Analysis of Softwood Lignans by Comprehensive Two-Dimensional Liquid Chromatography
Abstract
:1. Introduction
2. Results and Discussion
2.1. Column Screening and Selection of LC × LC Conditions
2.2. Quantitative Analysis and Method Validation
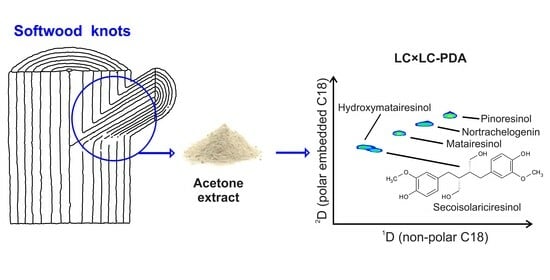
2.3. Analysis of Coniferous Knotwood Extracts
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Materials and Extraction
3.3. Isolation and Characterization of Hydroxymatairesinol and Nortrachelogenin
3.4. Comprehensive Two-Dimensional Liquid Chromatography
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- MacRae, W.D.; Towers, G.H.N. Biological activities of lignans. Phytochemistry 1984, 23, 1207–1220. [Google Scholar] [CrossRef]
- Saleem, M.; Hyoung, J.K.; Ali, M.S.; Yong, S.L. An update on bioactive plant lignans. Nat. Prod. Rep. 2005, 22, 696–716. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Flores, F.G.; Dobado, J.A.; Isac-García, J.; Martín-MartíNez, F.J. Lignin and Lignans as Renewable Raw Materials: Chemistry, Technology and Applications; John Wiley & Sons: Chichester, UK, 2015; pp. 313–454. [Google Scholar] [CrossRef]
- Willför, S.M.; Ahotupa, M.O.; Hemming, J.E.; Reunanen, M.H.T.; Eklund, P.C.; Sjöholm, R.E.; Eckerman, C.S.E.; Pohjamo, S.P.; Holmbom, B.R. Antioxidant Activity of Knotwood Extractives and Phenolic Compounds of Selected Tree Species. J. Agric. Food Chem. 2003, 51, 7600–7606. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Tsvetkov, D.E.; Varshney, V.K.; Nifantiev, N.E. Chemical constituents from temperate and subtropical trees with reference to knotwood. Ind. Crop. Prod. 2020, 145, 112077. [Google Scholar] [CrossRef]
- Ul’yanovskii, N.V.; Onuchina, A.A.; Faleva, A.V.; Gorbova, N.S.; Kosyakov, D.S. Comprehensive Characterization of Chemical Composition and Antioxidant Activity of Lignan-Rich Coniferous Knotwood Extractives. Antioxidants 2022, 11, 2338. [Google Scholar] [CrossRef] [PubMed]
- Willfor, S.M.; Smeds, A.I.; Holmbom, B.R. Chromatographic analysis of lignans. J. Chromatogr. A 2006, 1112, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Kuehnl, S.; Schroecksnadel, S.; Temml, V.; Gostner, J.M.; Schennach, H.; Schuster, D.; Schwaiger, S.; Rollinger, J.M.; Fuchs, D.; Stuppner, H. Lignans from Carthamus tinctorius suppress tryptophan breakdown via indoleamine 2,3-dioxygenase. Phytomedicine 2013, 20, 1190–1195. [Google Scholar] [CrossRef] [PubMed]
- Ekiert, R.J.; Szopa, A.; Ekiert, H.; Krzek, J.; Dzik, E. Analysis of lignans in Schisandra chinensis fruits, leaves, biomasses from in vitro cultures and food supplements. J. Funct. Foods 2013, 5, 1576–1581. [Google Scholar] [CrossRef]
- Srivastava, V.; Singh, M.; Malasoni, R.; Shanker, K.; Verma, R.K.; Gupta, M.M.; Gupta, A.K.; Khanuja, S.P.S. Separation and quantification of lignans in Phyllanthus species by a simple chiral densitometric method. J. Sep. Sci. 2008, 31, 47–55. [Google Scholar] [CrossRef]
- Sukumar, D.; Arimboor, R.; Arumughan, C. HPTLC fingerprinting and quantification of lignans as markers in sesame oil and its polyherbal formulations. J. Pharm. Biomed. Anal. 2008, 47, 795–801. [Google Scholar] [CrossRef]
- Sarajlija, H.; Čukelj Mustač, N.; Novotni, D.; Mršić, G.; Brncic, M.; Curic, D. Preparation of Flaxseed for Lignan Determination by Gas Chromatography-Mass Spectrometry Method. Czech J. Food Sci. 2012, 30, 45. [Google Scholar] [CrossRef]
- Bonzanini, F.; Bruni, R.; Palla, G.; Serlataite, N.; Caligiani, A. Identification and distribution of lignans in Punica granatum L. fruit endocarp, pulp, seeds, wood knots and commercial juices by GC–MS. Food Chem. 2009, 117, 745–749. [Google Scholar] [CrossRef]
- Kebbi-Benkeder, Z.; Colin, F.; Dumarçay, S.; Gérardin, P. Quantification and characterization of knotwood extractives of 12 European softwood and hardwood species. Ann. For. Sci. 2015, 72, 277–284. [Google Scholar] [CrossRef]
- Anikeenko, E.A.; Ul’yanovskii, N.V.; Shavrina, I.S.; Kosyakov, D.S. Laser Desorption/Ionization of Low-Molecular-Weight Lignin Oligomers. J. Anal. Chem. 2020, 75, 1814–1824. [Google Scholar] [CrossRef]
- Sicilia, T.; Niemeyer, H.B.; Honig, D.M.; Metzler, M. Identification and stereochemical characterization of lignans in flaxseed and pumpkin seeds. J. Agric. Food Chem. 2003, 51, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Meagher, L.P.; Beecher, G.R.; Flanagan, V.P.; Li, B.W. Isolation and characterization of the lignans, isolariciresinol and pinoresinol, in flaxseed meal. J. Agric. Food Chem. 1999, 47, 3173–3180. [Google Scholar] [CrossRef] [PubMed]
- Avula, B.; Choi, Y.W.; Srinivas, P.V.; Khan, I.A. Quantitative determination of lignan constituents from Schisandra chinensis by liquid chromatography. Chromatographia 2005, 61, 515–518. [Google Scholar] [CrossRef]
- Gu, W.; Wei, N.; Wang, Z. LC analysis of lignans from Schisandras sphenanthera Rehd. et Wils. Chromatographia 2008, 67, 979–983. [Google Scholar] [CrossRef]
- Milder, I.E.J.; Arts, I.C.W.; Venema, D.P.; Lasaroms, J.J.P.; Wähälä, K.; Hollman, P.C.H. Optimization of a liquid chromatoraphy-tandem mass spectrometry method for quantification of the plant lignans secoisolariciresinol, matairesinol, lariciresinol, and pinoresinol in foods. J. Agric. Food Chem. 2004, 52, 4643–4651. [Google Scholar] [CrossRef]
- Popova, I.E.; Hall, C.; Kubatova, A. Determination of lignans in flaxseed using liquid chromatography with time-of-flight mass spectrometry. J. Chromatogr. A 2009, 1216, 217–229. [Google Scholar] [CrossRef]
- Cacciola, F.; Rigano, F.; Dugo, P.; Mondello, L. Comprehensive two-dimensional liquid chromatography as a powerful tool for the analysis of food and food products. Trends Anal. Chem. 2020, 127, 115894. [Google Scholar] [CrossRef]
- Stoll, D.R.; Li, X.; Wang, X.; Carr, P.W.; Porter, S.E.G.; Rutan, S.C. Fast, comprehensive two-dimensional liquid chromatography. J. Chromatogr. A 2007, 1168, 3–43. [Google Scholar] [CrossRef] [PubMed]
- Cacciola, F.; Jandera, P.; Mondello, L. Comparison of high-temperature gradient heart-cutting and comprehensive LCxLC systems for the separation of phenolic antioxidants. Chromatographia 2007, 66, 661–667. [Google Scholar] [CrossRef]
- Chen, X.; Jiang, Z.; Zhu, Y.; Tan, J. Use of two-dimensional liquid chromatography combined with diode-array and mass spectrometric detection for analysis of compounds in Flos Lonicera. Chromatographia 2007, 65, 141–147. [Google Scholar] [CrossRef]
- Wang, S.; Wang, C.; Zhao, X.; Mao, S.; Wu, Y.; Fan, G. Comprehensive two-dimensional high performance liquid chromatography system with immobilized liposome chromatography column and monolithic column for separation of the traditional Chinese medicine Schisandra chinensis. Anal. Chim. Acta 2012, 713, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Camenzuli, M.; Schoenmakers, P.J. A new measure of orthogonality for multi-dimensional chromatography. Anal. Chim. Acta 2014, 838, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Gilar, M.; Olivova, P.; Daly, A.E.; Gebler, J.C. Orthogonality of Separation in Two-Dimensional Liquid Chromatography. Anal. Chem. 2005, 77, 6426–6434. [Google Scholar] [CrossRef] [PubMed]
- Lazzari, E.; Arena, K.; Caramão, E.B.; Mondello, L.; Herrero, M. Comprehensive two-dimensional liquid chromatography-based quali-quantitative screening of aqueous phases from pyrolysis bio-oils. Electrophoresis 2021, 42, 58–67. [Google Scholar] [CrossRef]
- Willför, S.; Hemming, J.; Reunanen, M.; Holmbom, B. Phenolic and lipophilic extractives in Scots pine knots and stemwood. Holzforschung 2003, 57, 359–372. [Google Scholar] [CrossRef]
- Jomová, K.; Hudecova, L.; Lauro, P.; Simunkova, M.; Alwasel, S.H.; Alhazza, I.M.; Valko, M. A Switch between Antioxidant and Prooxidant Properties of the Phenolic Compounds Myricetin, Morin, 3′,4′-Dihydroxyflavone, Taxifolin and 4-Hydroxy-Coumarin in the Presence of Copper (II) Ions: A Spectroscopic, Absorption Titration and DNA Damage Study. Molecules 2019, 24, 4335. [Google Scholar] [CrossRef]
- Alperth, F.; Schneebauer, A.; Kunert, O.; Bucar, F. Phytochemical Analysis of Pinus cembra Heartwood—UHPLC-DAD-ESI-MSn with Focus on Flavonoids, Stilbenes, Bibenzyls and Improved HPLC Separation. Plants 2023, 12, 3388. [Google Scholar] [CrossRef]
- Bede, J.C.; Tobe, S.S. Insect juvenile hormones in plants. Stud. Nat. Prod. Chem. 2000, 22, 369–418. [Google Scholar] [CrossRef]
- Ul’yanovskii, N.V.; Onuchina, A.A.; Ovchinnikov, D.V.; Faleva, A.V.; Gorbova, N.S.; Kosyakov, D.S. Analytical and Preparative Separation of Softwood Lignans by Supercritical Fluid Chromatography. Separations 2023, 10, 449. [Google Scholar] [CrossRef]



| Stationary Phase | AO (%) | R2 | |
|---|---|---|---|
| 1D | 2D | ||
| Shim-pack XR-ODS II | Nucleodur C18 Pyramid | 39 | 0.84 |
| Nucleodur C18 Isis | Nucleodur C18 Pyramid | 34 | 0.92 |
| Nucleodur PFP | Nucleodur C18 Pyramid | 40 | 0.85 |
| Nucleodur PolarTec | Nucleodur PFP | 38 | 0.88 |
| Shim-pack XR-ODS II | Nucleodur PFP | 38 | 0.87 |
| Nucleodur PolarTec | Nucleodur C18 Pyramid | 36 | 0.88 |
| Analyte | Retention Time, min | Linear Range, mg L−1 | a | b | R2 | LOD, mg L−1 | LOQ, mg L−1 | |
|---|---|---|---|---|---|---|---|---|
| 1D | 2D | |||||||
| HMR | 18.6 | 0.62 | 0.45–20 | 15389 | −4511 | >0.999 | 0.13 | 0.44 |
| Secoisolariciresinol | 20.6 | 0.61 | 0.35–20 | 20407 | −5854 | >0.999 | 0.16 | 0.54 |
| Nortrachelogenin | 23.7 | 0.67 | 0.36–20 | 16557 | −1944 | >0.999 | 0.11 | 0.37 |
| Pinoresinol | 27.7 | 0.70 | 0.29–20 | 37764 | −10824 | >0.999 | 0.29 | 0.95 |
| Matairesinol | 30.7 | 0.73 | 0.31–20 | 21436 | −2998 | >0.999 | 0.08 | 0.27 |
| Analyte | Concentration, mg L−1 | Intra-Day Assay (n = 6) | Inter-Day Assay (n = 6) | ||||
|---|---|---|---|---|---|---|---|
| Found, mg L−1 | Accuracy, % | Precision, % | Found, mg L−1 | Accuracy, % | Precision, % | ||
| HMR | 0.50 | 0.46 ± 0.03 | 91 | 4.66 | 0.45 ± 0.02 | 90 | 3.14 |
| Secoisolariciresinol | 0.50 | 0.51 ± 0.08 | 102 | 11.1 | 0.46 ± 0.04 | 92 | 6.11 |
| Nortrachelogenin | 0.50 | 0.49 ± 0.05 | 97 | 7.29 | 0.45 ± 0.07 | 89 | 11.1 |
| Pinoresinol | 1.00 | 1.00 ± 0.02 | 99 | 1.43 | 0.51 ± 0.09 | 101 | 12.6 |
| Matairesinol | 0.50 | 0.54 ± 0.07 | 107 | 9.25 | 0.55 ± 0.07 | 109 | 9.08 |
| Analyte | Spiked, mg L−1 | Found, mg L−1 | Recovery, % |
|---|---|---|---|
| HMR | 1.0 | 0.82 ± 0.06 | 82 |
| 10 | 8.4 ± 0.8 | 84 | |
| Secoisolariciresinol | 1.0 | 0.82 ± 0.15 | 82 |
| 10 | 9.0 ± 0.4 | 90 | |
| Nortrachelogenin | 1.0 | 0.85 ± 0.08 | 85 |
| 10 | 9.3 ± 0.7 | 93 | |
| Pinoresinol | 1.0 | 8.8 ± 0.7 | 88 |
| 10 | 9.8 ± 0.2 | 98 | |
| Matairesinol | 1.0 | 8.9 ± 1.0 | 89 |
| 10 | 9.0 ± 0.4 | 90 |
| Analyte | Larch | Fir | Spruce | Pine |
|---|---|---|---|---|
| HMR | 1.2 ± 0.2 | 0.17 ± 0.04 | 100 ± 10 | 0.23 ± 0.03 |
| Secoisolariciresinol | 17 ± 6 | 20 ± 2 | 6.1 ± 0.7 | 0.24 ± 0.08 |
| Nortrachelogenin | 3.3 ± 0.4 | - | - | 8.0 ± 0.9 |
| Pinoresinol | - | 0.78 ± 0.14 | 0.31 ± 0.05 | - |
| Matairesinol | 0.55 ± 0.07 | 0.75 ± 0.17 | 1.0 ± 0.2 | 0.65 ± 0.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falev, D.I.; Voronov, I.S.; Onuchina, A.A.; Faleva, A.V.; Ul’yanovskii, N.V.; Kosyakov, D.S. Analysis of Softwood Lignans by Comprehensive Two-Dimensional Liquid Chromatography. Molecules 2023, 28, 8114. https://doi.org/10.3390/molecules28248114
Falev DI, Voronov IS, Onuchina AA, Faleva AV, Ul’yanovskii NV, Kosyakov DS. Analysis of Softwood Lignans by Comprehensive Two-Dimensional Liquid Chromatography. Molecules. 2023; 28(24):8114. https://doi.org/10.3390/molecules28248114
Chicago/Turabian StyleFalev, Danil I., Ilya S. Voronov, Alexandra A. Onuchina, Anna V. Faleva, Nikolay V. Ul’yanovskii, and Dmitry S. Kosyakov. 2023. "Analysis of Softwood Lignans by Comprehensive Two-Dimensional Liquid Chromatography" Molecules 28, no. 24: 8114. https://doi.org/10.3390/molecules28248114
APA StyleFalev, D. I., Voronov, I. S., Onuchina, A. A., Faleva, A. V., Ul’yanovskii, N. V., & Kosyakov, D. S. (2023). Analysis of Softwood Lignans by Comprehensive Two-Dimensional Liquid Chromatography. Molecules, 28(24), 8114. https://doi.org/10.3390/molecules28248114