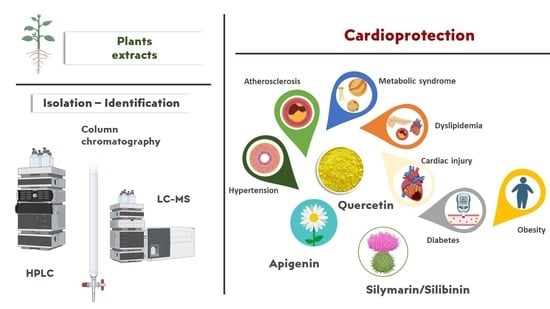
Bio-Actives from Natural Products with Potential Cardioprotective Properties: Isolation, Identification, and Pharmacological Actions of Apigenin, Quercetin, and Silibinin
Abstract
:1. Introduction
2. Literature Search Strategy
3. Results and Discussion
3.1. Chemical Structure, Plant Origin (Family), Methods of Isolation, and Identification
3.1.1. Apigenin
Methods for Isolation: Column Chromatography and Preparative HPLC
Methods for Identification: HPLC and LC-MS Analysis
3.1.2. Quercetin
Methods for Isolation: Column Chromatography and Preparative HPLC
Methods for Identification: HPLC and LC-MS Analysis
3.1.3. Silymarin Extract and Constituents
Methods for Isolation and Identification
| Botanical Name (Family) | Extract/Residue-Fraction | Plant Parts | Method/Solvents | References |
|---|---|---|---|---|
| Apigenin | ||||
| Ailanthus excelsa Roxb. [A. excelsus Roxb.] (Simaroubaceae) | 70% Methanol/Ethyl acetate (isolation) | L. | CC (Sephadex LH-20) | [22] |
| Chrysanthemum morifolium Ramat. (Asteraceae) | Aqueous, Ethanol (identification) | Fl. | LC-MS | [36] |
| Cynara cardunculus L. (Asteraceae) | Aqueous (identification) | L. | HPLC analysis | [35] |
| Gentiana veitchiorum Hemsl. (Gentianaceae) | 70% Methanol (identification) | Fl. | HPLC-MS/MS 0.1% formic acid/water and methanol | [31] |
| 70% Methanol (isolation) | CC (silica gel)/CHCl3-MeOH (100:1 to 1:1), Semi-prep HPLC/MeCN-H2O | |||
| Matricaria recutita L. (Asteraceae) | 70% Methanol (isolation) | L. | CC (Sephadex LH-20)/acetone | [30] |
| Merremia tridentata (L.) Hallier f. (Convolvulaceae) | Aqueous, 50% Ethanol (isolation; identification) | Stem; R. | CC (silica gel)/MeOH, CHCl3 | [34] |
| HPLC-DAD | ||||
| Petroselinum crispum (Mill.) Nym. ex A.W. Hill (Apiaceae) | Aqueous/Ethyl acetate (isolation) | L. | CC (Sephadex LH-20)/EtOH | [26] |
| Premna foetida Renw. ex Blume (Lamiaceae) | Methanol (identification) | L. | RP-HPLC/0.1% H3PO4: ACN (gradient system) | [27] |
| Chloroform (isolation) | CC | |||
| Platycodon grandiflorum (Jacq.) A. DC. [P. grandiflorum A. DC.] (Campanulaceae) | Ethanol/Ethyl acetate (isolation) | Fl. | CC (silica gel)/CH2Cl2: MeOH (19:1 to 9:1) | [25] |
| Morus indica L. (Moraceae) | 80% Methanol (isolation) | L. | prep-HPLC | [32] |
| Sophora alopecuroides L. (Leguminosae) | 75% Ethanol/Ethyl acetate (isolation) | A.p.; R.; S. | CC (Sephadex LH-20)/MeOH | [33] |
| Teucrium polium L. (Lamiaceae) | Methanol (isolation) | A.p. | CC (silica gel)/different solvent systems | [23,24] |
| CC (Sephadex LH-20)/MeOH | ||||
| Ziziphora clinopodioides Lam. (Lamiaceae) | Hydroalcoholic (80% Ethanol:20% Water)/Dichloromethane (isolation) | Whole plant | CC (Sephadex LH-20) Flash CC (silica gel) | [28] |
| Quercetin | ||||
| Acacia arabica (Lam.) Willd. (Leguminosae) | Hot water (isolation) | B. | RP-HPLC | [59] |
| Allium victorialis L. (Alliaceae; Liliaceae p) | 50% Ethanol/Ethyl acetate (isolation) | L. | CC | [44] |
| Anacardium humile A.St.-Hil. (Anacardiaceae) | 98% Ethanol (identification) | L. | HPLC-ESI-MS/MS)/water acidified with formic acid (0.1% v/v) and MeOH | [66] |
| Artemisia capillaris Thunb. (Asteraceae) | Methanol (isolation) | Whole plant | CC | [43] |
| Bauhinia megalandra Griseb. (Leguminosae) | Methanol/Ethyl acetate-acetone (8:2) (isolation) | L. | CC (Sephadex LH-20) | [74] |
| Bauhinia strychnifolia Craib. (Leguminosae) | Ethanol/Ethyl acetate (isolation; identification) | Stem | CC (Sephadex LH-20) | [61] |
| LC-QTOF/MS | ||||
| Bryophyllum pinnatum (Lam.) Oken (Crassulaceae) | Methanol/Ethyl acetate (isolation) | L. | CC (silica gel)/ MeOH:EtOAc:H2O (5:3:2) | [53] |
| Carya illinoinensis (Wangenh.) K. Koch (Juglandaceae) | 70% Ethanol (isolation) | B. | CC | [40] |
| Cordia boissieri A.DC. (Boraginaceae) | Hydroalcoholic/Ethyl acetate (isolation) | L. | CC (polyamide, Sephadex LH-20) | [51] |
| Coreopsis lanceolata L. (Asteraceae) | Methanol/Ethyl acetate (isolation) | Fl. | RP-CC/MeOH:H2O; CH3CN:H2O, CC (Sephadex LH-20)/MeOH | [57] |
| Coreopsis tinctoria Nutt. (Asteraceae) | Ethanol (isolation) | Flower buds | ODS-RP-18 column/MeOH: H2O, CC (Sephadex LH-20)/MeOH | [46] |
| Crataegus pinnatifida Bge. var. major N.E.Br. [C. pinnatifida f. major (N.E.Br.) W.Lee] (Rosaceae) | 70% Ethanol | L. | CC | [60] |
| Cuscuta pedicellata Ledeb. (Convolvulaceae) | Ethanol (isolation) | Whole plant | CC | [48] |
| Cyclocarya paliurus (Batal.) Iljinsk. (Juglandaceae; Cyclocaryaceae p) | 75% Ethanol/Chloroform (isolation) | B. | CC (silica gel, Sephadex LH-20) | [41] |
| Cynanchum acutum L. (Asclepiadaceae; Apocynaceae p) | Methanol/Ethyl acetate (isolation) | Whole plant | CC (Sephadex LH-20) | [58] |
| Dillenia indica Blanco (Dilleniaceae) | Methanol/Ethyl acetate (isolation) | L. | CC | [45] |
| Geigeria alata (DC), Oliv. and Hiern. [G. alata Benth. and Hook.f. ex Oliv.] (Asteraceae) | 80% Ethanol/Chloroform, Ethyl acetate (isolation) | n.d. | CC (silica gel)/DCM:MeOH | [56] |
| Lactuca serriola L. (Asteraceae) | Methanol (isolation) | A.p. | n.d. | [55] |
| Leonurus cardiaca L. (Lamiaceae) | 70% Ethanol (identification) | A.p. | HPLC | [63] |
| Mandevilla moricandiana Woodson (Apocynaceae) | Hydroalcoholic (70% Ethanol: 30% Water)/Ethyl acetate (identification) | L. | UHPLC-DAD-ESI-MSn | [65] |
| Phyllanthus emblica L. (Euphorbiaceae) | Methanol (isolation) | Fr. | CC (silica gel)/CHCl3: MeOH | [54] |
| Polygonum hyrcanicum Rech.f. (Polygonaceae) | Methanol/Ethyl acetate (isolation) | A.p. | CC (silica gel, Sephadex LH-20) | [42] |
| Pueraria thomsonii Benth (Fabaceae) | 75% Ethanol/Ethyl acetate (isolation; identification) | L. | CC (silica gel, SHP-20P) | [62] |
| HPLC-DAD | ||||
| Sarcopyramis nepalensis Wall. (Melastomataceae) | 70% Ethanol/Ethyl acetate (isolation) | Whole plant | CC (Sephadex LH-20/MeOH) | [47] |
| Sophora alopecuroides L. (Leguminosae) | 75% Ethanol/Ethyl acetate (isolation) | A.p.; R.; S. | CC (Sephadex LH-20)/MeOH | [33] |
| Tetracera indica Merr. [T. indica (Christm. and Panz.) Merr.] (Dilleniaceae) | Ethanol/Ethyl acetate (isolation) | Stems | CC (Silica gel, Sephadex LH-20) | [50] |
| Toona sinensis (A.Juss.) M.Roem. (Meliaceae) | 80% Ethanol/Chloroform, Ethyl acetate (isolation) | L. | CC (silica gel)/n-hexane:EtOAc:MeOH, capillary electrophoresis using silica gel CC | [49] |
| Ugni molinae Turcz. (Myrtaceae) | Aqueous (identification) | Fr. | HPLC/1% HCOOH: ACN | [64] |
| Xenophyllum poposum (Phil.) V.A.Funk (Asteraceae) | Hydroalcoholic (Ethanol:Water, 1:1)/Ethyl acetate (isolation; identification) | A.p. | CC | [52] |
| HPLC-DAD-MS/MS | ||||
| Flavonolignans and extracts of Silybum marianum (L.) Gaertn. (Asteraceae) | ||||
| Silymarin constituents | n.d. (identification) | n.d. | HPLC-DAD/H2O + 0.1% HCOOH; MeOH + 0.1% HCOOH | [69] |
| Silychristin | n.d. (isolation) | prep-HPLC | [69] | |
| S. marianum | Ethyl acetate (identification) | S. | HPLC/H3PO4: MeOH: H2O (0.5:35:65–0.5:50:50 v/v/v) | [72] |
| S. marianum | Ethanol:Water (1:1) (identification) | S. | HPLC-DAD/ water with 0.1% formic acid; MeOH (1:1) | [73] |
3.2. Physicochemical and Biopharmaceutical Properties
3.2.1. Apigenin
3.2.2. Quercetin
3.2.3. Silymarin Extract and Constituents
3.3. Bio-Actives’ Cardiovascular Prevention Activity Based on Preclinical and Clinical Studies
3.3.1. Hypertension
3.3.2. Diabetes
3.3.3. Dyslipidemia
3.3.4. Atherosclerosis
3.3.5. Obesity
3.3.6. Cardiac Injury
3.3.7. Metabolic Syndrome
| Cardiovascular Disease | Mechanism | Bio-Active | References |
|---|---|---|---|
| Hypertension | ↓SBP and DBP | quercetin | [96,109,110] |
| ↓ADMA | silibinin | [18] | |
| ↓CXCR4 and SDF-1 | |||
| ↓PAH | |||
| ↓ROS, ↓oxidative stress, and ↓MCP-1 | apigenin, quercetin | [11,93,94,95,96] | |
| ↓overproduction of eNOS and cNOS | [21,103,104,105] | ||
| ↓lipid peroxides | [97] | ||
| Activation of AMPK/SIRT1 | [98,99] | ||
| ACE inhibition | [22,102] | ||
| Inhibition of calcium exchange | [46,64] | ||
| ↑NO production/bioavailability and vasorelaxation | quercetin, silibinin | [29,35,41,94,95,96] | |
| ↓inflammatory cytokines (IL-1β, IL-6, IL-10, TNF-α, and MCP-1) | apigenin, quercetin, silibinin | [11,18,95] | |
| Diabetes | Restoration of Bcl-2/Bax levels | apigenin | [126] |
| ↓ TNF-α and IL-6 | [125] | ||
| ↓ CK-MB and LDH | [124] | ||
| ↑insulin release and sensitivity | [24] | ||
| Inhibition of PKCβII activation | [104] | ||
| ↓ICAM-1 and E-selectin | [36] | ||
| ↓ROS oxidative stress | quercetin | [118] | |
| ↑glucose uptake via GLUT4 stimulation | [115,116,117,119] | ||
| DPP-IV inhibition | [57] | ||
| ↓ TNF-α, IL-1β, and IFNγ | silibinin/silymarin | [122,123,133] | |
| ↓pancreatic protein damage and creatinine levels | [135] | ||
| ↓blood glucose levels | apigenin, quercetin | [32,54] | |
| Inhibition of myocardial fibrosis and cardiac remodeling | apigenin, silymarin | [122,126,128,129,130] | |
| Inhibition of lipid peroxidation, ↓MDA, and ↑GSH/GSSG ratio | quercetin, silymarin | [72,118,131,132] | |
| ↓NF-κB/p65 and Akt phosphorylation | apigenin, quercetin, silibinin | [36,49,58,95,123,126] | |
| Dyslipidemia | Restoration of HDL, LOX-1, and Bcl-2/Bax levels | quercetin | [139,142,143] |
| ↓ICAM-1, ↓IL-6, and ↓VCAM-1 | [144,145,146] | ||
| ↓lipid accumulation | apigenin, quercetin | [139,146] | |
| ↓BW | [140,141] | ||
| ↓levels of TC, TG, and LDL | apigenin, quercetin, silymarin | [140,141] | |
| Atherosclerosis | ↓proinflammatory cytokines | apigenin | [11,155,159,190] |
| ↓ICAM-1 and MCP-1 | quercetin | [156,158] | |
| ↓elastin degradation, ↓macrophage infiltration, and ↓MMP-9 and VCAM-1 expression | [168,169,170] | ||
| ↓LDL oxidation | silymarin | [165] | |
| Induction of autophagy and foam cell formation | apigenin, quercetin | [154,155,156,157] | |
| ↓atherosclerotic plaque formation | quercetin, silymarin | [17,166] | |
| ↑ABCA1 and ABCA1-mediated cholesterol efflux | apigenin, quercetin, silymarin | [159,160,161,162,163,164] | |
| ↓inflammation via TLR-4/NF-κB signaling pathway | |||
| Obesity | ↓BW | apigenin, quercetin | [140,176,177] |
| ↑AMPK phosphorylation | apigenin | [173] | |
| ↓fatty acid-binding protein 4 and stearoyl-CoA desaturase | |||
| Downregulation of MAPK, ERK, and JNK | quercetin | [177] | |
| ↑glucose uptake | quercetin, silymarin | [59,69,160,178] | |
| ↓fasting blood glucose levels | quercetin | [42,43,45,61,62] | |
| ↓activity of pancreatic lipase and fatty acid synthase | apigenin | [174] | |
| Cardiac injury | Inhibition of cardiomyocyte apoptosis via the PI3K/Akt and SIRT1/TMBIM6 pathways | quercetin | [12,182,183] |
| Stimulation of mitophagy events | [184] | ||
| Impedes Ca2+ influx via L-type Ca2+ channels | [186] | ||
| Inhibition of MAPK phosphorylation and MDA, LDH, and CK release | [187] | ||
| ↓MAPK | [194] | ||
| Anti-platelet activity | apigenin, quercetin | [26] | |
| ↓LDL oxidation | [27] | ||
| ↓myocardial infract size | apigenin, quercetin, silibinin | [189,191,192] | |
| ↑SOD activity | |||
| ↓ER and oxidative stress, reverse of inflammation via the NF-kB pathway | silibinin | [193] | |
| Metabolic syndrome | ↑insulin secretion and sensitization | quercetin | [120,121] |
| ↓plasma lipid content | silymarin | [200] | |
| ↑NAD+ levels in liver | apigenin, silymarin | [75,195] | |
| ↓inflammatory cytokines | [75,196,197] | ||
| ↓ROS production and oxidative stress in β pancreatic cells |
| Cardiovascular Disease | Study Design | Main Outcomes | Bio-Active | Ref. |
|---|---|---|---|---|
| Hypertension | Meta-analysis: Seven RCTs, 587 pts, HTN, healthy individuals | ↓SBP | quercetin | [112] |
| Meta-analysis: Ten RCTs, 841 pts, HTN, healthy individuals | ↓SBP and DBP | [113] | ||
| Cohort study, 15,662 pts, healthy individuals | No effect on hypertension incidence | [114] | ||
| Diabetes | Non-controlled pilot study, 15 pts, T2DM | ↓glycosylated hemoglobin, ↓basal insulin, ↓TSH, ↓usCRP, ↓both SBP, ↓DBP | quercetin | [137] |
| Meta-analysis: Ten clinical trials, 700 pts, healthy, T2DM, NAFLD | ↓FBG, ↓HbA1c, ↓insulin, ↓TC, ↓TG, ↓LDL, ↑HDL | silymarin | [15] | |
| Meta-analysis: Five RCTs, 270 pts, healthy, T2DM | ↓FBG, ↓HbA1c | [138] | ||
| Dyslipidemia | Meta-analysis: Five RCTs, 442 pts, healthy, T2DM, HTN, hyperlipidemia | ↓TG | quercetin | [147] |
| Meta-analysis: Sixteen RCTs, 1575 pts, healthy, HTN, T2DM, hypercholesterolemic | ↓TC, ↔TG, ↓LDL | [148] | ||
| Double-blinded, placebo-controlled cross-over study, 175 pts, overweight with high-CVD risk | ↓LDL | [149] | ||
| Randomized, double-blinded, placebo-controlled cross-over trial, 70 pts, overweight-to-obese patients with pre-hypertension | ↔FBG, ↔LDL | [150] | ||
| Meta-analysis: Five RCTs, 270 pts, healthy, T2DM | ↔lipid levels | silymarin | [138] | |
| Meta-analysis: Eight RCTs, 195 pts, T2DM | ↓FBG, ↓HbA1c, ↓LDL, ↓MDA, ↑HDL | [139] | ||
| Meta-analysis: Ten RCTs, 620 pts, hyperlipidemic | ↓TC, ↓TG, ↓LDL, ↑HDL | [152] | ||
| Obesity | Randomized, placebo-controlled, double-blind trial, 110 pts, MS | ↓BW, ↓SBP, ↓DBP, ↓TC, ↓LDL, ↓fasting plasma insulin | quercetin | [179] |
| Double-blind crossover study, 49 pts, healthy with different APOE isoforms | ↓waist circumference, ↓TG, ↑HDL | [180] | ||
| Meta-analysis: Seven RCTs, 896 pts, healthy, obese, HTN | ↓SBP, ↓DBP, ↔BW, ↔BMI, ↔waist circumference, ↔waist-to-hip ratio | [181] | ||
| Double-blinded, placebo-controlled cross-over study, 172 pts, overweight, high-CVD risk phenotype | ↓SBP, ↓ox-LDL, ↔TNF-a, ↔C-reactive protein | [149] | ||
| Metabolic syndrome | Meta-analysis: Eighteen RCTs, 987 pts, HTN, overweight, MS, T2DM, NAFLD | ↓SBP, ↓DBP, ↓TC, ↓TG, ↓LDL, ↑HDL, ↓glucose levels | quercetin | [202] |
| Meta-analysis: Nine RCTs, 781 pts, HTN, T2DM, obesity, PCOS | ↔FBG, ↔HbA1c, ↓insulin, | [203] |
4. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Thiriet, M. Cardiovascular Disease: An Introduction. In Vasculopathies; Biomathematical and Biomechanical Modeling of the Circulatory and Ventilatory Systems; Springer: Cham, Switzerland, 2018; Volume 8, pp. 1–90. ISBN 978-3-319-89314-3. [Google Scholar]
- Cardiovascular Diseases (CVDs). Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 17 December 2022).
- Popiolek-Kalisz, J.; Fornal, E. The Impact of Flavonols on Cardiovascular Risk. Nutrients 2022, 14, 1973. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Jones, D.M.; Hong, Y.; Labarthe, D.; Mozaffarian, D.; Appel, L.J.; Van Horn, L.; Greenlund, K.; Daniels, S.; Nichol, G.; Tomaselli, G.F.; et al. Defining and Setting National Goals for Cardiovascular Health Promotion and Disease Reduction: The American Heart Association’s Strategic Impact Goal Through 2020 and Beyond. Circulation 2010, 121, 586–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cook, N.C.; Samman, S. Flavonoids—Chemistry, Metabolism, Cardioprotective Effects, and Dietary Sources. J. Nutr. Biochem. 1996, 7, 66–76. [Google Scholar] [CrossRef]
- Testai, L.; Martelli, A.; Cristofaro, M.; Breschi, M.C.; Calderone, V. Cardioprotective Effects of Different Flavonoids against Myocardial Ischaemia/Reperfusion Injury in Langendorff-Perfused Rat Hearts. JPP 2013, 65, 750–756. [Google Scholar] [CrossRef] [PubMed]
- Ciumărnean, L.; Milaciu, M.V.; Runcan, O.; Vesa, Ș.C.; Răchișan, A.L.; Negrean, V.; Perné, M.-G.; Donca, V.I.; Alexescu, T.-G.; Para, I.; et al. The Effects of Flavonoids in Cardiovascular Diseases. Molecules 2020, 25, 4320. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.-H.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Peterson, J.J.; Dwyer, J.T.; Jacques, P.F.; McCullough, M.L. Associations between Flavonoids and Cardiovascular Disease Incidence or Mortality in European and US Populations. Nutr. Rev. 2012, 70, 491–508. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Li, X.; Wang, H. Protective Roles of Apigenin Against Cardiometabolic Diseases: A Systematic Review. Front. Nutr. 2022, 9, 875826. [Google Scholar] [CrossRef]
- Gao, H.-L.; Yu, X.-J.; Hu, H.-B.; Yang, Q.-W.; Liu, K.-L.; Chen, Y.-M.; Zhang, Y.; Zhang, D.-D.; Tian, H.; Zhu, G.-Q.; et al. Apigenin Improves Hypertension and Cardiac Hypertrophy Through Modulating NADPH Oxidase-Dependent ROS Generation and Cytokines in Hypothalamic Paraventricular Nucleus. Cardiovasc. Toxicol. 2021, 21, 721–736. [Google Scholar] [CrossRef]
- Patel, R.V.; Mistry, B.M.; Shinde, S.K.; Syed, R.; Singh, V.; Shin, H.-S. Therapeutic Potential of Quercetin as a Cardiovascular Agent. Eur. J. Med. Chem. 2018, 155, 889–904. [Google Scholar] [CrossRef]
- Papakyriakopoulou, P.; Velidakis, N.; Khattab, E.; Valsami, G.; Korakianitis, I.; Kadoglou, N.P. Potential Pharmaceutical Applications of Quercetin in Cardiovascular Diseases. Pharmaceuticals 2022, 15, 1019. [Google Scholar] [CrossRef]
- Biedermann, D.; Vavříková, E.; Cvak, L.; Křen, V. Chemistry of Silybin. Nat. Prod. Rep. 2014, 31, 1138–1157. [Google Scholar] [CrossRef]
- Kadoglou, N.P.E.; Panayiotou, C.; Vardas, M.; Balaskas, N.; Kostomitsopoulos, N.G.; Tsaroucha, A.K.; Valsami, G. A Comprehensive Review of the Cardiovascular Protective Properties of Silibinin/Silymarin: A New Kid on the Block. Pharmaceuticals 2022, 15, 538. [Google Scholar] [CrossRef]
- Marmouzi, I.; Bouyahya, A.; Ezzat, S.M.; El Jemli, M.; Kharbach, M. The Food Plant Silybum marianum (L.) Gaertn.: Phytochemistry, Ethnopharmacology and Clinical Evidence. J. Ethnopharmacol. 2021, 265, 113303. [Google Scholar] [CrossRef]
- Radjabian, T.; Huseini, H.F. Anti-Hyperlipidemic and Anti-Atherosclerotic Activities of Silymarins from Cultivated and Wild Plants of Silybum marianum L. With Different Content of Flavonolignans. Iran. J. Pharmacol. Ther. 2010, 9, 6367. [Google Scholar]
- Zhang, T.; Kawaguchi, N.; Yoshihara, K.; Hayama, E.; Furutani, Y.; Kawaguchi, K.; Tanaka, T.; Nakanishi, T. Silibinin Efficacy in a Rat Model of Pulmonary Arterial Hypertension Using Monocrotaline and Chronic Hypoxia. Respir. Res. 2019, 20, 79. [Google Scholar] [CrossRef] [Green Version]
- International Plant Names Index (IPNI). Available online: https://www.ipni.org/ (accessed on 11 December 2022).
- Sung, B.; Chung, H.Y.; Kim, N.D. Role of Apigenin in Cancer Prevention via the Induction of Apoptosis and Autophagy. J. Cancer Prev. 2016, 21, 216–226. [Google Scholar] [CrossRef] [Green Version]
- Paredes, M.; Romecín, P.; Atucha, N.; O’Valle, F.; Castillo, J.; Ortiz, M.; García-Estañ, J. Beneficial Effects of Different Flavonoids on Vascular and Renal Function in L-NAME Hypertensive Rats. Nutrients 2018, 10, 484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loizzo, M.R.; Said, A.; Tundis, R.; Rashed, K.; Statti, G.A.; Hufner, A.; Menichini, F. Inhibition of Angiotensin Converting Enzyme (ACE) by Flavonoids Isolated From Ailanthus excelsa (Roxb) (Simaroubaceae). Phytother. Res. 2007, 21, 32–36. [Google Scholar] [CrossRef]
- Esmaeili, M.; Zohari, F.; Sadeghi, H. Antioxidant and Protective Effects of Major Flavonoids from Teucrium polium on β -Cell Destruction in a Model of Streptozotocin-Induced Diabetes. Planta Med. 2009, 75, 1418–1420. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, M.A.; Sadeghi, H. Pancreatic Β-Cell Protective Effect of Rutin and Apigenin Isolated from Teucrium polium. Pharmacologyonline 2009, 2, 341–353. [Google Scholar]
- Jang, D.S.; Lee, Y.M.; Jeong, I.H.; Kim, J.S. Constituents of the Flowers of Platycodon grandiflorum with Inhibitory Activity on Advanced Glycation End Products and Rat Lens Aldose Reductase in Vitro. Arch. Pharm. Res. 2010, 33, 875–880. [Google Scholar] [CrossRef]
- Chaves, D.S.A.; Frattani, F.S.; Assafim, M.; de Almeida, A.P.; Zingali, R.B.; Costa, S.S. Phenolic Chemical Composition of Petroselinum crispum Extract and Its Effect on Haemostasis. Nat. Prod. Commun. 2011, 6, 1934578X1100600. [Google Scholar] [CrossRef] [Green Version]
- Dianita, R.; Jantan, I. Inhibition of Human Platelet Aggregation and Low-Density Lipoprotein Oxidation by Premna foetida Extract and Its Major Compounds. Molecules 2019, 24, 1469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senejoux, F.; Demougeot, C.; Kerram, P.; Aisa, H.A.; Berthelot, A.; Bévalot, F.; Girard-Thernier, C. Bioassay-Guided Isolation of Vasorelaxant Compounds from Ziziphora clinopodioides Lam. (Lamiaceae). Fitoterapia 2012, 83, 377–382. [Google Scholar] [CrossRef]
- Senejoux, F.; Girard, C.; Kerram, P.; Aisa, H.A.; Berthelot, A.; Bévalot, F.; Demougeot, C. Mechanisms of Vasorelaxation Induced by Ziziphora clinopodioides Lam. (Lamiaceae) Extract in Rat Thoracic Aorta. J. Ethnopharmacol. 2010, 132, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.H.; Wang, Z.; Guillen Quispe, Y.N.; Lim, S.S.; Yu, J.M. Evaluation of Aldose Reductase, Protein Glycation, and Antioxidant Inhibitory Activities of Bioactive Flavonoids in Matricaria recutita L. and Their Structure-Activity Relationship. J. Diabetes Res. 2018, 2018, 3276162. [Google Scholar] [CrossRef] [Green Version]
- Dou, X.; Zhou, Z.; Ren, R.; Xu, M. Apigenin, Flavonoid Component Isolated from Gentiana veitchiorum Flower Suppresses the Oxidative Stress through LDLR-LCAT Signaling Pathway. Biomed. Pharmacother. 2020, 128, 110298. [Google Scholar] [CrossRef]
- Anandan, S.; Urooj, A. Hypoglycemic Effects of Apigenin from Morus indica in Streptozotocin Induced Diabetic Rats. IJCRR. 2021, 13, 100–105. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Y.; Huang, Q.; Duan, H.; Zhao, G.; Liu, L.; Li, Y. Flavonoids from Sophora alopecuroides L. Improve Palmitate-Induced Insulin Resistance by Inhibiting PTP1B Activity in Vitro. Bioorg. Med. Chem. Lett. 2021, 35, 127775. [Google Scholar] [CrossRef] [PubMed]
- Vo Van, L.; Pham, E.C.; Nguyen, C.V.; Duong, N.T.N.; Vi Le Thi, T.; Truong, T.N. In Vitro and in Vivo Antidiabetic Activity, Isolation of Flavonoids, and in Silico Molecular Docking of Stem Extract of Merremia tridentata (L.). Biomed. Pharmacother. 2022, 146, 112611. [Google Scholar] [CrossRef]
- Rossoni, G.; Grande, S.; Galli, C.; Visioli, F. Wild Artichoke Prevents the Age-Associated Loss of Vasomotor Function. J. Agric. Food Chem. 2005, 53, 10291–10296. [Google Scholar] [CrossRef] [PubMed]
- Lii, C.-K.; Lei, Y.-P.; Yao, H.-T.; Hsieh, Y.-S.; Tsai, C.-W.; Liu, K.-L.; Chen, H.-W. Chrysanthemum morifolium Ramat. Reduces the Oxidized LDL-Induced Expression of Intercellular Adhesion Molecule-1 and E-Selectin in Human Umbilical Vein Endothelial Cells. J. Ethnopharmacol. 2010, 128, 213–220. [Google Scholar] [CrossRef]
- Suganthy, N.; Devi, K.P.; Nabavi, S.F.; Braidy, N.; Nabavi, S.M. Bioactive Effects of Quercetin in the Central Nervous System: Focusing on the Mechanisms of Actions. Biomed. Pharmacother. 2016, 84, 892–908. [Google Scholar] [CrossRef] [PubMed]
- Materska, M. Quercetin And Its Derivatives: Chemical Structure And Bioactivity—A Review. Pol. J. Food Nutr. Sci. 2008, 58, 407–413. [Google Scholar]
- Khan, F.; Niaz, K.; Maqbool, F.; Ismail Hassan, F.; Abdollahi, M.; Nagulapalli Venkata, K.; Nabavi, S.; Bishayee, A. Molecular Targets Underlying the Anticancer Effects of Quercetin: An Update. Nutrients 2016, 8, 529. [Google Scholar] [CrossRef] [Green Version]
- Abdallah, H.M.; Salama, M.M.; Abd-elrahman, E.H.; El-Maraghy, S.A. Antidiabetic Activity of Phenolic Compounds from Pecan Bark in Streptozotocin-Induced Diabetic Rats. Phytochem. Lett. 2011, 4, 337–341. [Google Scholar] [CrossRef]
- Li, S.; Li, J.; Guan, X.-L.; Li, J.; Deng, S.-P.; Li, L.-Q.; Tang, M.-T.; Huang, J.-G.; Chen, Z.-Z.; Yang, R.-Y. Hypoglycemic Effects and Constituents of the Barks of Cyclocarya paliurus and Their Inhibiting Activities to Glucosidase and Glycogen Phosphorylase. Fitoterapia 2011, 82, 1081–1085. [Google Scholar] [CrossRef]
- Moradi-Afrapoli, F.; Asghari, B.; Saeidnia, S.; Ajani, Y.; Mirjani, M.; Malmir, M.; Dolatabadi Bazaz, R.; Hadjiakhoondi, A.; Salehi, P.; Hamburger, M.; et al. In Vitro α-Glucosidase Inhibitory Activity of Phenolic Constituents from Aerial Parts of Polygonum hyrcanicum. DARU J. Pharm. Sci. 2012, 20, 37. [Google Scholar] [CrossRef] [Green Version]
- Nurul Islam, M.; Jung, H.A.; Sohn, H.S.; Kim, H.M.; Choi, J.S. Potent α-Glucosidase and Protein Tyrosine Phosphatase 1B Inhibitors from Artemisia capillaris. Arch. Pharm. Res. 2013, 36, 542–552. [Google Scholar] [CrossRef]
- Kim, Y.S.; Jung, D.H.; Lee, I.S.; Choi, S.-J.; Yu, S.Y.; Ku, S.-K.; Kim, M.-H.; Kim, J.S. Effects of Allium victorialis Leaf Extracts and Its Single Compounds on Aldose Reductase, Advanced Glycation End Products and TGF-Β1 Expression in Mesangial Cells. BMC Complement. Altern. Med. 2013, 13, 251. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Kumar, V.; Prakash, O. Enzymes Inhibition and Antidiabetic Effect of Isolated Constituents from Dillenia indica. Biomed. Res. Int. 2013, 2013, 382063. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-H.; Zhao, J.; Jin, H.-T.; Cao, Y.; Ming, T.; Zhang, L.-L.; Hu, M.-Y.; Hamlati, H.; Pang, S.-B.; Ma, X.-P. Vasorelaxant Effects of the Extracts and Some Flavonoids from the Buds of Coreopsis tinctoria. Pharm. Biol. 2013, 51, 1158–1164. [Google Scholar] [CrossRef]
- Tan, C.; Zuo, J.; Yi, X.; Wang, P.; Luo, C.; Hu, Y.; Yi, H.; Qiao, W. Phenolic Constituents from Sarcopyramis nepalensis and Their α-Glucosidase Inhibitory Activity. Afr. J. Trad. Compl. Alt. Med. 2015, 12, 156. [Google Scholar] [CrossRef] [Green Version]
- Zekry, S.H.; Abo-elmatty, D.M.; Zayed, R.A.; Radwan, M.M.; ElSohly, M.A.; Hassanean, H.A.; Ahmed, S.A. Effect of Metabolites Isolated from Cuscuta pedicellata on High Fat Diet-Fed Rats. Med. Chem. Res. 2015, 24, 1964–1973. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, H.; Wang, M.; Zhang, J. Quercetin Isolated from Toona sinensis Leaves Attenuates Hyperglycemia and Protects Hepatocytes in High-Carbohydrate/High-Fat Diet and Alloxan Induced Experimental Diabetic Mice. J. Diabetes Res. 2016, 2016, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasan, M.M.; Ahmed, Q.U.; Soad, S.Z.M.; Latip, J.; Taher, M.; Syafiq, T.M.F.; Sarian, M.N.; Alhassan, A.M.; Zakaria, Z.A. Flavonoids from Tetracera indica Merr. Induce Adipogenesis and Exert Glucose Uptake Activities in 3T3-L1 Adipocyte Cells. BMC Complement. Altern. Med. 2017, 17, 431. [Google Scholar] [CrossRef] [Green Version]
- Owis, A.I.; Abo-youssef, A.M.; Osman, A.H. Leaves of Cordia Boissieri, A. DC. as a Potential Source of Bioactive Secondary Metabolites for Protection against Metabolic Syndrome-Induced in Rats. Z. Naturforsch. C 2017, 72, 107–118. [Google Scholar] [CrossRef]
- Cifuentes, F.; Palacios, J.; Kuzmicic, J.; Carvajal, L.; Muñoz, F.; Quispe, C.; Nwokocha, C.R.; Morales, G.; Norambuena-Soto, I.; Chiong, M.; et al. Vasodilator and Hypotensive Effects of Pure Compounds and Hydroalcoholic Extract of Xenophyllum poposum (Phil) V.A Funk (Compositae) on Rats. Phytomedicine 2018, 50, 99–108. [Google Scholar] [CrossRef]
- Ibitoye, O.B.; Olofinsan, K.A.; Teralı, K.; Ghali, U.M.; Ajiboye, T.O. Bioactivity-Guided Isolation of Antidiabetic Principles from the Methanolic Leaf Extract of Bryophyllum pinnatum. J. Food Biochem. 2018, 42, e12627. [Google Scholar] [CrossRef]
- Srinivasan, P.; Vijayakumar, S.; Kothandaraman, S.; Palani, M. Anti-Diabetic Activity of Quercetin Extracted from Phyllanthus emblica L. Fruit: In Silico and in Vivo Approaches. J. Pharm. Anal. 2018, 8, 109–118. [Google Scholar] [CrossRef]
- Hussein, N.; Amen, Y.; Abdel Bar, F.; Halim, A.F.; Saad, H.-E.A. Antioxidants and α-Glucosidase Inhibitors from Lactuca serriola L. Rec. Nat. Prod. 2020, 14, 410–415. [Google Scholar] [CrossRef]
- Fadul, E.; Nizamani, A.; Rasheed, S.; Adhikari, A.; Yousuf, S.; Parveen, S.; Gören, N.; Alhazmi, H.A.; Choudhary, M.I.; Khalid, A. Anti-Glycating and Anti-Oxidant Compounds from Traditionally Used Anti-Diabetic Plant Geigeria alata (DC) Oliv. & Hiern. Nat. Prod. Res. 2020, 34, 2456–2464. [Google Scholar] [CrossRef]
- Kim, B.-R.; Paudel, S.; Nam, J.-W.; Jin, C.; Lee, I.-S.; Han, A.-R. Constituents of Coreopsis lanceolata Flower and Their Dipeptidyl Peptidase IV Inhibitory Effects. Molecules 2020, 25, 4370. [Google Scholar] [CrossRef]
- Abdelhameed, R.F.A.; Ibrahim, A.K.; Elfaky, M.A.; Habib, E.S.; Mahamed, M.I.; Mehanna, E.T.; Darwish, K.M.; Khodeer, D.M.; Ahmed, S.A.; Elhady, S.S. Antioxidant and Anti-Inflammatory Activity of Cynanchum acutum L. Isolated Flavonoids Using Experimentally Induced Type 2 Diabetes Mellitus: Biological and In Silico Investigation for NF-ΚB Pathway/MiR-146a Expression Modulation. Antioxidants 2021, 10, 1713. [Google Scholar] [CrossRef] [PubMed]
- Ansari, P.; Flatt, P.R.; Harriott, P.; Hannan, J.M.A.; Abdel-Wahab, Y.H.A. Identification of Multiple Pancreatic and Extra-Pancreatic Pathways Underlying the Glucose-Lowering Actions of Acacia arabica Bark in Type-2 Diabetes and Isolation of Active Phytoconstituents. Plants 2021, 10, 1190. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Wang, M.; Wang, S.; Zhang, J.; Du, Y.; Zhao, Y.; Zheng, X.; Ma, B. Phenolic Compounds from the Leaves of Crataegus pinnatifida Bge. var. major N.E.Br. And Their Lipid-Lowering Effects. Bioorg. Med. Chem. Lett. 2021, 47, 128211. [Google Scholar] [CrossRef]
- Praparatana, R.; Maliyam, P.; Barrows, L.R.; Puttarak, P. Flavonoids and Phenols, the Potential Anti-Diabetic Compounds from Bauhinia strychnifolia Craib. Stem. Molecules 2022, 27, 2393. [Google Scholar] [CrossRef]
- Zhang, S.-S.; Zhang, N.-N.; Guo, S.; Liu, S.-J.; Hou, Y.-F.; Li, S.; Ho, C.-T.; Bai, N.-S. Glycosides and Flavonoids from the Extract of Pueraria thomsonii Benth Leaf Alleviate Type 2 Diabetes in High-Fat Diet plus Streptozotocin-Induced Mice by Modulating the Gut Microbiota. Food Funct. 2022, 13, 3931–3945. [Google Scholar] [CrossRef]
- Bernatoniene, J.; Kopustinskiene, D.; Jakstas, V.; Majiene, D.; Baniene, R.; Kuršvietiene, L.; Masteikova, R.; Savickas, A.; Toleikis, A.; Trumbeckaite, S. The Effect of Leonurus cardiaca Herb Extract and Some of Its Flavonoids on Mitochondrial Oxidative Phosphorylation in the Heart. Planta Med. 2014, 80, 525–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jofré, I.; Pezoa, C.; Cuevas, M.; Scheuermann, E.; Freires, I.A.; Rosalen, P.L.; de Alencar, S.M.; Romero, F. Antioxidant and Vasodilator Activity of Ugni molinae Turcz. (Murtilla) and Its Modulatory Mechanism in Hypotensive Response. Oxid. Med. Cell. Longev. 2016, 2016, 6513416. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, L.L.D.M.; Leão, V.d.F.; de Melo, C.M.; de Machado, T.B.; Amaral, A.C.F.; da Silva, L.L.; Simas, N.K.; Muzitano, M.F.; Leal, I.C.R.; Raimundo, J.M. Ethyl Acetate Fraction and Isolated Phenolics Derivatives from Mandevilla moricandiana Identified by UHPLC-DAD-ESI-MSn with Pharmacological Potential for the Improvement of Obesity-Induced Endothelial Dysfunction. Pharmaceutics 2021, 13, 1173. [Google Scholar] [CrossRef]
- De Lima Júnior, J.P.; Franco, R.R.; Saraiva, A.L.; Moraes, I.B.; Espindola, F.S. Anacardium humile St. Hil as a Novel Source of Antioxidant, Antiglycation and α-Amylase Inhibitors Molecules with Potential for Management of Oxidative Stress and Diabetes. J. Ethnopharmacol. 2021, 268, 113667. [Google Scholar] [CrossRef]
- Karkanis, A.; Bilalis, D.; Efthimiadou, A. Cultivation of Milk Thistle (Silybum marianum L. Gaertn.), a Medicinal Weed. Ind. Crops Prod. 2011, 34, 825–830. [Google Scholar] [CrossRef]
- Lee, D.Y.-W.; Liu, Y. Molecular Structure and Stereochemistry of Silybin A, Silybin B, Isosilybin A, and Isosilybin B, Isolated from Silybum marianum (Milk Thistle). J. Nat. Prod. 2003, 66, 1171–1174. [Google Scholar] [CrossRef] [PubMed]
- Pferschy-Wenzig, E.-M.; Atanasov, A.G.; Malainer, C.; Noha, S.M.; Kunert, O.; Schuster, D.; Heiss, E.H.; Oberlies, N.H.; Wagner, H.; Bauer, R.; et al. Identification of Isosilybin A from Milk Thistle Seeds as an Agonist of Peroxisome Proliferator-Activated Receptor Gamma. J. Nat. Prod. 2014, 77, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Dewick, P. Medicinal Natural Products A Biosynthtic Approach, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2002. [Google Scholar]
- Křen, V.; Valentová, K. Silybin and Its Congeners: From Traditional Medicine to Molecular Effects. Nat. Prod. Rep. 2022, 39, 1264–1281. [Google Scholar] [CrossRef]
- Palomino, O.; Gouveia, N.; Ramos, S.; Martín, M.; Goya, L. Protective Effect of Silybum marianum and Silibinin on Endothelial Cells Submitted to High Glucose Concentration. Planta Med. 2016, 83, 97–103. [Google Scholar] [CrossRef] [PubMed]
- İnceören, N.; Emen, S.; Çeken Toptancı, B.; Kızıl, G.; Kızıl, M. In Vitro Inhibition of Advanced Glycation End Product Formation by Ethanol Extract of Milk Thistle (Silybum marianum L.) Seed. S. Afr. J. Bot. 2022, 149, 682–692. [Google Scholar] [CrossRef]
- Estrada, O.; Hasegawa, M.; Gonzalez-Mujíca, F.; Motta, N.; Perdomo, E.; Solorzano, A.; Méndez, J.; Méndez, B.; Zea, E.G. Evaluation of Flavonoids From Bauhinia megalandra Leaves as Inhibitors of Glucose-6-Phosphatase System. Phytother. Res. 2005, 19, 859–863. [Google Scholar] [CrossRef]
- Kashyap, P.; Shikha, D.; Thakur, M.; Aneja, A. Functionality of Apigenin as a Potent Antioxidant with Emphasis on Bioavailability, Metabolism, Action Mechanism and in Vitro and in Vivo Studies: A Review. J. Food Biochem. 2022, 46, e13950. [Google Scholar] [CrossRef]
- Tang, D.; Chen, K.; Huang, L.; Li, J. Pharmacokinetic Properties and Drug Interactions of Apigenin, a Natural Flavone. Expert Opin. Drug Metab. Toxicol. 2017, 13, 323–330. [Google Scholar] [CrossRef]
- Alam, W.; Rocca, C.; Khan, H.; Hussain, Y.; Aschner, M.; De Bartolo, A.; Amodio, N.; Angelone, T.; Cheang, W.S. Current Status and Future Perspectives on Therapeutic Potential of Apigenin: Focus on Metabolic-Syndrome-Dependent Organ Dysfunction. AOs 2021, 10, 1643. [Google Scholar] [CrossRef]
- DeRango-Adem, E.F.; Blay, J. Does Oral Apigenin Have Real Potential for a Therapeutic Effect in the Context of Human Gastrointestinal and Other Cancers? Front. Pharmacol. 2021, 12, 681477. [Google Scholar] [CrossRef]
- El Daibani, A.A.; Xi, Y.; Luo, L.; Mei, X.; Zhou, C.; Yasuda, S.; Liu, M.-C. Sulfation of Hesperetin, Naringenin and Apigenin by the Human Cytosolic Sulfotransferases: A Comprehensive Analysis. Nat. Prod. Res. 2020, 34, 797–803. [Google Scholar] [CrossRef]
- Bak, M.J.; Das Gupta, S.; Wahler, J.; Suh, N. Role of Dietary Bioactive Natural Products in Estrogen Receptor-Positive Breast Cancer. Semin. Cancer Biol. 2016, 40–41, 170–191. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Shang, P.; Li, D. Luteolin: A Flavonoid That Has Multiple Cardio-Protective Effects and Its Molecular Mechanisms. Front. Pharmacol. 2017, 8, 692. [Google Scholar] [CrossRef] [Green Version]
- Farkhondeh, T.; Samarghandian, S.; Bafandeh, F. The Cardiovascular Protective Effects of Chrysin: A Narrative Review on Experimental Researches. CHAMC 2019, 17, 17–27. [Google Scholar] [CrossRef]
- Zhou, Y.; Suo, W.; Zhang, X.; Lv, J.; Liu, Z.; Liu, R. Roles and Mechanisms of Quercetin on Cardiac Arrhythmia: A Review. Biomed. Pharmacother. 2022, 153, 113447. [Google Scholar] [CrossRef]
- Alsaidan, O.A.; Pattanayak, P.; Awasthi, A.; Alruwaili, N.K.; Zafar, A.; Almawash, S.; Gulati, M.; Singh, S.K. Quality by Design-Based Optimization of Formulation Parameters to Develop Quercetin Nanosuspension for Improving Its Biopharmaceutical Properties. S. Afr. J. Bot. 2022, 149, 798–806. [Google Scholar] [CrossRef]
- Rao, L. A Review on Quercetin: Assessment of the Pharmacological Potentials and Various Formulations Strategies. Int. J. Pharm. Sci. Rev. Res. 2020, 64, 139–144. [Google Scholar] [CrossRef]
- Dabeek, W.M.; Marra, M.V. Dietary Quercetin and Kaempferol: Bioavailability and Potential Cardiovascular-Related Bioactivity in Humans. Nutrients 2019, 11, 2288. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-Reyes, D.; Morales, A.I.; Prieto, M. Transit and Metabolic Pathways of Quercetin in Tubular Cells: Involvement of Its Antioxidant Properties in the Kidney. Antioxidants 2021, 10, 909. [Google Scholar] [CrossRef]
- Di Costanzo, A.; Angelico, R. Formulation Strategies for Enhancing the Bioavailability of Silymarin: The State of the Art. Molecules 2019, 24, 2155. [Google Scholar] [CrossRef] [Green Version]
- Sornsuvit, C.; Hongwiset, D.; Yotsawimonwat, S.; Toonkum, M.; Thongsawat, S.; Taesotikul, W. The Bioavailability and Pharmacokinetics of Silymarin SMEDDS Formulation Study in Healthy Thai Volunteers. Evid. Based Complement. Altern. Med. 2018, 2018, 1507834. [Google Scholar] [CrossRef] [Green Version]
- Kellici, T.F.; Ntountaniotis, D.; Leonis, G.; Chatziathanasiadou, M.; Chatzikonstantinou, A.V.; Becker-Baldus, J.; Glaubitz, C.; Tzakos, A.G.; Viras, K.; Chatzigeorgiou, P.; et al. Investigation of the Interactions of Silibinin with 2-Hydroxypropyl-β-Cyclodextrin through Biophysical Techniques and Computational Methods. Mol. Pharm. 2015, 12, 954–965. [Google Scholar] [CrossRef]
- Tvrdý, V.; Pourová, J.; Jirkovský, E.; Křen, V.; Valentová, K.; Mladěnka, P. Systematic Review of Pharmacokinetics and Potential Pharmacokinetic Interactions of Flavonolignans from Silymarin. Med. Res. Rev. 2021, 41, 2195–2246. [Google Scholar] [CrossRef]
- Ma, J.; Chen, X. Advances in Pathogenesis and Treatment of Essential Hypertension. Front. Cardiovasc. Med. 2022, 9, 1003852. [Google Scholar] [CrossRef]
- Xu, D.; Hu, M.-J.; Wang, Y.-Q.; Cui, Y.-L. Antioxidant Activities of Quercetin and Its Complexes for Medicinal Application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef] [Green Version]
- Pereira, S.C.; Parente, J.M.; Belo, V.A.; Mendes, A.S.; Gonzaga, N.A.; do Vale, G.T.; Ceron, C.S.; Tanus-Santos, J.E.; Tirapelli, C.R.; Castro, M.M. Quercetin Decreases the Activity of Matrix Metalloproteinase-2 and Ameliorates Vascular Remodeling in Renovascular Hypertension. Atherosclerosis 2018, 270, 146–153. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.G.; Lee, G.B.; Vinayagam, R.; Do, G.S.; Oh, S.Y.; Yang, S.J.; Kwon, J.B.; Singh, M. Anti-Inflammatory, Antioxidative, and Nitric Oxide-Scavenging Activities of a Quercetin Nanosuspension with Polyethylene Glycol in LPS-Induced RAW 264.7 Macrophages. Molecules 2022, 27, 7432. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Han, T.; Fan, Y.; Wu, S.; Wang, F.; Wang, C. Quercetin Improves Vascular Endothelial Function through Promotion of Autophagy in Hypertensive Rats. Life Sci. 2020, 258, 118106. [Google Scholar] [CrossRef]
- Haleagrahara, N.; Chakravarthi, S.; Bangra Kulur, A.; Yee, T.M. Plant Flavone Apigenin Protects against Cyclosporine-Induced Histological and Biochemical Changes in the Kidney in Rats. Biomed. Prev. Nutr. 2014, 4, 589–593. [Google Scholar] [CrossRef]
- Shen, Y.; Croft, K.D.; Hodgson, J.M.; Kyle, R.; Lee, I.-L.E.; Wang, Y.; Stocker, R.; Ward, N.C. Quercetin and Its Metabolites Improve Vessel Function by Inducing ENOS Activity via Phosphorylation of AMPK. Biochem. Pharmacol. 2012, 84, 1036–1044. [Google Scholar] [CrossRef]
- Chen, X.; Zheng, L.; Zhang, B.; Deng, Z.; Li, H. Synergistic Protection of Quercetin and Lycopene against Oxidative Stress via SIRT1-Nox4-ROS Axis in HUVEC Cells. Curr. Res. Nutr. Food Sci. 2022, 5, 1985–1993. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.-L.; Hsu, C.-N. AMP-Activated Protein Kinase as a Reprogramming Strategy for Hypertension and Kidney Disease of Developmental Origin. Int. J. Mol. Sci. 2018, 19, 1744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iside, C.; Scafuro, M.; Nebbioso, A.; Altucci, L. SIRT1 Activation by Natural Phytochemicals: An Overview. Front. Pharmacol. 2020, 11, 1225. [Google Scholar] [CrossRef]
- Häckl, L.P.N.; Cuttle, G.; Sanches Dovichi, S.; Lima-Landman, M.T.; Nicolau, M. Inhibition of Angiotensin-Converting Enzyme by Quercetin Alters the Vascular Response to Bradykinin and Angiotensin I. Pharmacology 2002, 65, 182–186. [Google Scholar] [CrossRef]
- Palmieri, D.; Perego, P.; Palombo, D. Apigenin Inhibits the TNFα-Induced Expression of ENOS and MMP-9 via Modulating Akt Signalling through Oestrogen Receptor Engagement. Mol. Cell Biochem. 2012, 371, 129–136. [Google Scholar] [CrossRef]
- Qin, W.; Ren, B.; Wang, S.; Liang, S.; He, B.; Shi, X.; Wang, L.; Liang, J.; Wu, F. Apigenin and Naringenin Ameliorate PKCβII-Associated Endothelial Dysfunction via Regulating ROS/Caspase-3 and NO Pathway in Endothelial Cells Exposed to High Glucose. Vasc. Pharmacol. 2016, 85, 39–49. [Google Scholar] [CrossRef]
- Jin, B.; Qian, L.; Chen, S.; Li, J.; Wang, H.; Bruce, I.C.; Lin, J.; Xia, Q. Apigenin Protects Endothelium-Dependent Relaxation of Rat Aorta against Oxidative Stress. Eur. J. Pharmacol. 2009, 616, 200–205. [Google Scholar] [CrossRef]
- Wei, X.; Gao, P.; Pu, Y.; Li, Q.; Yang, T.; Zhang, H.; Xiong, S.; Cui, Y.; Li, L.; Ma, X.; et al. Activation of TRPV4 by Dietary Apigenin Antagonizes Renal Fibrosis in Deoxycorticosterone Acetate (DOCA)–Salt-Induced Hypertension. Clin. Sci. 2017, 131, 567–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demirci, B.; Dost, T.; Gokalp, F.; Birincioglu, M. Silymarin Improves Vascular Function of Aged Ovariectomized Rats: Silymarin And Postmenopausal Endothelium. Phytother. Res. 2014, 28, 868–872. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-K.; Hong, Y.; Huang, Z.-Q. Protective Effects of Silybin on Human Umbilical Vein Endothelial Cell Injury Induced by H2O2 in Vitro. Vasc. Pharmacol. 2005, 43, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Duarte, J.; Pérez-Palencia, R.; Vargas, F.; Ocete, M.A.; Pérez-Vizcaino, F.; Zarzuelo, A.; Tamargo, J. Antihypertensive Effects of the Flavonoid Quercetin in Spontaneously Hypertensive Rats. Br. J. Pharmacol. 2001, 133, 117–124. [Google Scholar] [CrossRef]
- Elbarbry, F.; Abdelkawy, K.; Moshirian, N.; Abdel-Megied, A.M. The Antihypertensive Effect of Quercetin in Young Spontaneously Hypertensive Rats; Role of Arachidonic Acid Metabolism. Int. J. Mol. Sci. 2020, 21, 6554. [Google Scholar] [CrossRef]
- Li Volti, G.; Salomone, S.; Sorrenti, V.; Mangiameli, A.; Urso, V.; Siarkos, I.; Galvano, F.; Salamone, F. Effect of Silibinin on Endothelial Dysfunction and ADMA Levels in Obese Diabetic Mice. Cardiovasc. Diabetol. 2011, 10, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serban, M.; Sahebkar, A.; Zanchetti, A.; Mikhailidis, D.P.; Howard, G.; Antal, D.; Andrica, F.; Ahmed, A.; Aronow, W.S.; Muntner, P.; et al. Effects of Quercetin on Blood Pressure: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2016, 5, e002713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popiolek-Kalisz, J.; Fornal, E. The Effects of Quercetin Supplementation on Blood Pressure—Meta-Analysis. Curr. Probl. Cardiol. 2022, 47, 101350. [Google Scholar] [CrossRef]
- Yao, Z.; Dai, K.; Meng, G.; Zhang, Q.; Liu, L.; Wu, H.; Gu, Y.; Sun, S.; Wang, X.; Jia, Q.; et al. Low Dietary Quercetin Intake by Food Frequency Questionnaire Analysis Is Not Associated with Hypertension Occurrence. Clin. Nutr. 2021, 40, 3748–3753. [Google Scholar] [CrossRef]
- Eid, H.M.; Martineau, L.C.; Saleem, A.; Muhammad, A.; Vallerand, D.; Benhaddou-Andaloussi, A.; Nistor, L.; Afshar, A.; Arnason, J.T.; Haddad, P.S. Stimulation of AMP-Activated Protein Kinase and Enhancement of Basal Glucose Uptake in Muscle Cells by Quercetin and Quercetin Glycosides, Active Principles of the Antidiabetic Medicinal Plant Vaccinium Vitis-Idaea. Mol. Nutr. Food Res. 2010, 54, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Eid, H.; Nachar, A.; Thong, F.; Sweeney, G.; Haddad, P. The Molecular Basis of the Antidiabetic Action of Quercetin in Cultured Skeletal Muscle Cells and Hepatocytes. Pharmacogn. Mag. 2015, 11, 74. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.; Yamashita, Y.; Nakamura, A.; Croft, K.; Ashida, H. Quercetin and Its Metabolite Isorhamnetin Promote Glucose Uptake through Different Signalling Pathways in Myotubes. Sci. Rep. 2019, 9, 2690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alam, M.M.; Meerza, D.; Naseem, I. Protective Effect of Quercetin on Hyperglycemia, Oxidative Stress and DNA Damage in Alloxan Induced Type 2 Diabetic Mice. Life Sci. 2014, 109, 8–14. [Google Scholar] [CrossRef]
- Mokashi, P.; Khanna, A.; Pandita, N. Flavonoids from Enicostema Littorale Blume Enhances Glucose Uptake of Cells in Insulin Resistant Human Liver Cancer (HepG2) Cell Line via IRS-1/PI3K/Akt Pathway. Biomed. Pharmacother. 2017, 90, 268–277. [Google Scholar] [CrossRef]
- Boydens, C.; Pauwels, B.; Vanden Daele, L.; Van de Voorde, J. Protective Effect of Resveratrol and Quercetin on in Vitro-Induced Diabetic Mouse Corpus Cavernosum. Cardiovasc. Diabetol. 2016, 15, 46. [Google Scholar] [CrossRef] [Green Version]
- Xie, J.; Song, W.; Liang, X.; Zhang, Q.; Shi, Y.; Liu, W.; Shi, X. Protective Effect of Quercetin on Streptozotocin-Induced Diabetic Peripheral Neuropathy Rats through Modulating Gut Microbiota and Reactive Oxygen Species Level. Biomed. Pharmacother. 2020, 127, 110147. [Google Scholar] [CrossRef]
- Chu, C.; Gao, X.; Li, X.; Zhang, X.; Ma, R.; Jia, Y.; Li, D.; Wang, D.; Xu, F. Involvement of Estrogen Receptor-α in the Activation of Nrf2-Antioxidative Signaling Pathways by Silibinin in Pancreatic β-Cells. Biomol. Ther. 2020, 28, 163–171. [Google Scholar] [CrossRef]
- Mohammadi, H.; Manouchehri, H.; Changizi, R.; Bootorabi, F.; Khorramizadeh, M.R. Concurrent Metformin and Silibinin Therapy in Diabetes: Assessments in Zebrafish (Danio Rerio) Animal Model. J. Diabetes Metab. Disord. 2020, 19, 1233–1244. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, U.; Chandrayan, G.; Patil, C.; Arya, D.; Suchal, K.; Agrawal, Y.; Ojha, S.; Goyal, S. The Protective Effect of Apigenin on Myocardial Injury in Diabetic Rats Mediating Activation of the PPAR-γ Pathway. Int. J. Mol. Sci. 2017, 18, 756. [Google Scholar] [CrossRef] [Green Version]
- Malik, S.; Suchal, K.; Khan, S.I.; Bhatia, J.; Kishore, K.; Dinda, A.K.; Arya, D.S. Apigenin Ameliorates Streptozotocin-Induced Diabetic Nephropathy in Rats via MAPK-NF-ΚB-TNF-α and TGF-Β1-MAPK-Fibronectin Pathways. Am. J. Physiol. Renal Physiol. 2017, 313, F414–F422. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.-J.; Fan, Y.-L.; Liao, H.-H.; Liu, Y.; Chen, S.; Ma, Z.-G.; Zhang, N.; Yang, Z.; Deng, W.; Tang, Q.-Z. Apigenin Alleviates STZ-Induced Diabetic Cardiomyopathy. Mol. Cell Biochem. 2017, 428, 9–21. [Google Scholar] [CrossRef]
- Meng, S.; Yang, F.; Wang, Y.; Qin, Y.; Xian, H.; Che, H.; Wang, L. Silymarin Ameliorates Diabetic Cardiomyopathy via Inhibiting TGF-Β1/Smad Signaling: Silymarin Ameliorates DCM. Cell Biol. Int. 2019, 43, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Wadhwa, K.; Pahwa, R.; Kumar, M.; Kumar, S.; Sharma, P.C.; Singh, G.; Verma, R.; Mittal, V.; Singh, I.; Kaushik, D.; et al. Mechanistic Insights into the Pharmacological Significance of Silymarin. Molecules 2022, 27, 5327. [Google Scholar] [CrossRef]
- Ingles, J.; Goldstein, J.; Thaxton, C.; Caleshu, C.; Corty, E.W.; Crowley, S.B.; Dougherty, K.; Harrison, S.M.; McGlaughon, J.; Milko, L.V.; et al. Evaluating the Clinical Validity of Hypertrophic Cardiomyopathy Genes. Circ. Genom. Precis. Med. 2019, 12, e002460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzarotto, F.; Tayal, U.; Buchan, R.J.; Midwinter, W.; Wilk, A.; Whiffin, N.; Govind, R.; Mazaika, E.; de Marvao, A.; Dawes, T.J.W.; et al. Reevaluating the Genetic Contribution of Monogenic Dilated Cardiomyopathy. Circulation 2020, 141, 387–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreland, N.; La Grange, L.; Montoya, R. Impact of in Utero Exposure to EtOH on Corpus Callosum Development and Paw Preference in Rats: Protective Effects of Silymarin. BMC Complement. Altern. Med. 2002, 2, 10. [Google Scholar] [CrossRef] [Green Version]
- Malekinejad, H.; Rezabakhsh, A.; Rahmani, F.; Hobbenaghi, R. Silymarin Regulates the Cytochrome P450 3A2 and Glutathione Peroxides in the Liver of Streptozotocin-Induced Diabetic Rats. Phytomedicine 2012, 19, 583–590. [Google Scholar] [CrossRef]
- Yang, J.; Sun, Y.; Xu, F.; Liu, W.; Hayashi, T.; Onodera, S.; Tashiro, S.; Ikejima, T. Involvement of Estrogen Receptors in Silibinin Protection of Pancreatic β-Cells from TNFα- or IL-1β-Induced Cytotoxicity. Biomed. Pharmacother. 2018, 102, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Soto, C.; Recoba, R.; Barrón, H.; Alvarez, C.; Favari, L. Silymarin Increases Antioxidant Enzymes in Alloxan-Induced Diabetes in Rat Pancreas. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2003, 136, 205–212. [Google Scholar] [CrossRef]
- Miranda, L.M.O.; da Cunha Agostini, L.; de Lima, W.G.; Camini, F.C.; Costa, D.C. Silymarin Attenuates Hepatic and Pancreatic Redox Imbalance Independent of Glycemic Regulation in the Alloxan-Induced Diabetic Rat Model. Biomed. Environ. Sci. 2020, 33, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Manson, J.E.; Buring, J.E.; Sesso, H.D.; Liu, S. Associations of Dietary Flavonoids with Risk of Type 2 Diabetes, and Markers of Insulin Resistance and Systemic Inflammation in Women: A Prospective Study and Cross-Sectional Analysis. J. Am. Coll. Nutr. 2005, 24, 376–384. [Google Scholar] [CrossRef]
- Sales, D.S.; Carmona, F.; de Azevedo, B.C.; Taleb-Contini, S.H.; Bartolomeu, A.C.D.; Honorato, F.B.; Martinez, E.Z.; Pereira, A.M.S. Eugenia punicifolia (Kunth) DC. as an Adjuvant Treatment for Type-2 Diabetes Mellitus: A Non-Controlled, Pilot Study. Phytother. Res. 2014, 28, 1816–1821. [Google Scholar] [CrossRef]
- Voroneanu, L.; Nistor, I.; Dumea, R.; Apetrii, M.; Covic, A. Silymarin in Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Diabetes Res. 2016, 2016, 5147468. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Guo, T.; Deng, R.; Liu, L.; Yu, Y. Apigenin Ameliorates Insulin Resistance and Lipid Accumulation by Endoplasmic Reticulum Stress and SREBP-1c/SREBP-2 Pathway in Palmitate-Induced HepG2 Cells and High-Fat Diet–Fed Mice. J. Pharmacol. Exp. Ther. 2021, 377, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.H.; Cho, I.; Ahn, J.; Jeon, T.-I.; Ha, T.-Y. Quercetin Reduces High-Fat Diet-Induced Fat Accumulation in the Liver by Regulating Lipid Metabolism Genes: Anti-Obesity Effect Of Quercetin. Phytother. Res. 2013, 27, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, R.A.; Elshikh, M.S.; Mohamed, M.O.; Darweesh, M.F.; Hussein, D.S.; Almutairi, S.M.; Embaby, A.S. Quercetin Mitigates the Adverse Effects of High Fat Diet on Pancreatic and Renal Tissues in Adult Male Albino Rats. J. King Saud Univ. Sci. 2022, 34, 101946. [Google Scholar] [CrossRef]
- Ren, B.; Qin, W.; Wu, F.; Wang, S.; Pan, C.; Wang, L.; Zeng, B.; Ma, S.; Liang, J. Apigenin and Naringenin Regulate Glucose and Lipid Metabolism, and Ameliorate Vascular Dysfunction in Type 2 Diabetic Rats. Eur. J. Pharmacol. 2016, 773, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Li, Y.-C.; Du, C.; Wang, L.-N.; Xiao, Y.-H. Effects of Apigenin on the Expression of LOX-1, Bcl-2, and Bax in Hyperlipidemia Rats. Chem. Biodivers. 2021, 18, e2100049. [Google Scholar] [CrossRef]
- Gobalakrishnan, S. Effect of Silybin on Lipid Profile in Hypercholesterolaemic Rats. J. Clin. Diagn. Res. 2016, 10, FF01. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Zhao, P.; Huang, J.; Zhao, Y.; Wang, Y.; Li, Y.; Li, Y.; Fan, S.; Ma, Y.-M.; Tong, Q.; et al. Silymarin Ameliorates Metabolic Dysfunction Associated with Diet-Induced Obesity via Activation of Farnesyl X Receptor. Front. Pharmacol. 2016, 7, 345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.-H.; Jiang, L.-Y.; Wang, Y.-C.; Ma, D.-F.; Li, X. Quercetin Attenuates Atherosclerosis via Modulating Oxidized LDL-Induced Endothelial Cellular Senescence. Front. Pharmacol. 2020, 11, 512. [Google Scholar] [CrossRef] [PubMed]
- Sahebkar, A. Effects of Quercetin Supplementation on Lipid Profile: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Crit. Rev. Food Sci. Nutr. 2017, 57, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Tabrizi, R.; Tamtaji, O.R.; Mirhosseini, N.; Lankarani, K.B.; Akbari, M.; Heydari, S.T.; Dadgostar, E.; Asemi, Z. The Effects of Quercetin Supplementation on Lipid Profiles and Inflammatory Markers among Patients with Metabolic Syndrome and Related Disorders: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Crit. Rev. Food Sci. Nutr. 2020, 60, 1855–1868. [Google Scholar] [CrossRef]
- Egert, S.; Bosy-Westphal, A.; Seiberl, J.; Kürbitz, C.; Settler, U.; Plachta-Danielzik, S.; Wagner, A.E.; Frank, J.; Schrezenmeir, J.; Rimbach, G.; et al. Quercetin Reduces Systolic Blood Pressure and Plasma Oxidised Low-Density Lipoprotein Concentrations in Overweight Subjects with a High-Cardiovascular Disease Risk Phenotype: A Double-Blinded, Placebo-Controlled Cross-over Study. Br. J. Nutr. 2009, 102, 1065–1074. [Google Scholar] [CrossRef] [Green Version]
- Brüll, V.; Burak, C.; Stoffel-Wagner, B.; Wolffram, S.; Nickenig, G.; Müller, C.; Langguth, P.; Alteheld, B.; Fimmers, R.; Naaf, S.; et al. Effects of a Quercetin-Rich Onion Skin Extract on 24 h Ambulatory Blood Pressure and Endothelial Function in Overweight-to-Obese Patients with (Pre-)Hypertension: A Randomised Double-Blinded Placebo-Controlled Cross-over Trial. Br. J. Nutr. 2015, 114, 1263–1277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadi, A.; Pourmasoumi, M.; Mohammadi, H.; Symonds, M.; Miraghajani, M. The Effects of Silymarin Supplementation on Metabolic Status and Oxidative Stress in Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis of Clinical Trials. Complement. Ther. Med. 2018, 41, 311–319. [Google Scholar] [CrossRef]
- Mohammadi, H.; Hadi, A.; Arab, A.; Moradi, S.; Rouhani, M.H. Effects of Silymarin Supplementation on Blood Lipids: A Systematic Review and Meta-analysis of Clinical Trials. Phytother. Res. 2019, 33, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Ravari, S.S.; Talaei, B.; Gharib, Z. The Effects of Silymarin on Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Obes. Med. 2021, 26, 100368. [Google Scholar] [CrossRef]
- Wang, Q.; Zeng, P.; Liu, Y.; Wen, G.; Fu, X.; Sun, X. Inhibition of Autophagy Ameliorates Atherogenic Inflammation by Augmenting Apigenin-Induced Macrophage Apoptosis. Int. Immunopharmacol. 2015, 27, 24–31. [Google Scholar] [CrossRef]
- Clayton, Z.S.; Hutton, D.A.; Brunt, V.E.; VanDongen, N.S.; Ziemba, B.P.; Casso, A.G.; Greenberg, N.T.; Mercer, A.N.; Rossman, M.J.; Campisi, J.; et al. Apigenin Restores Endothelial Function by Ameliorating Oxidative Stress, Reverses Aortic Stiffening, and Mitigates Vascular Inflammation with Aging. Am. J. Physiol. Heart Circ. Physiol. 2021, 321, H185–H196. [Google Scholar] [CrossRef]
- Huwait, E.A.; Saddeek, S.Y.; Al-Massabi, R.F.; Almowallad, S.J.; Pushparaj, P.N.; Kalamegam, G. Antiatherogenic Effects of Quercetin in the THP-1 Macrophage Model In Vitro, With Insights Into Its Signaling Mechanisms Using In Silico Analysis. Front. Pharmacol. 2021, 12, 698138. [Google Scholar] [CrossRef]
- Cui, Y.; Hou, P.; Li, F.; Liu, Q.; Qin, S.; Zhou, G.; Xu, X.; Si, Y.; Guo, S. Quercetin Improves Macrophage Reverse Cholesterol Transport in Apolipoprotein E-Deficient Mice Fed a High-Fat Diet. Lipids Health Dis. 2017, 16, 9. [Google Scholar] [CrossRef] [Green Version]
- Bhaskar, S.; Sudhakaran, P.R.; Helen, A. Quercetin Attenuates Atherosclerotic Inflammation and Adhesion Molecule Expression by Modulating TLR-NF-ΚB Signaling Pathway. Cell. Immunol. 2016, 310, 131–140. [Google Scholar] [CrossRef]
- Ren, K.; Jiang, T.; Zhou, H.-F.; Liang, Y.; Zhao, G.-J. Apigenin Retards Atherogenesis by Promoting ABCA1-Mediated Cholesterol Efflux and Suppressing Inflammation. Cell. Physiol. Biochem. 2018, 47, 2170–2184. [Google Scholar] [CrossRef]
- Wang, L.; Rotter, S.; Ladurner, A.; Heiss, E.; Oberlies, N.; Dirsch, V.; Atanasov, A. Silymarin Constituents Enhance ABCA1 Expression in THP-1 Macrophages. Molecules 2015, 21, 55. [Google Scholar] [CrossRef] [Green Version]
- Puteri, M.U.; Azmi, N.U.; Kato, M.; Saputri, F.C. PCSK9 Promotes Cardiovascular Diseases: Recent Evidence about Its Association with Platelet Activation-Induced Myocardial Infarction. Life 2022, 12, 190. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.-L.; Zhao, C.-H.; Yao, X.-L.; Zhang, H. Quercetin Attenuates High Fructose Feeding-Induced Atherosclerosis by Suppressing Inflammation and Apoptosis via ROS-Regulated PI3K/AKT Signaling Pathway. Biomed. Pharmacother. 2017, 85, 658–671. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Cao, H.; Shen, D.; Li, S.; Yan, L.; Chen, C.; Xing, S.; Dou, F. Quercetin Protects against Atherosclerosis by Regulating the Expression of PCSK9, CD36, PPARγ, LXRα and ABCA1. Int. J. Mol. Med. 2019, 44, 893–902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Cao, H.; Shen, D.; Chen, C.; Xing, S.; Dou, F.; Jia, Q. Effect of Quercetin on Atherosclerosis Based on Expressions of ABCA1, LXR-α and PCSK9 in ApoE-/- Mice. Chin. J. Integr. Med. 2020, 26, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Surai, P. Silymarin as a Natural Antioxidant: An Overview of the Current Evidence and Perspectives. Antioxidants 2015, 4, 204–247. [Google Scholar] [CrossRef] [Green Version]
- Garelnabi, M.; Mahini, H.; Wilson, T. Quercetin Intake with Exercise Modulates Lipoprotein Metabolism and Reduces Atherosclerosis Plaque Formation. J. Int. Soc. Sports Nutr. 2014, 11, 22. [Google Scholar] [CrossRef] [Green Version]
- Saragusti, A.C.; Ortega, M.G.; Cabrera, J.L.; Estrin, D.A.; Marti, M.A.; Chiabrando, G.A. Inhibitory Effect of Quercetin on Matrix Metalloproteinase 9 Activity Molecular Mechanism and Structure–Activity Relationship of the Flavonoid–Enzyme Interaction. Eur. J. Pharmacol. 2010, 644, 138–145. [Google Scholar] [CrossRef]
- Kondo, M.; Izawa-Ishizawa, Y.; Goda, M.; Hosooka, M.; Kagimoto, Y.; Saito, N.; Matsuoka, R.; Zamami, Y.; Chuma, M.; Yagi, K.; et al. Preventive Effects of Quercetin against the Onset of Atherosclerosis-Related Acute Aortic Syndromes in Mice. Int. J. Mol. Sci. 2020, 21, 7226. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Xu, M.; Lopes-Virella, M.F.; Huang, Y. Quercetin Inhibits Matrix Metalloproteinase-1 Expression in Human Vascular Endothelial Cells through Extracellular Signal-Regulated Kinase. Arch. Biochem. 2001, 391, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Myoung, H.-J.; Kim, G.; Nam, K.-W. Apigenin Isolated from the Seeds of Perilla frutescens Britton var crispa (Benth.) Inhibits Food Intake in C57BL/6J Mice. Arch. Pharm. Res. 2010, 33, 1741–1746. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Fujimori, K. Antiadipogenic Effect of Dietary Apigenin through Activation of AMPK in 3T3-L1 Cells. J. Agric. Food Chem. 2011, 59, 13346–13352. [Google Scholar] [CrossRef]
- Gómez-Zorita, S.; Lasa, A.; Abendaño, N.; Fernández-Quintela, A.; Mosqueda-Solís, A.; Garcia-Sobreviela, M.P.; Arbonés-Mainar, J.M.; Portillo, M.P. Phenolic Compounds Apigenin, Hesperidin and Kaempferol Reduce in Vitro Lipid Accumulation in Human Adipocytes. J. Transl. Med. 2017, 15, 237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, B.O.; Che, D.N.; Shin, J.Y.; Kang, H.J.; Kim, J.H.; Jang, S.I. Anti-obesity Effects of Enzyme-treated Celery Extract in Mice Fed with High-fat Diet. J. Food Biochem. 2020, 44, e13105. [Google Scholar] [CrossRef]
- Sun, T.; Ding, W.; Xu, T.; Ao, X.; Yu, T.; Li, M.; Liu, Y.; Zhang, X.; Hou, L.; Wang, J. Parkin Regulates Programmed Necrosis and Myocardial Ischemia/Reperfusion Injury by Targeting Cyclophilin-D. Antioxid. Redox. Signal. 2019, 31, 1177–1193. [Google Scholar] [CrossRef]
- Zhao, L.; Zheng, M.; Cai, H.; Chen, J.; Lin, Y.; Wang, F.; Wang, L.; Zhang, X.; Liu, J. The Activity Comparison of Six Dietary Flavonoids Identifies That Luteolin Inhibits 3T3-L1 Adipocyte Differentiation through Reducing ROS Generation. J. Nutr. Biochem. 2022, 112, 109208. [Google Scholar] [CrossRef]
- Qiao, Y.; Zhang, Z.; Zhai, Y.; Yan, X.; Zhou, W.; Liu, H.; Guan, L.; Peng, L. Apigenin Alleviates Obesity-Associated Metabolic Syndrome by Regulating the Composition of the Gut Microbiome. Front. Microbiol. 2021, 12, 805827. [Google Scholar] [CrossRef]
- Ahn, J.; Lee, H.; Kim, S.; Park, J.; Ha, T. The Anti-Obesity Effect of Quercetin Is Mediated by the AMPK and MAPK Signaling Pathways. Biochem. Biophys. Res. Commun. 2008, 373, 545–549. [Google Scholar] [CrossRef]
- Fang, X.-K.; Gao, J.; Zhu, D.-N. Kaempferol and Quercetin Isolated from Euonymus alatus Improve Glucose Uptake of 3T3-L1 Cells without Adipogenesis Activity. Life Sci. 2008, 82, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Shatylo, V.; Antoniuk-Shcheglova, I.; Naskalova, S.; Bondarenko, O.; Havalko, A.; Krasnienkov, D.; Zabuga, O.; Kukharskyy, V.; Guryanov, V.; Vaiserman, A. Cardio-Metabolic Benefits of Quercetin in Elderly Patients with Metabolic Syndrome. PharmaNutrition 2021, 15, 100250. [Google Scholar] [CrossRef]
- Pfeuffer, M.; Auinger, A.; Bley, U.; Kraus-Stojanowic, I.; Laue, C.; Winkler, P.; Rüfer, C.E.; Frank, J.; Bösch-Saadatmandi, C.; Rimbach, G.; et al. Effect of Quercetin on Traits of the Metabolic Syndrome, Endothelial Function and Inflammation in Men with Different APOE Isoforms. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Liao, D.; Dong, Y.; Pu, R. Effect of Quercetin Supplementation on Plasma Lipid Profiles, Blood Pressure, and Glucose Levels: A Systematic Review and Meta-Analysis. Nutr. Rev. 2020, 78, 615–626. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhang, Z.Z.; Wu, Y.; Ke, J.J.; He, X.H.; Wang, Y.L. Quercetin Postconditioning Attenuates Myocardial Ischemia/Reperfusion Injury in Rats through the PI3K/Akt Pathway. Braz. J. Med. Biol. Res. 2013, 46, 861–867. [Google Scholar] [CrossRef] [Green Version]
- Jing, Z.; Wang, Z.; Li, X.; Li, X.; Cao, T.; Bi, Y.; Zhou, J.; Chen, X.; Yu, D.; Zhu, L.; et al. Protective Effect of Quercetin on Posttraumatic Cardiac Injury. Sci. Rep. 2016, 6, 30812. [Google Scholar] [CrossRef] [Green Version]
- Chang, X.; Zhang, T.; Meng, Q.; ShiyuanWang; Yan, P.; Wang, X.; Luo, D.; Zhou, X.; Ji, R. Quercetin Improves Cardiomyocyte Vulnerability to Hypoxia by Regulating SIRT1/TMBIM6-Related Mitophagy and Endoplasmic Reticulum Stress. Oxid. Med. Cell Long. 2021, 2021, 5529913. [Google Scholar] [CrossRef]
- Bali, E.; Ergin, V.; Rackova, L.; Bayraktar, O.; Küçükboyacı, N.; Karasu, Ç. Olive Leaf Extracts Protect Cardiomyocytes against 4-Hydroxynonenal-Induced Toxicity In Vitro: Comparison with Oleuropein, Hydroxytyrosol, and Quercetin. Planta Med. 2014, 80, 984–992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, X.; Chen, M.; Zeng, H.; Liu, P.; Zhu, X.; Zhou, F.; Liu, J.; Zhang, J.; Dong, Z.; Tang, Y.; et al. Quercetin Attenuates Ethanol-Induced Iron Uptake and Myocardial Injury by Regulating the Angiotensin II-L-Type Calcium Channel. Mol. Nutr. Food Res. 2018, 62, 1700772. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, R.; Retamal, C.; Schupper, D.; Vergara-Hernández, D.; Saha, S.; Profumo, E.; Buttari, B.; Saso, L. Antioxidant Cardioprotection against Reperfusion Injury: Potential Therapeutic Roles of Resveratrol and Quercetin. Molecules 2022, 27, 2564. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Li, Z.; Xu, L.; Sun, A.; Fu, X.; Zhang, L.; Jing, L.; Lu, A.; Dong, Y.; Jia, Z. Protective Effect of Apigenin on Ischemia/Reperfusion Injury of the Isolated Rat Heart. Cardiovasc. Toxicol. 2015, 15, 241–249. [Google Scholar] [CrossRef]
- Yang, X.; Yang, J.; Hu, J.; Li, X.; Zhang, X.; Li, Z. Apigenin Attenuates Myocardial Ischemia/Reperfusion Injury via the Inactivation of P38 Mitogen-activated Protein Kinase. Mol. Med. Rep. 2015, 12, 6873–6878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Zhang, H.; Liu, Z.; Ma, Z.; An, D.; Xu, D. Apigenin Attenuates Myocardial Infarction-Induced Cardiomyocyte Injury by Modulating Parkin-Mediated Mitochondrial Autophagy. J. Biosci. 2020, 45, 75. [Google Scholar] [CrossRef]
- Rao, P. Cardioprotective Activity of Silymarin in Ischemia-Reperfusion-Induced Myocardial Infarction in Albino Rats. Exp. Clin. Cardiol. 2007, 12, 179. [Google Scholar]
- Albadrani, G.M.; BinMowyna, M.N.; Bin-Jumah, M.N.; El–Akabawy, G.; Aldera, H.; AL-Farga, A.M. Quercetin Prevents Myocardial Infarction Adverse Remodeling in Rats by Attenuating TGF-Β1/Smad3 Signaling: Different Mechanisms of Action. Saudi J. Biol. Sci. 2021, 28, 2772–2782. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Lin, H.; Wang, Q.; Hou, J.-W.; Mao, Z.-J.; Li, Y.-G. Protective Role of Silibinin against Myocardial Ischemia/Reperfusion Injury-Induced Cardiac Dysfunction. Int. J. Biol. Sci. 2020, 16, 1972–1988. [Google Scholar] [CrossRef]
- Tan, X.; Xian, W.; Li, X.; Chen, Y.; Geng, J.; Wang, Q.; Gao, Q.; Tang, B.; Wang, H.; Kang, P. Mechanisms of Quercetin against Atrial Fibrillation Explored by Network Pharmacology Combined with Molecular Docking and Experimental Validation. Sci. Rep. 2022, 12, 9777. [Google Scholar] [CrossRef]
- Bouderba, S.; Sanchez-Martin, C.; Villanueva, G.R.; Detaille, D.; Koceïr, E.A. Beneficial Effects of Silibinin against the Progression of Metabolic Syndrome, Increased Oxidative Stress, and Liver Steatosis in Psammomys Obesus, a Relevant Animal Model of Human Obesity and Diabetes. J. Diabetes 2014, 6, 184–192. [Google Scholar] [CrossRef]
- Prakash, P.; Singh, V.; Jain, M.; Rana, M.; Khanna, V.; Barthwal, M.K.; Dikshit, M. Silymarin Ameliorates Fructose Induced Insulin Resistance Syndrome by Reducing de Novo Hepatic Lipogenesis in the Rat. Eur. J. Pharmacol. 2014, 727, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Alex, R.; Bellner, L.; Raffaele, M.; Licari, M.; Vanella, L.; Stec, D.E.; Abraham, N.G. Milk Thistle Seed Cold Press Oil Attenuates Markers of the Metabolic Syndrome in a Mouse Model of Dietary-induced Obesity. J. Food Biochem. 2020, 44, e13522. [Google Scholar] [CrossRef] [PubMed]
- Mariee, A.D.; Abd-Allah, G.M.; El-Beshbishy, H.A. Protective Effect of Dietary Flavonoid Quercetin against Lipemic-Oxidative Hepatic Injury in Hypercholesterolemic Rats. Pharm. Biol. 2012, 50, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.-R.; Chen, Z.; Fang, K.; Xu, J.-X.; Ge, J.-F. Protective Effect of Quercetin against the Metabolic Dysfunction of Glucose and Lipids and Its Associated Learning and Memory Impairments in NAFLD Rats. Lipids Health Dis. 2021, 20, 164. [Google Scholar] [CrossRef] [PubMed]
- Wat, E.; Wang, Y.; Chan, K.; Law, H.W.; Koon, C.M.; Lau, K.M.; Leung, P.C.; Yan, C.; Lau, C.B.S. An in Vitro and in Vivo Study of a 4-Herb Formula on the Management of Diet-Induced Metabolic Syndrome. Phytomedicine 2018, 42, 112–125. [Google Scholar] [CrossRef]
- Hosseini, A.; Razavi, B.M.; Banach, M.; Hosseinzadeh, H. Quercetin and Metabolic Syndrome: A Review. Phytother. Res. 2021, 35, 5352–5364. [Google Scholar] [CrossRef]
- Menezes, R.; Rodriguez-Mateos, A.; Kaltsatou, A.; González-Sarrías, A.; Greyling, A.; Giannaki, C.; Andres-Lacueva, C.; Milenkovic, D.; Gibney, E.; Dumont, J.; et al. Impact of Flavonols on Cardiometabolic Biomarkers: A Meta-Analysis of Randomized Controlled Human Trials to Explore the Role of Inter-Individual Variability. Nutrients 2017, 9, 117. [Google Scholar] [CrossRef] [Green Version]
- Ostadmohammadi, V.; Milajerdi, A.; Ayati, E.; Kolahdooz, F.; Asemi, Z. Effects of Quercetin Supplementation on Glycemic Control among Patients with Metabolic Syndrome and Related Disorders: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Phytother. Res. 2019, 33, 1330–1340. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomou, E.-M.; Papakyriakopoulou, P.; Skaltsa, H.; Valsami, G.; Kadoglou, N.P.E. Bio-Actives from Natural Products with Potential Cardioprotective Properties: Isolation, Identification, and Pharmacological Actions of Apigenin, Quercetin, and Silibinin. Molecules 2023, 28, 2387. https://doi.org/10.3390/molecules28052387
Tomou E-M, Papakyriakopoulou P, Skaltsa H, Valsami G, Kadoglou NPE. Bio-Actives from Natural Products with Potential Cardioprotective Properties: Isolation, Identification, and Pharmacological Actions of Apigenin, Quercetin, and Silibinin. Molecules. 2023; 28(5):2387. https://doi.org/10.3390/molecules28052387
Chicago/Turabian StyleTomou, Ekaterina-Michaela, Paraskevi Papakyriakopoulou, Helen Skaltsa, Georgia Valsami, and Nikolaos P. E. Kadoglou. 2023. "Bio-Actives from Natural Products with Potential Cardioprotective Properties: Isolation, Identification, and Pharmacological Actions of Apigenin, Quercetin, and Silibinin" Molecules 28, no. 5: 2387. https://doi.org/10.3390/molecules28052387
APA StyleTomou, E. -M., Papakyriakopoulou, P., Skaltsa, H., Valsami, G., & Kadoglou, N. P. E. (2023). Bio-Actives from Natural Products with Potential Cardioprotective Properties: Isolation, Identification, and Pharmacological Actions of Apigenin, Quercetin, and Silibinin. Molecules, 28(5), 2387. https://doi.org/10.3390/molecules28052387