |
| CAF-3 | 35 | 0.1191 | 322.76 | 12.87 | 86.4 |
| CAF-4 | 26 | 0.1092 | 234.81 | 12.41 | 81.5 |
| Solution | SS-1 | SS-2 | SS-3 | SS-4 |
|---|---|---|---|---|
| Added water (mL) | 0 | 3 | 6 | 9 |
| Corresponding sample name | CAF-1 | CAF-2 | CAF-3 | CAF-4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, J.; Liu, L.; Zhu, R.; Song, Q.; Yu, H.; Jiang, P.; Miao, C.; Du, Y.; Fu, R.; Wang, Y.; et al. Recycling Coal Fly Ash for Super-Thermal-Insulating Aerogel Fiber Preparation with Simultaneous Al2O3 Extraction. Molecules 2023, 28, 7978. https://doi.org/10.3390/molecules28247978
Gu J, Liu L, Zhu R, Song Q, Yu H, Jiang P, Miao C, Du Y, Fu R, Wang Y, et al. Recycling Coal Fly Ash for Super-Thermal-Insulating Aerogel Fiber Preparation with Simultaneous Al2O3 Extraction. Molecules. 2023; 28(24):7978. https://doi.org/10.3390/molecules28247978
Chicago/Turabian StyleGu, Jie, Lipeng Liu, Rongrong Zhu, Qiqi Song, Hanqing Yu, Pengjie Jiang, Changqing Miao, Yuxiang Du, Rui Fu, Yaxiong Wang, and et al. 2023. "Recycling Coal Fly Ash for Super-Thermal-Insulating Aerogel Fiber Preparation with Simultaneous Al2O3 Extraction" Molecules 28, no. 24: 7978. https://doi.org/10.3390/molecules28247978
APA StyleGu, J., Liu, L., Zhu, R., Song, Q., Yu, H., Jiang, P., Miao, C., Du, Y., Fu, R., Wang, Y., Hao, Y., & Sai, H. (2023). Recycling Coal Fly Ash for Super-Thermal-Insulating Aerogel Fiber Preparation with Simultaneous Al2O3 Extraction. Molecules, 28(24), 7978. https://doi.org/10.3390/molecules28247978