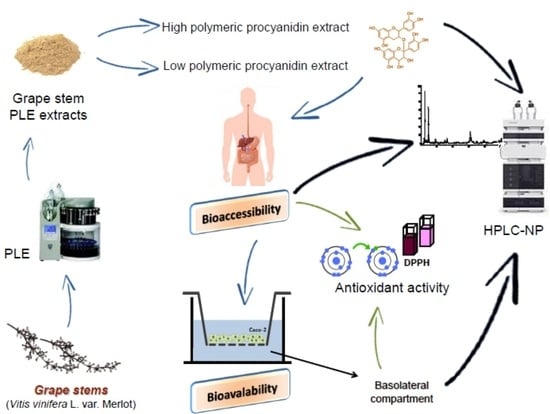
Implication of the Polymeric Phenolic Fraction and Matrix Effect on the Antioxidant Activity, Bioaccessibility, and Bioavailability of Grape Stem Extracts
Abstract
:1. Introduction
2. Results
2.1. Characterization of PLE Extracts
2.2. In Vitro Gastrointestinal Digestion: Antioxidant Activity and TPC
2.3. In Vitro Gastrointestinal Digestion: Phenolic Compound Composition
2.4. In Vitro Transepithelial Transport of Extracts
2.5. In Vitro Transepithelial Transport: Phenolic Compound Transport
3. Discussion
3.1. Characterization of PLE Extracts
3.2. In Vitro Gastrointestinal Digestion of Extracts
3.3. In Vitro Transepithelial Transport
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Plant Material
4.3. Pressurized Liquid Extraction (PLE)
4.4. Composition Analysis
4.5. HPLC-PAD Analysis of Phenolic Composition
4.6. Total Polymeric Procyanidin Content
4.7. Determination of Mean Degree of Procyanidin Polymerization (mDP)
4.8. Mono-Oligomeric and Polymeric Fraction Isolation
4.9. Total Phenolic Content (TPC)
4.10. Antioxidant Activity: DPPH Assay
4.11. In Vitro Gastrointestinal Digestion
4.12. In Vitro Transepithelial Transport
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ben Khadher, T.; Aydi, S.; Mars, M.; Bouajila, J. Study on the Chemical Composition and the Biological Activities of Vitis vinifera Stem Extracts. Molecules 2022, 27, 3109. [Google Scholar] [CrossRef]
- Nieto, J.A.; Jaime, L.; Arranz, E.; Reglero, G.; Santoyo, S. Winemaking by products as anti-inflammatory food ingredients. Food Agric. Immunol. 2017, 28, 1507–1518. [Google Scholar] [CrossRef] [Green Version]
- Domínguez-Perles, R.; Teixeira, A.; Rosa, E.; Barros, A. Assessment of (poly)phenols in grape (Vitis vinifera L.) stems by using food/pharma industry compatible solvents and response surface methodology. Food Chem. 2014, 164, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Tabart, J.; Kevers, C.; Pincemail, J.; Defraigne, J.O.; Dommes, J. Comparative antioxidant capacities of phenolic compounds measured by various tests. Food Chem. 2009, 113, 1226–1233. [Google Scholar] [CrossRef]
- Gomes, T.M.; Toaldo, I.M.; da Silva Haas, I.C.; Burin, V.M.; Caliari, V.; Luna, A.S.; Santos, J.; Bordignon-Luiz, M.T. Differential contribution of grape peel, pulp, and seed to bioaccessibility of micronutrients and major polyphenolic compounds of red and white grapes through simulated human digestion. J. Funct. Foods 2019, 52, 699–708. [Google Scholar] [CrossRef]
- Rocchetti, G.; Rizzi, C.; Cervini, M.; Rainero, G.; Bianchi, F.; Giuberti, G.; Lucini, L.; Simonato, B. Impact of Grape Pomace Powder on the Phenolic Bioaccessibility and on in vitro Starch Digestibility of Wheat Based Bread. Foods 2021, 10, 507. [Google Scholar] [CrossRef]
- Jara-Palacios, M.J.; Gonçalves, S.; Hernanz, D.; Heredia, F.J.; Romano, A. Effects of in vitro gastrointestinal digestion on phenolic compounds and antioxidant activity of different white winemaking byproducts extracts. Food Res. Int. 2018, 109, 433–439. [Google Scholar] [CrossRef]
- Ferreyra, S.; Bottini, R.; Fontana, A. Tandem absorbance and fluorescence detection following liquid chromatography for the profiling of multiclass phenolic compounds in different winemaking products. Food Chem. 2021, 338, 128030. [Google Scholar] [CrossRef]
- Ferreyra, S.; Torres-Palazzolo, C.; Bottini, R.; Camargo, A.; Fontana, A. Assessment of in-vitro bioaccessibility and antioxidant capacity of phenolic compounds extracts recovered from grapevine bunch stem and cane by-products. Food Chem. 2021, 348, 129063. [Google Scholar] [CrossRef]
- Lingua, M.S.; Theumer, M.G.; Kruzynski, P.; Wunderlin, D.A.; Baroni, M.V. Bioaccessibility of polyphenols and antioxidant properties of the white grape by simulated digestion and Caco-2 cell assays: Comparative study with its winemaking product. Food Res. Int. 2019, 122, 496–505. [Google Scholar] [CrossRef]
- Konishi, Y.; Kobayasi, S.; Shimizu, M. Transepithelial transport of p-coumaric acid and gallic acid in caco-2 cell monolayers. Biosci. Biotechnol. Biochem. 2003, 67, 2317–2324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deprez, S.; Mila, I.; Huneau, J.F.; Tome, D.; Scalbert, A. Transport of procyanidin dimer, trimer and polymer across monolayers of human intestinal epithelial caco-2 cells. Antioxid. Redox Signal. 2001, 3, 957–967. [Google Scholar] [CrossRef]
- Vaz, A.; Odriozola-Serrano, I.; Oms-Oliu, G.; Martin-Belloso, O. Physicochemical Properties and Bioaccessibility of Phenolic Compounds of Dietary Fibre Concentrates from Vegetable By-Products. Foods 2022, 11, 2578. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, Y.; Li, S.; Chen, M.; Xiao, J.; Xie, B.; Sun, Z. Oligomer procyanidins from lotus seedpod regulate lipid homeostasis partially by modifying fat emulsification and digestion. J. Agric. Food Chem. 2019, 67, 4524–4534. [Google Scholar] [CrossRef]
- He, Z.; Zhu, H.; Xu, M.; Zeng, M.; Qin, F.; Chen, J. Complexation of bovine β-lactoglobulin with malvidin-3-O-glucoside and its effect on the stability of grape skin anthocyanin extracts. Food Chem. 2016, 209, 234–240. [Google Scholar] [CrossRef]
- Rodríguez-Roque, M.J.; Rojas-Graü, M.A.; Elez-Martínez, P.; Martín-Belloso, O. In vitro bioaccessibility of health-related compounds as affected by the formulation of fruit juice- and milk-based beverages. Food Res. Int. 2014, 62, 771–778. [Google Scholar] [CrossRef]
- Nieto, J.A.; Santoyo, S.; Prodanov, M.; Reglero, G.; Jaime, L. Valorisation of grape stems as a source of phenolic antioxidants by using a sustainable extraction methodology. Foods 2020, 9, 604. [Google Scholar] [CrossRef]
- Arranz, E.; Santoyo, S.; Jaime, L.; Fornari, T.; Reglero, G.; Guri, A.; Corredig, M. Improved bioavailability of supercritical rosemary extract through encapsulation in different delivery systems after in vitro digestion. J. Funct. Foods 2015, 13, 384–390. [Google Scholar] [CrossRef] [Green Version]
- González-Centeno, M.R.; Rosselló, C.; Simal, S.; Garau, M.C.; López, F.; Femenia, A. Physico-chemical properties of cell wall materials obtained from ten grape varieties and their byproducts: Grape pomaces and stems. LWT-Food Sci. Technol. 2010, 43, 1580–1586. [Google Scholar] [CrossRef]
- Mangione, R.; Simões, R.; Pereira, H.; Catarino, S.; Ricardo-da-Silva, J.; Miranda, I.; Ferreira-Dias, S. Potential Use of Grape Stems and Pomaces from Two Red Grapevine Cultivars as Source of Oligosaccharides. Processes 2022, 10, 1896. [Google Scholar] [CrossRef]
- Spigno, G.; Maggi, L.; Amendola, D.; Dragoni, M.; de Faveri, D.M. Influence of cultivar on the lignocellulosic fractionation of grape stalks. Ind. Crops Prod. 2013, 46, 283–289. [Google Scholar] [CrossRef]
- De Sá, M.; Justino, V.; Spranger, M.I.; Zhao, Y.Q.; Hanc, L.; Sun, B. Extraction yields and anti-oxidant activity of procyanidins from different parts of grape pomace: Effect of mechanical treatments. Phytochem. Anal. 2014, 25, 134–140. [Google Scholar] [CrossRef]
- Souquet, J.M.; Labarbe, B.; Le Guernevé, C.; Cheynier, V.; Moutounet, M. Phenolic composition of grape stems. J. Agric. Food Chem. 2000, 48, 1076–1080. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Dong, W.; Long, Y.; Zhao, J.; Hu, R.; Zhang, Y.; Zhu, K. Evaluation of the impact of different drying methods on the phenolic compounds, antioxidant activity, and in vitro digestion of green coffee beans. Food Sci. Nutr. 2019, 7, 1084–1095. [Google Scholar] [CrossRef]
- Tagliazucchi, D.; Verzelloni, E.; Bertolini, D.; Conte, A. In vitro bio-accessibility and antioxidant activity of grape polyphenols. Food Chem. 2010, 120, 599–606. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, S.J.; Yim, D.G.; Hur, S.J. Changes in the content and bioavailability of onion quercetin and grape resveratrol during in vitro human digestion. Foods 2020, 9, 694. [Google Scholar] [CrossRef] [PubMed]
- Garbetta, A.; Nicassio, L.; D’Antuono, I.; Cardinali, A.; Linsalata, V.; Attolico, G.; Minervini, F. Influence of in vitro digestion process on polyphenolic profile of skin grape (cv. Italia) and on antioxidant activity in basal or stressed conditions of human intestinal cell line (HT-29). Food Res. Int. 2018, 106, 878–884. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Chen, F.; Zhao, G. Effects of molecular structure of polyphenols on their noncovalent interactions with oat b-glucan. J. Agric. Food Chem. 2013, 61, 4533–4538. [Google Scholar] [CrossRef] [PubMed]
- Ortega, N.; Reguant, J.; Romero, M.P.; Maciᾲ, A.; Motilva, M.J. Effect of fat content on the digestibility and bioaccessibility of cocoa polyphenol by an in vitro-digestion model. J. Agric. Food Chem. 2009, 57, 5743–5749. [Google Scholar] [CrossRef]
- McDougall, G.J.; Fyffe, S.; Dobson, P.; Stewart, D. Anthocyanins from red wine—Their stability under simulated gastrointestinal digestion. Phytochemistry 2005, 66, 2540–2548. [Google Scholar] [CrossRef] [PubMed]
- Serra, A.; Macia, A.; Romero, M.P.; Valls, J.; Bladé, C.; Arola, L.; Motilva, M.J. Bioavailability of procyanidin dimers and trimers and matrix food effects in in vitro and in vivo models. Br. J. Nutr. 2010, 103, 944–952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toro-Uribe, S.; López-Giraldo, L.J.; Alvarez-Rivera, G.; Ibáñez, E.; Herrero, M. Insight of Stability of Procyanidins in Free and Liposomal Form under an in vitro Digestion Model: Study of Bioaccessibility, Kinetic Release Profile, Degradation, and Antioxidant Activity. J. Agric. Food Chem. 2019, 67, 1990–2003. [Google Scholar] [CrossRef] [PubMed]
- Krook, M.A.; Hagerman, A.E. Stability of polyphenols epigallocatechin gallate and pentagalloyl glucose in a simulated digestive system. Food Res. Int. 2012, 49, 112–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neilson, A.; Hopf, A.; Cooper, B.; Pereira, M.A.; Bomser, J.A.; Ferruzzi, M.G. Catechin degradation with concurrent formation of homo- and heterocatechin dimers during in vitro digestion. J. Agric. Food Chem. 2007, 55, 8941–8949. [Google Scholar] [CrossRef]
- Laurent, C.; Besançon, P.; Caporiccio, B. Flavonoids from a grape seed extract interact with digestive secretions and intestinal cells as assessed in an in vitro digestion/Caco-2 cell culture model. Food Chem. 2007, 100, 1704–1712. [Google Scholar] [CrossRef]
- Durán-Castañeda, A.C.; Cardenas-Castro, A.P.; Pérez-Jiménez, J.; Pérez-Carvajal, A.M.; Sánchez-Burgos, J.A.; Mateos, R.; Sáyago-Ayerdi, S.G. Bioaccessibility of phenolic compounds in Psidium guajava L. varieties and P. friedrichsthalianum Nied. after gastrointestinal digestion. Food Chem. 2023, 400, 134046. [Google Scholar] [CrossRef]
- Wang, J.; Xie, B.; Sun, Z. Anion carboxymethylated β-glucan alleviates undesirable binding between procyanidins and β-galactosidase. Food Chem. 2021, 344, 128686. [Google Scholar] [CrossRef]
- Zhong, H.; Xue, Y.; Lu, X.; Shao, Q.; Cao, Y.; Wu, Z.; Chen, G. The effects of different degrees of procyanidin polymerization on the nutrient absorption and digestive enzyme activity in mice. Molecules 2018, 23, 2916. [Google Scholar] [CrossRef] [Green Version]
- Abia, R.; Fry, S.C. Degradation and metabolism of 14C-labelled proanthocyanidins from carob (Ceratonia siliqua) pods in the gastrointestinal tract of the rat. J. Sci. Food Agric. 2001, 81, 1156–1165. [Google Scholar] [CrossRef]
- Gonthier, M.P.; Donovan, J.L.; Texier, O.; Felgines, C.; Remesy, C.; Scalbert, A. Metabolism of dietary procyanidins in rats. Free. Radic. Biol. Med. 2003, 35, 837–844. [Google Scholar] [CrossRef]
- Arimboor, R.; Arumugham, C. Sea buckthorn (Hippophae rhamnoides) procyanidins inhibit in vitro enzymatic hydrolysis of protein. J. Food Sci. 2011, 76, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Crespy, V.; Morand, C.; Besson, C.; Cotelle, N.; Vézin, H.; Demigné, C.; Rémésy, C. The splanchnic metabolism of flavonoids highly differed according to the nature of the compound. Am. J. Physiol. Gastrointest. 2003, 284, G980–G988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-Juaristi, M.; Sarria, B.; Goya, L.; Bravo-Clemente, L.; Mateos, R. Experimental confounding factors affecting stability, transport and metabolism of flavanols and hydroxycinnamic acids in Caco-2 cells. Food Res. Int. 2020, 129, 108797. [Google Scholar] [CrossRef] [PubMed]
- Said, I.H.; Truex, J.D.; Heidorn, C.; Retta, M.B.; Petrov, D.D.; Haka, S.; Kuhnert, N. LC-MS/MS based molecular networking approach for the identification of cocoa phenolic metabolites in human urine. Food Res. Int. 2020, 132, 109119. [Google Scholar] [CrossRef]
- Pereira-Caro, G.; Gaillet, S.; Ordóñez, J.L.; Mena, P.; Bresciani, L.; Bindon, K.A.; Del Rio, D.; Rounet, J.M.; Moreno Rojas, J.M.; Crozier, A. Bioavailability of red wine and grape seed proanthocyanidins in rats. Food Func. 2020, 11, 3986–4001. [Google Scholar] [CrossRef]
- Rosa, N.N.; Dufour, C.; Lullien-Pellerin, V.; Micard, V. Exposure or release of ferulic acid from wheat aleurone: Impact on its antioxidant capacity. Food Chem. 2013, 141, 2355–2362. [Google Scholar] [CrossRef]
- Rodríguez-González, S.; Pérez-Ramírez, I.F.; Castaño-Tostado, E.; Amaya-Llano, S.; Rodríguez-García, M.E.; Reynoso-Camacho, R. Improvement of physicochemical properties and phenolic compounds bioavailability by concentrating dietary fiber of peach (Prunus persica) juice by-product. J. Sci. Food Agric. 2018, 98, 3109–3118. [Google Scholar]
- Association of Official Analytical Chemists; Association of Official Agricultural Chemists. Official Methods of Analysis, 16th ed.; Association of Official Analytical Chemist: Washington, DC, USA, 1995. [Google Scholar]
- Ribeiro-Smiderle, F.; Morales, D.; Gil-Ramírez, A.; de Jesus, L.I.; Gilbert-López, B.; Iacominia, M.; Soler-Rivas, C. Evaluation of microwave-assisted and pressurized liquid extractions to obtain β-d-glucans from mushrooms. Carbohyd. Polym. 2017, 156, 165–174. [Google Scholar] [CrossRef]
- Lee, S.G.; Prosky, L.; De Vries, J.W. Determination of total, soluble and insoluble dietary fibre in foods–enzymatic-gravimetric method, MES-TRIS buffer: Collaborative study. J. AOAC Int. 1992, 75, 395–416. [Google Scholar] [CrossRef]
- Sun, B.; Leandro, C.; Ricardo-da-Silva, J.M.; Spranger, M.I. Separation of grape and wine procyanidins according to their degree of polymerization. J. Agric. Food Chem. 1998, 46, 1390–1396. [Google Scholar] [CrossRef]
- Sun, B.; Ricardo-da-Silva, J.M.; Spranger, M.I. Critical factors of vainillin assay for catechins and procyanidins. J. Agric. Food Chem. 1998, 46, 4267–4274. [Google Scholar] [CrossRef]
- Prodanov, M.; Garrido, I.; Vacas, V.; Lebrón-Aguilar, R.; Dueñas, M.; Gómez-Cordovés, C.; Bartolomé, B. Ultrafiltration as alternative purification procedure for the characterization of low and high molecular-mass phenolics from almond skins. Anal. Chim. Acta 2008, 609, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Soler-Rivas, C.; Marín, F.R.; Santoyo, S.; García-Risco, M.R.; Señorans, F.J.; Reglero, G. Testing and enhancing the in vitro bioaccessibility and bioavailability of Rosmarinus officinalis extracts with high levels of antioxidant abietanes. J. Agric. Food Chem. 2010, 58, 1144–1152. [Google Scholar] [CrossRef] [PubMed]


| Main Components | HPE | LPE |
|---|---|---|
| Proteins | 14.3 ± 0.1 * | 1.6 ± 0.0 |
| Fat | 13.9 ± 0.7 | 35.7 ± 1.9 * |
| Soluble sugars | 24.8 ± 1.3 | 28.7 ± 0.9 * |
| Hemicelluloses | 7.4 ± 0.6 * | - |
| Ash | 15.8 ± 0.6 * | 1.2 ± 0.1 |
| Total phenolic content | 18.5 ± 0.3 * | 8.7 ± 0.0 |
| Procyanidins | 8.1 ± 0.7 * | 1.1 ± 0.1 |
| Phenolic Compounds | HPE | LPE |
|---|---|---|
| Hydroxybenzoic acids | ||
| Gallic acid | 0.541 ± 0.029 | 0.726 ± 0.038 * |
| Protocatechuic acid | 0.008 ± 0.000 | 0.017 ± 0.000 * |
| Monogalloyl glucoside | <LoQ | 0.001 ± 0.000 * |
| 4-Hydroxybenzoic acid | 0.048 ± 0.001 | 0.173 ± 0.001 * |
| Vanillic acid | 0.224 ± 0.010 | 0.505 ± 0.016 * |
| Syringic acid | 0.202 ± 0.015 | 0.489 ± 0.020 * |
| Ethyl gallate | 0.010 ± 0.001 | 0.015 ± 0.002 |
| Ellagic acid | 0.073 ± 0.004 | 0.130 ± 0.007 * |
| Hydroxycinnamic acids | ||
| Caftaric acid | 0.357 ± 0.003 | 0.183 ± 0.001 * |
| Caffeic acid | 0.006 ± 0.000 | 0.014 ± 0.001 * |
| 3-Coumaric acid | 0.003 ± 0.000 | <LoD |
| Stilbenes | ||
| trans-Piceid | 0.016 ± 0.000 | 0.049 ± 0.002 * |
| trans-Resveratrol | 0.141 ± 0.003 | 1.215 ± 0.036 * |
| Flavan-3-ols | ||
| Catechin | 2.422 ± 0.034 | 6.662 ± 0.381 * |
| Epicatechin | 1.293 ± 0.039 | 1.435 ± 0.073 * |
| Epicatechin gallate | 0.245 ± 0.005 | 0.466 ± 0.009 * |
| Dimer B1 | 1.410 ± 0.034 | 1.028 ± 0.055 * |
| Dimer B2 | 0.349 ± 0.025 | Co |
| Flavonols | ||
| Quercetin-3-O-galactoside | 0.047 ± 0.000 | 0.046 ± 0.001 |
| Quercetin-3-O-rutinoside | 0.029 ± 0.000 | 0.048 ± 0.005 * |
| Quercetin-3-O-glucuronide | 1.425 ± 0.001 | 0.368 ± 0.012 * |
| Quercetin-3-O-glucoside | 0.106 ± 0.004 | 0.167 ± 0.009 * |
| Quercetin | 0.005 ± 0.000 | 0.019 ± 0.001 * |
| Σ Phenolic compounds | 8.96 ± 0.008 | 13.76 ± 0.013 * |
| Phenolic Compounds | Initial | Oral | Stomach | Intestinal |
|---|---|---|---|---|
| Hydroxybenzoic acids | ||||
| Gallic acid | 0.622 ± 0.003 b | 0.645 ± 0.001 a | 0.540 ± 0.002 c | 0.270 ± 0.024 d |
| Protocatechuic acid | 0.010 ± 0.000 a | 0.011 ± 0.000 a | 0.010 ± 0.000 a | 0.011 ± 0.002 a |
| Monogalloyl glucoside | 0.012 ± 0.001 a | 0.010 ± 0.000 b | <LoD | <LoD |
| 4-Hydroxybenzoic acid | 0.058 ± 0.003 c | 0.060 ± 0.002 c | 0.068 ± 0.002 b | 0.074 ± 0.004 a |
| Vanillic acid | 0.209 ± 0.013 a | 0.201 ± 0.019 b | Co | Co |
| Syringic acid | 0.277 ± 0.004 b | 0.261 ± 0.012 c | 0.291 ± 0.002 a | 0.295 ± 0.008 a |
| Ethyl gallate | 0.010 ± 0.001 c | 0.010 ± 0.002 c | 0.014 ± 0.000 b | 0.021 ± 0.001 a |
| Ellagic acid | 0.077 ± 0.004 a | 0.072 ± 0.002 b | 0.037 ± 0.001 d | 0.046 ± 0.001 c |
| Hydroxycinnamic acids | ||||
| Caftaric acid | 0.165 ± 0.002 b | 0.173 ± 0.000 a | 0.148 ± 0.001 c | 0.130 ± 0.005 d |
| Caffeic acid | 0.006 ± 0.000 b | 0.006 ± 0.000 b | 0.011 ± 0.000 a | Co |
| 3-Coumaric acid | 0.003 ± 0.000 a | 0.003 ± 0.000 a | <LoD | <LoD |
| Stilbenes | ||||
| trans-Piceid | 0.014 ± 0.000 a | 0.015 ± 0.000 a | 0.014 ± 0.000 b | 0.014 ± 0.000 b |
| trans-Resveratrol | 0.263 ± 0.004 a | 0.273 ± 0.001 a | 0.218 ± 0.001 b | 0.199 ± 0.015 c |
| Flavan-3-ols | ||||
| Catechin | 2.749 ± 0.010 a | 2.755 ± 0.169 a | 1.885 ± 0.002 b | 0.368 ± 0.037 c |
| Epicatechin | 0.851 ± 0.003 a | 0.892 ± 0.023 a | 0.683 ± 0.001 b | 0.052 ± 0.029 c |
| Epicatechin gallate | 0.230 ± 0.003 a | 0.234 ± 0.004 a | <LoD | <LoD |
| Dimer B1 | 1.562 ± 0.004 a | 1.577 ± 0.055 a | 1.434 ± 0.004 b | 0.248 ± 0.044 c |
| Dimer B2 | 0.508 ± 0.027 a | 0.521 ± 0.010 a | 0.293 ± 0.007 b | Co |
| Flavonols | ||||
| Quercetin-3-O-galactoside | 0.018 ± 0.001 a | 0.018 ± 0.001 a | 0.005 ± 0.001 c | 0.009 ± 0.003 b |
| Quercetin-3-O-rutinoside | 0.019 ± 0.001 a | 0.018 ± 0.001 a | 0.015 ± 0.001 b | 0.014 ± 0.002 b |
| Quercetin-3-O-glucuronide | 0.784 ± 0.010 a | 0.797 ± 0.006 a | 0.555 ± 0.002 c | 0.629 ± 0.051 b |
| Quercetin-3-O-glucoside | 0.106 ± 0.004 a | 0.110 ± 0.004 a | 0.082 ± 0.002 b | 0.073 ± 0.003 c |
| Quercetin | 0.004 ± 0.000 a | 0.005 ± 0.000 a | 0.001 ± 0.000 b | < LoD |
| Σ Phenolic compounds | 8.557 ± 0.006 a | 8.670 ± 0.013 a | 6.287 ± 0.001 b | 2.454 ± 0.010 c |
| Phenolic Compounds | Initial | Oral | Stomach | Intestinal |
|---|---|---|---|---|
| Hydroxybenzoic acids | ||||
| Gallic acid | 0.726 ± 0.038 a,b | 0.735 ± 0.042 a,b | 0.788 ± 0.033 a | 0.671 ± 0.047 b |
| Protocatechuic acid | 0.017 ± 0.002 a | 0.017 ± 0.000 a | 0.019 ± 0.000 a | 0.017 ± 0.002 a |
| Monogalloyl glucoside | 0.005 ± 0.002 a | 0.005 ± 0.000 a | < LoD | < LoD |
| 4-Hydroxybenzoic acid | 0.173 ± 0.011 b | 0.182 ± 0.009 a,b | 0.168 ± 0.011 b | 0.194 ± 0.004 a |
| Vanillic acid | 0.505 ± 0.016 a | 0.493 ± 0.012 a | Co | Co |
| Syringic acid | 0.489 ± 0.020 b | 0.535 ± 0.018 a | 0.507 ± 0.002 a,b | 0.485 ± 0.029 b |
| Ethyl gallate | 0.015 ± 0.002 c | 0.014 ± 0.001 c | 0.019 ± 0.000 b | 0.021 ± 0.000 a |
| Ellagic acid | 0.131 ± 0.007 a | 0.130 ± 0.009 a | 0.012 ± 0.000 c | 0.035 ± 0.005 b |
| Hydroxycinnamic acids | ||||
| Caftaric acid | 0.018 ± 0.001 a | 0.018 ± 0.001 a | 0.019 ± 0.000 a | 0.018 ± 0.002 a |
| Caffeic acid | 0.015 ± 0.003 a | 0.018 ± 0.001 a | 0.012 ± 0.000 b | 0.011 ± 0.002 b |
| 3-Coumaric acid | <LoD | <LoD | <LoD | <LoD |
| Stilbenes | ||||
| trans-Piceid | 0.049 ± 0.002 a | 0.050 ± 0.003 a | 0.047 ± 0.000 a | 0.041 ± 0.002 b |
| trans-Resveratrol | 1.215 ± 0.036 b | 1.366 ± 0.121 a,b | 1.415 ± 0.035 a | 1.499 ± 0.116 a |
| Flavan-3-ols | ||||
| Catechin | 6.662 ± 0.380 a | 6.954 ± 0.383 a | 3.754 ± 0.004 b | 2.102 ± 0.046 c |
| Epicatechin | 1.435 ± 0.073 a | 1.478 ± 0.008 a | 1.200 ± 0.008 b | 0.718 ± 0.060 c |
| Epicatechin gallate | 0.466 ± 0.009 a | 0.490 ± 0.022 a | <LoQ | 0.064 ± 0.003 b |
| Dimer B1 | 1.028 ± 0.055 b | 0.891 ± 0.076 b | 1.201 ± 0.007 a | 0.720 ± 0.095 c |
| Dimer B2 | Co | Co | Co | Co |
| Flavonols | ||||
| Quercetin-3-O-galactoside | 0.046 ± 0.001 a | 0.046 ± 0.004 a | 0.018 ± 0.000 c | 0.026 ± 0.001 b |
| Quercetin-3-O-rutinoside | 0.048 ± 0.005 a | 0.047 ± 0.008 a | 0.027 ± 0.001 c | 0.036 ± 0.000 b |
| Quercetin-3-O-glucuronide | 0.386 ± 0.012 a | 0.402 ± 0.002 a | 0.243 ± 0.002 c | 0.287 ± 0.013 b |
| Quercetin-3-O-glucoside | 0.167 ± 0.009 a | 0.163 ± 0.006 a | 0.167 ± 0.001 a | 0.163 ± 0.005 a |
| Quercetin | 0.009 ± 0.000 a | 0.010 ± 0.002 a | 0.001 ± 0.000 c | 0.003 ± 0.000 b |
| Σ Phenolic compounds | 13.604 ± 0.030 a | 14.046 ± 0.032 a | 9.605 ± 0.004 b | 7.111 ± 0.019 c |
| Extracts | HPE | LPE | ||||
|---|---|---|---|---|---|---|
| Phenolic Compounds | Digested | Apical | Basolateral | Digested | Apical | Basolateral |
| Hydroxybenzoic acids | ||||||
| Gallic acid | 0.270 ± 0.024 a | 0.217 ± 0.002 b | 0.064 ± 0.008 c | 0.671 ± 0.047 a | 0.279 ± 0.001 b | 0.132 ± 0.008 c |
| Protocatechuic acid | 0.011 ± 0.002 a | 0.008 ± 0.000 b | 0.002 ± 0.000 c | 0.017 ± 0.002 a | Co | 0.011 ± 0.001 b |
| Monogalloyl glucoside | <LoD | <LoD | <LoD | <LoD | <LoD | <LoD |
| 4-Hydroxybenzoic acid | 0.074 ± 0.004 a | 0.049 ± 0.012 b | 0.028 ± 0.004 c | 0.194 ± 0.004 a | 0.082 ± 0.003 c | 0.122 ± 0.012 b |
| Vanillic acid | Co | <LoD | <LoD | Co | Co | 0.301 ± 0.017 |
| Syringic acid | 0.295 ± 0.008 a | 0.212 ± 0.006 b | 0.076 ± 0.002 c | 0.485 ± 0.029 a | 0.345 ± 0.026 b | 0.218 ± 0.013 c |
| Ethyl gallate | 0.021 ± 0.000 a | 0.009 ± 0.002 b | <LoD | 0.021 ± 0.000 a | 0.010 ± 0.003 b | 0.004 ± 0.000 c |
| Ellagic acid | 0.046 ± 0.001 a | 0.032 ± 0.002 b | <LoD | 0.035 ± 0.005 a | 0.013 ± 0.000 b | <LoD |
| Hydroxycinnamic acids | ||||||
| Caftaric acid | 0.130 ± 0.005 a | 0.071 ± 0.003 b | 0.017 ± 0.001 c | 0.018 ± 0.002 | <LoQ | <LoQ |
| Caffeic acid | Co | <LoD | <LoD | 0.011 ± 0.002 a | <LoQ | 0.12 ± 0.001 b |
| 3-Coumaric acid | <LoD | <LoD | <LoD | <LoD | <LoD | <LoD |
| Stilbenes | ||||||
| trans-Piceid | 0.014 ± 0.000 a | 0.013 ± 0.000 b | 0.003 ± 0.000 c | 0.041 ± 0.002 a | 0.030 ± 0.000 b | 0.011 ± 0.001 c |
| trans-Resveratrol | 0.199 ± 0.015 a | 0.006 ± 0.000 c | 0.019 ± 0.000 b | 1.499 ± 0.116 a | 0.211 ± 0.005 c | 0.325 ± 0.010 b |
| Flavan-3-ols | ||||||
| Catechin | 0.368 ± 0.037 a | Co | 0.215 ± 0.001 b | 2.102 ± 0.046 a | 1.249 ± 0.064 b | 0.688 ± 0.006 c |
| Epicatechin | 0.052 ± 0.029 | <LoD | <LoD | 0.718 ± 0.060 a | 0.330 ± 0.060 b | 0.174 ± 0.010 c |
| Epicatechin gallate | <LoD | <LoD | <LoD | 0.064 ± 0.003 | <LoD | <LoD |
| Dimer B1 | 0.248 ± 0.044 a | 0.113 ± 0.008 b | <LoD | 0.720 ± 0.095 a | 0.127 ± 0.019 b | 0.083 ± 0.002 c |
| Dimer B2 | Co | <LoD | <LoD | Co | <LoD | <LoD |
| Flavonols | ||||||
| Quercetin-3-O-galactoside | 0.009 ± 0.003 a | 0.009 ± 0.000 a | <LoD | 0.026 ± 0.001 a | 0.018 ± 0.001 b | 0.002 ± 0.000 c |
| Quercetin-3-O-rutinoside | 0.014 ± 0.002 a | 0.013 ± 0.000 a | <LoD | 0.036 ± 0.000 a | 0.025 ± 0.001 b | <LoD |
| Quercetin-3-O-glucuronide | 0.629 ± 0.051 a | 0.507 ± 0.004 b | 0.040 ± 0.001 c | 0.287 ± 0.013 a | 0.238 ± 0.005 b | 0.056 ± 0.008 c |
| Quercetin-3-O-glucoside | 0.073 ± 0.003 a | 0.038 ± 0.000 b | <LoQ | 0.163 ± 0.005 a | 0.061 ± 0.003 b | 0.047 ± 0.002 c |
| Quercetin | <LoD | <LoD | <LoD | 0.003 ± 0.000 a | 0.001 ± 0.000 b | <LoQ |
| Σ Phenolic compounds | 2.454 ± 0.010 a | 1.434 ± 0.001 b | 0.536 ± 0.001 c | 7.111 ± 0.017 a | 3.087 ± 0.008 b | 2.274 ± 0.004 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nieto, J.A.; Fernández-Jalao, I.; Siles-Sánchez, M.d.l.N.; Santoyo, S.; Jaime, L. Implication of the Polymeric Phenolic Fraction and Matrix Effect on the Antioxidant Activity, Bioaccessibility, and Bioavailability of Grape Stem Extracts. Molecules 2023, 28, 2461. https://doi.org/10.3390/molecules28062461
Nieto JA, Fernández-Jalao I, Siles-Sánchez MdlN, Santoyo S, Jaime L. Implication of the Polymeric Phenolic Fraction and Matrix Effect on the Antioxidant Activity, Bioaccessibility, and Bioavailability of Grape Stem Extracts. Molecules. 2023; 28(6):2461. https://doi.org/10.3390/molecules28062461
Chicago/Turabian StyleNieto, Juan Antonio, Irene Fernández-Jalao, María de las Nieves Siles-Sánchez, Susana Santoyo, and Laura Jaime. 2023. "Implication of the Polymeric Phenolic Fraction and Matrix Effect on the Antioxidant Activity, Bioaccessibility, and Bioavailability of Grape Stem Extracts" Molecules 28, no. 6: 2461. https://doi.org/10.3390/molecules28062461
APA StyleNieto, J. A., Fernández-Jalao, I., Siles-Sánchez, M. d. l. N., Santoyo, S., & Jaime, L. (2023). Implication of the Polymeric Phenolic Fraction and Matrix Effect on the Antioxidant Activity, Bioaccessibility, and Bioavailability of Grape Stem Extracts. Molecules, 28(6), 2461. https://doi.org/10.3390/molecules28062461