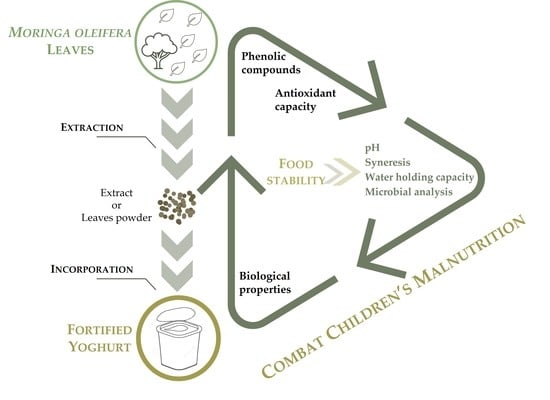
Incorporation of Moringa oleifera Leaf Extract in Yoghurts to Mitigate Children’s Malnutrition in Developing Countries
Abstract
:1. Introduction
2. Results and Discussion
2.1. Extraction of Bioactive Compounds from M. oleifera Leaves
2.2. Characterization of M. oleifera Extract
2.3. Characterization of Fortified Yoghurts
2.3.1. Physicochemical Properties
2.3.2. Antioxidant and Antibacterial Activities
2.3.3. Microbial Analysis
3. Materials and Methods
3.1. Samples and Reagents
3.1.1. Samples
3.1.2. Reagents
3.1.3. Analytical Standards for HPLC-DAD Analysis
3.2. Extraction of Bioactive Compounds from Moring Leaf Powder
3.3. Characterization of M. oleifera Extract
3.3.1. Total Phenolic Content
3.3.2. Antioxidant Capacity
3.3.3. Antibacterial Activity
3.3.4. Analysis of Phenolic Compounds by HPLC-DAD
3.4. Incorporation of M. oleifera Extract in Yoghurt
3.4.1. Yoghurt Production
3.4.2. pH Determination
3.4.3. Syneresis
3.4.4. Water Holding Capacity
3.4.5. Viscosity
3.4.6. Total Phenolic Content and Antioxidant Capacity
3.4.7. Antibacterial Activity
3.4.8. Microbial Safety
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Azlan, U.K.; Mediani, A.; Rohani, E.R.; Tong, X.; Han, R.; Misnan, N.M.; Jam, F.A.; Bunawan, H.; Sarian, M.N.; Hamezah, H.S. A Comprehensive Review with Updated Future Perspectives on the Ethnomedicinal and Pharmacological Aspects of Moringa oleifera. Molecules 2022, 27, 5765. [Google Scholar] [CrossRef] [PubMed]
- Omodanisi, E.I.; Aboua, Y.G.; Oguntibeju, O.O. Assessment of the Anti-Hyperglycaemic, Anti-Inflammatory and Antioxidant Activities of the Methanol Extract of Moringa oleifera in Diabetes-Induced Nephrotoxic Male Wistar Rats. Molecules 2017, 22, 439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panda, S.; Kar, A.; Sharma, P.; Sharma, A. Cardioprotective potential of N,α-l-rhamnopyranosyl vincosamide, an indole alkaloid, isolated from the leaves of Moringa oleifera in isoproterenol induced cardiotoxic rats: In vivo and in vitro studies. Bioorg. Med. Chem. Lett. 2013, 23, 959–962. [Google Scholar] [CrossRef] [PubMed]
- Arulselvan, P.; Tan, W.S.; Gothai, S.; Muniandy, K.; Fakurazi, S.; Esa, N.M.; Alarfaj, A.A.; Kumar, S.S. Anti-Inflammatory Potential of Ethyl Acetate Fraction of Moringa oleifera in Downregulating the NF-κB Signaling Pathway in Lipopolysaccharide-Stimulated Macrophages. Molecules 2016, 21, 1452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peixoto, J.R.O.; Silva, G.C.; Costa, R.A.; Fontenelle, J.R.L.D.S.; Vieira, G.H.F.; Filho, A.A.F.; Vieira, R.H.S.D.F. In vitro antibacterial effect of aqueous and ethanolic Moringa leaf extracts. Asian Pac. J. Trop. Med. 2011, 4, 201–204. [Google Scholar] [CrossRef] [Green Version]
- Luetragoon, T.; Sranujit, R.P.; Noysang, C.; Thongsri, Y.; Potup, P.; Suphrom, N.; Nuengchamnong, N.; Usuwanthim, K. Anti-Cancer Effect of 3-Hydroxy-β-Ionone Identified from Moringa oleifera Lam. Leaf on Human Squamous Cell Carcinoma 15 Cell Line. Molecules 2020, 25, 3563. [Google Scholar] [CrossRef]
- Paula, P.C.; Sousa, D.O.B.; Oliveira, J.T.A.; Carvalho, A.F.U.; Alves, B.G.T.; Pereira, M.L.; Farias, D.F.; Viana, M.P.; Santos, F.A.; Morais, T.C.; et al. A Protein Isolate from Moringa oleifera Leaves Has Hypoglycemic and Antioxidant Effects in Alloxan-Induced Diabetic Mice. Molecules 2017, 22, 271. [Google Scholar] [CrossRef] [Green Version]
- Singh, D.; Arya, P.V.; Aggarwal, V.P.; Gupta, R.S. Evaluation of Antioxidant and Hepatoprotective Activities of Moringa oleifera Lam. Leaves in Carbon Tetrachloride-Intoxicated Rats. Antioxidants 2014, 3, 569–591. [Google Scholar] [CrossRef] [Green Version]
- Zeb, A. Concept, mechanism, and applications of phenolic antioxidants in foods. J. Food Biochem. 2020, 44, e13394. [Google Scholar] [CrossRef]
- Nizioł-Łukaszewska, Z.; Furman-Toczek, D.; Bujak, T.; Wasilewski, T.; Hordyjewicz-Baran, Z. Moringa oleifera L. Extracts as Bioactive Ingredients That Increase Safety of Body Wash Cosmetics. Dermatol. Res. Pract. 2020, 2020, 8197902. [Google Scholar] [CrossRef]
- Vonghirundecha, P.; Chusri, S.; Meunprasertdee, P.; Kaewmanee, T. Microencapsulated functional ingredients from a Moringa oleifera leaf polyphenol-rich extract: Characterization, antioxidant properties, in vitro simulated digestion, and storage stability. LWT 2021, 154, 112820. [Google Scholar] [CrossRef]
- Ademiluyi, A.O.; Aladeselu, O.H.; Oboh, G.; Boligon, A.A. Drying alters the phenolic constituents, antioxidant properties, α-amylase, and α-glucosidase inhibitory properties of Moringa (Moringa oleifera) leaf. Food Sci. Nutr. 2018, 6, 2123–2133. [Google Scholar] [CrossRef] [Green Version]
- Kashyap, P.; Kumar, S.; Riar, C.S.; Jindal, N.; Baniwal, P.; Guiné, R.P.F.; Correia, P.M.R.; Mehra, R.; Kumar, H. Recent Advances in Drumstick (Moringa oleifera) Leaves Bioactive Compounds: Composition, Health Benefits, Bioaccessibility, and Dietary Applications. Antioxidants 2022, 11, 402. [Google Scholar] [CrossRef]
- Mohammed, E.A.; Abdalla, I.G.; Alfawaz, M.A.; Mohammed, M.A.; Al Maiman, S.A.; Osman, M.A.; Yagoub, A.E.A.; Hassan, A.B. Effects of Extraction Solvents on the Total Phenolic Content, Total Flavonoid Content, and Antioxidant Activity in the Aerial Part of Root Vegetables. Agriculture 2022, 12, 1820. [Google Scholar] [CrossRef]
- Irfan, S.; Ranjha, M.M.A.N.; Nadeem, M.; Safdar, M.N.; Jabbar, S.; Mahmood, S.; Murtaza, M.A.; Ameer, K.; Ibrahim, S.A. Antioxidant Activity and Phenolic Content of Sonication- and Maceration-Assisted Ethanol and Acetone Extracts of Cymbopogon citratus Leaves. Separations 2022, 9, 244. [Google Scholar] [CrossRef]
- Lohvina, H.; Sándor, M.; Wink, M. Effect of Ethanol Solvents on Total Phenolic Content and Antioxidant Properties of Seed Extracts of Fenugreek (Trigonella foenum-graecum L.) Varieties and Determination of Phenolic Composition by HPLC-ESI-MS. Diversity 2021, 14, 7. [Google Scholar] [CrossRef]
- Jiménez-Moreno, N.; Volpe, F.; Moler, J.A.; Esparza, I.; Ancín-Azpilicueta, C. Impact of Extraction Conditions on the Phenolic Composition and Antioxidant Capacity of Grape Stem Extracts. Antioxidants 2019, 8, 597. [Google Scholar] [CrossRef] [Green Version]
- Hikmawanti, N.P.E.; Fatmawati, S.; Asri, A.W. The Effect of Ethanol Concentrations as The Extraction Solvent on Antioxidant Activity of Katuk (Sauropus androgynus (L.) Merr.) Leaves Extracts. IOP Conf. Ser. Earth Environ. Sci. 2021, 755, 012060. [Google Scholar] [CrossRef]
- Zhao, B.; Deng, J.; Li, H.; He, Y.; Lan, T.; Wu, D.; Gong, H.; Zhang, Y.; Chen, Z. Optimization of Phenolic Compound Extraction from Chinese Moringa oleifera Leaves and Antioxidant Activities. J. Food Qual. 2019, 2019, 5346279. [Google Scholar] [CrossRef] [Green Version]
- Pollini, L.; Tringaniello, C.; Ianni, F.; Blasi, F.; Manes, J.; Cossignani, L. Impact of Ultrasound Extraction Parameters on the Antioxidant Properties of Moringa oleifera Leaves. Antioxidants 2020, 9, 277. [Google Scholar] [CrossRef]
- Islam, Z.; Islam, S.M.R.; Hossen, F.; Mahtab-Ul-Islam, K.; Hasan, R.; Karim, R. Moringa oleifera is a Prominent Source of Nutrients with Potential Health Benefits. Int. J. Food Sci. 2021, 2021, 6627265. [Google Scholar] [CrossRef] [PubMed]
- Dhakad, A.K.; Ikram, M.; Sharma, S.; Khan, S.; Pandey, V.V.; Singh, A. Biological, nutritional, and therapeutic significance of Moringa oleifera Lam. Phytother. Res. 2019, 33, 2870–2903. [Google Scholar] [CrossRef] [PubMed]
- Asare, G.A.; Gyan, B.; Bugyei, K.; Adjei, S.; Mahama, R.; Addo, P.; I-Nyarko, L.; Wiredu, E.K.; Nyarko, A. Toxicity potentials of the nutraceutical Moringa oleifera at supra-supplementation levels. J. Ethnopharmacol. 2012, 139, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, O.; Alam, S.S.; Fatima, M.; Altaf, I.; Khan, F.; Afzal, A. Comparative anti-influenza potential of Moringa oleifera leaves and amantadine in vitro. PPMJ 2017, 28, 127–131. [Google Scholar]
- Stohs, S.J.; Hartman, M.J. Review of the Safety and Efficacy of Moringa oleifera. Phytother. Res. 2015, 29, 796–804. [Google Scholar] [CrossRef]
- El-Gammal, R.E.; Ghoneim, G.A.; ElShehawy, S.M. Effect of Moringa Leaves Powder (Moringa oleifera) on Some Chemical and Physical Properties of Pan Bread. J. Food Dairy Sci. 2016, 7, 307–314. [Google Scholar] [CrossRef]
- Mushtaq, B.S.; Pasha, I.; Omer, R.; Hussain, M.B.; Tufail, T.; Shariati, M.A.; Derkanosova, A.A.; Shchetilina, I.P.; Popova, N.N.; Popov, E.S.; et al. Characterization of Moringa oleifera leaves and its utilization as value added ingredient in unleavened flat bread (chapatti). J. Microbiol. Biotechnol. Food Sci. 2018, 8, 750–755. [Google Scholar] [CrossRef] [Green Version]
- Giuberti, G.; Bresciani, A.; Cervini, M.; Frustace, A.; Marti, A. Moringa oleifera L. leaf powder as ingredient in gluten-free biscuits: Nutritional and physicochemical characteristics. Eur. Food Res. Technol. 2020, 247, 687–694. [Google Scholar] [CrossRef]
- Kamble, D.B.; Bashir, K.; Singh, R.; Rani, S. Effect of Moringa oleífera pod addition on the digestibility, cooking quality, and structural attributes of functional pasta. J. Food Process. Preserv. 2021, 46, e16163. [Google Scholar] [CrossRef]
- Dhawi, F.; El-Beltagi, H.S.; Aly, E.; Hamed, A. Antioxidant, Antibacterial Activities and Mineral Content of Buffalo Yoghurt Fortified with Fenugreek and Moringa oleifera Seed Flours. Foods 2020, 9, 1157. [Google Scholar] [CrossRef]
- Nadeem, M.; Abdullah, M.; Hussain, I. Improvement of the Oxidative Stability of Butter Oil by Blending with Moringa oleifera Oil. J. Food Process. Preserv. 2013, 38, 1491–1500. [Google Scholar] [CrossRef]
- Villarruel-López, A.; López-de la Mora, D.A.; Vázquez-Paulino, O.D.; Puebla-Mora, A.G.; Torres-Vitela, M.R.; Guerrero-Quiroz, L.A.; Nuño, K. Effect of Moringa oleifera consumption on diabetic rats. BMC Complement. Altern. Med. 2018, 18, 127. [Google Scholar] [CrossRef] [Green Version]
- Hagoel, L.; Vexler, A.; Kalich-Philosoph, L.; Earon, G.; Ron, I.; Shtabsky, A.; Marmor, S.; Lev-Ari, S. Combined Effect of Moringa oleifera and Ionizing Radiation on Survival and Metastatic Activity of Pancreatic Cancer Cells. Integr. Cancer Ther. 2019, 18, 1534735419828829. [Google Scholar] [CrossRef] [Green Version]
- Elgamily, H.; Moussa, A.; Elboraey, A.; El-Sayed, H.; Al-Moghazy, M.; Abdalla, A. Microbiological Assessment of Moringa oleifera Extracts and Its Incorporation in Novel Dental Remedies against Some Oral Pathogens. Open Access Maced. J. Med. Sci. 2016, 4, 585–590. [Google Scholar] [CrossRef] [Green Version]
- Bamagous, G.A.; Al Ghamdi, S.S.; Ibrahim, I.A.A.; Mahfoz, A.M.; Afify, M.A.; Alsugoor, M.H.; Shammah, A.A.; Arulselvan, P.; Rengarajan, T. Antidiabetic and antioxidant activity of ethyl acetate extract fraction of Moringa oleifera leaves in streptozotocin-induced diabetes rats via inhibition of inflammatory mediators. Asian Pac. J. Trop. Biomed. 2018, 8, 320. [Google Scholar] [CrossRef]
- Tiloke, C.; Phulukdaree, A.; Chuturgoon, A.A. The Antiproliferative Effect of Moringa oleifera Crude Aqueous Leaf Extract on Human Esophageal Cancer Cells. J. Med. Food 2016, 19, 398–403. [Google Scholar] [CrossRef]
- Bukar, A.; Uba, A.; Oyeyi, T. Antimicrobial profile of Moringa oleifera lam. Extracts against some food—Borne microorganisms. Bayero J. Pure Appl. Sci. 2010, 3, 43–48. [Google Scholar] [CrossRef]
- Kanlayavattanakul, M.; Lourith, N. Moringa leaf: An innovative source of antioxidative phenolics for cosmeceutical products. Sci. Hortic. 2022, 295, 110894. [Google Scholar] [CrossRef]
- OECD. FAO Dairy and Dairy Products. In OECD-FAO Agricultural Outlook 2022–2031; FAO; OECD: Paris, France, 2022; pp. 212–223. [Google Scholar] [CrossRef]
- FoodData Central. Available online: https://fdc.nal.usda.gov/fdc-app.html#/?query=dairy%20products (accessed on 6 January 2023).
- Clarke, H.J.; McCarthy, W.P.; O’Sullivan, M.G.; Kerry, J.P.; Kilcawley, K.N. Oxidative Quality of Dairy Powders: Influencing Factors and Analysis. Foods 2021, 10, 2315. [Google Scholar] [CrossRef]
- Rahaman, M.; Hossain, R.; Herrera-Bravo, J.; Islam, M.T.; Atolani, O.; Adeyemi, O.S.; Owolodun, O.A.; Kambizi, L.; Daştan, S.D.; Calina, D.; et al. Natural antioxidants from some fruits, seeds, foods, natural products, and associated health benefits: An update. Food Sci. Nutr. 2023, in press. [Google Scholar] [CrossRef]
- Djoko, S.W.; Kembauw, E.; Kapelle, I.B.D. Moringa oleifera milk powder as a supplementary food for malnutrition children (SUSUKE). IOP Conf. Ser. Earth Environ. Sci. 2021, 883, 012090. [Google Scholar] [CrossRef]
- Nadeem, M.; Abdullah, M.; Hussain, I.; Inayat, S.; Javid, A.; Zahoor, Y. Antioxidant potential of Moringa oleifera leaf extract for the stabilisation of butter at refrigeration temperature. Czech J. Food Sci. 2013, 31, 332–339. [Google Scholar] [CrossRef] [Green Version]
- Nadeem, M.; Javid, A.; Abdullah, M.; Arif, A.M.; Mahmood, T. Improving Nutritional Value of Butter Milk by Blending with Dry Leaves of Moringa oleifera. Pak. J. Nutr. 2012, 11, 812–816. [Google Scholar] [CrossRef] [Green Version]
- Bermudez-Beltrán, K.A.; Marzal-Bolaño, J.K.; Olivera-Martínez, A.B.; Espitia, P.J. Cape gooseberry Petit Suisse Cheese incorporated with moringa leaf powder and gelatin. LWT 2020, 123, 109101. [Google Scholar] [CrossRef]
- El-Fat, F.A.; Sal, H.H.; El, S.M.; El-S, H.S.; Abdel-Hady, H. Utilization of Natural Antimicrobial and Antioxidant of Moringa oleifera Leaves Extract in Manufacture of Cream Cheese. J. Biol. Sci. 2018, 18, 92–106. [Google Scholar] [CrossRef] [Green Version]
- Ademosun, A.O. Glycemic properties of soursop-based ice cream enriched with moringa leaf powder. Food Raw Mater. 2021, 9, 207–214. [Google Scholar] [CrossRef]
- Ademosun, A.O.; Oboh, G.; Ajeigbe, O.F. Influence of Moringa (Moringa oleifera) enriched ice creams on rats’ brain: Exploring the redox and cholinergic systems. Curr. Res. Nutr. Food Sci. 2022, 5, 366–373. [Google Scholar] [CrossRef]
- Zhang, T.; Jeong, C.H.; Cheng, W.N.; Bae, H.; Seo, H.G.; Petriello, M.C.; Han, S.G. Moringa extract enhances the fermentative, textural, and bioactive properties of yogurt. LWT 2018, 101, 276–284. [Google Scholar] [CrossRef]
- Saeed, M.; Ali, S.W.; Ramzan, S. Physicochemical analysis of mango flavored yogurt supplemented with Moringa oleifera leaf powder. J. Food Sci. Technol. 2021, 58, 4805–4814. [Google Scholar] [CrossRef]
- Van Tienen, A.; Hullegie, Y.M.; Hummelen, R.; Hemsworth, J.; Changalucha, J.; Reid, G. Development of a locally sustainable functional food for people living with HIV in Sub-Saharan Africa: Laboratory testing and sensory evaluation. Benef. Microbes 2011, 2, 193–198. [Google Scholar] [CrossRef]
- Olvera-Aguirre, G.; Mendoza-Taco, M.M.; Moo-Huchin, V.M.; Lee-Rangel, H.A.; Roque-Jiménez, J.A.; Gómez-Vázquez, A.; Dzib-Cauich, D.A.; Vargas-Bello-Pérez, E.; Chay-Canul, A.J. Effect of Extraction Type on Bioactive Compounds and Antioxidant Activity of Moringa oleifera Lam. Leaves. Agriculture 2022, 12, 1462. [Google Scholar] [CrossRef]
- Sulaiman, I.S.C.; Basri, M.; Masoumi, H.R.F.; Chee, W.J.; Ashari, S.E.; Ismail, M. Effects of temperature, time, and solvent ratio on the extraction of phenolic compounds and the anti-radical activity of Clinacanthus nutans Lindau leaves by response surface methodology. Chem. Central J. 2017, 11, 54. [Google Scholar] [CrossRef] [Green Version]
- Tai, T.K.; Thongklay, J.; Meunprasertdee, P.; Kornthattalim, P.; Kaaewmanee, T. A Comparison of Three Extraction Methods for Phenolic Compounds and Antioxidant Activities from Moringa oleifera Leaves. Chiang Mai J. Sci. 2018, 45, 2779–2789. [Google Scholar]
- Oboh, G.; Ademiluyi, A.O.; Ademosun, A.O.; Olasehinde, T.A.; Oyeleye, S.I.; Boligon, A.A.; Athayde, M.L. Phenolic Extract from Moringa oleifera Leaves Inhibits Key Enzymes Linked to Erectile Dysfunction and Oxidative Stress in Rats’ Penile Tissues. Biochem. Res. Int. 2015, 2015, 175950. [Google Scholar] [CrossRef] [Green Version]
- pH/Temperature Meter for Yogurt. Available online: https://www.mediray.co.nz/media/15607/ds_hanna_ph_ha-hi99164_1p0_0415.pdf (accessed on 24 January 2023).
- Ranasinghe, J.; Perera, W. Prevalence of Lactobacillus bulgaricus and Streptococcus thermophilus stability in commercially available yogurts in Sri lanka. Asian J. Med. Sci. 2016, 7, 97–101. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Wang, X.; Tian, Q.; Mao, X. Effect of casein to whey protein ratios on the protein interactions and coagulation properties of low-fat yogurt. J. Dairy Sci. 2016, 99, 7768–7775. [Google Scholar] [CrossRef] [Green Version]
- Arab, M.; Yousefi, M.; Khanniri, E.; Azari, M.; Ghasemzadeh-Mohammadi, V.; Mollakhalili-Meybodi, N. A comprehensive review on yogurt syneresis: Effect of processing conditions and added additives. J. Food Sci. Technol. 2022. [Google Scholar] [CrossRef]
- Sánchez, L.; Pérez, M.D.; Parrón, J.A. HPP in dairy products: Impact on quality and applications. In Present and Future of High Pressure Processing; Barba, F.J., Tonello-Samson, C., Puértolas, E., Lavilla, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 245–272. [Google Scholar] [CrossRef]
- Mehanna, N.S.; Hassan, Z.M.R.; El-Din, H.M.F.; Ali, A.A.-E.; Amarowicz, R.; El-Messery, T.M. Effect of Interaction Phenolic Compounds with Milk Proteins on Cell Line. Food Nutr. Sci. 2014, 5, 2130–2146. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.-Y.; Yoo, B. Effects of cross-linking on the rheological and thermal properties of sweet potato starch. Starch/Stärke 2010, 62, 577–583. [Google Scholar] [CrossRef]
- Amiri, Z.R.; Nemati, A.; Tirgarian, B.; Dehghan, B.; Nasiri, H. Influence of stinging nettle (Urtica dioica L.) extract-loaded nano-emulsion on the storage stability and antioxidant attributes of Doogh (Traditional Iranian yoghurt beverage). J. Food Meas. Charact. 2020, 15, 437–448. [Google Scholar] [CrossRef]
- De Souza, E.L.; De Albuquerque, T.M.R.; Dos Santos, A.S.; Massa, N.M.L.; Alves, J.L.D.B. Potential interactions among phenolic compounds and probiotics for mutual boosting of their health-promoting properties and food functionalities—A review. Crit. Rev. Food Sci. Nutr. 2018, 59, 1645–1659. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.M.; Santos, L. Incorporation of phenolic extracts from different by-products in yoghurts to create fortified and sustainable foods. Food Biosci. 2023, 51, 102293. [Google Scholar] [CrossRef]
- Stobiecka, M.; Król, J.; Brodziak, A. Antioxidant Activity of Milk and Dairy Products. Animals 2022, 12, 245. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Wilkins, K.; Bowers, M.; Wynn, C.; Ndegwa, E. Influence of PH and Temperature on Growth Characteristics of Leading Foodborne Pathogens in a Laboratory Medium and Select Food Beverages. Austin. Food Sci. 2018, 3, 1031. [Google Scholar]
- European Comission. Commission Regulation (EC) No 2073/2005 of 15 November 2005 on Microbiological Criteria for Foodstuffs. OJEU 2015, 48, 1–26. Available online: http://data.europa.eu/eli/reg/2005/2073/2020-03-08 (accessed on 12 January 2023).
- Silva, A.M.; Nouli, E.; Xekoukoulotakis, N.P.; Mantzavinos, D. Effect of key operating parameters on phenols degradation during H2O2-assisted TiO2 photocatalytic treatment of simulated and actual olive mill wastewaters. Appl. Catal. B Environ. 2007, 73, 11–22. [Google Scholar] [CrossRef]
- Bobo-García, G.; Davidov-Pardo, G.; Arroqui, C.; Vírseda, P.; Marín-Arroyo, M.R.; Navarro, M. Intra-laboratory validation of microplate methods for total phenolic content and antioxidant activity on polyphenolic extracts, and comparison with conventional spectrophotometric methods. J. Sci. Food Agric. 2014, 95, 204–209. [Google Scholar] [CrossRef]
- Xiao, F.; Xu, T.; Lu, B.; Liu, R. Guidelines for antioxidant assays for food components. Food Front. 2020, 1, 60–69. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, S.M.; Santos, L. From by-product to functional ingredient: Incorporation of avocado peel extract as an antioxidant and antibacterial agent. Innov. Food Sci. Emerg. Technol. 2022, 80, 103116. [Google Scholar] [CrossRef]
- Cho, W.-Y.; Kim, D.-H.; Lee, H.-J.; Yeon, S.-J.; Lee, C.-H. Quality characteristic and antioxidant activity of yogurt containing olive leaf hot water extract. CyTA J. Food 2020, 18, 43–50. [Google Scholar] [CrossRef] [Green Version]
- Bakirci, S.; Dagdemir, E.; Boran, O.S.; Hayaloglu, A.A. The effect of pumpkin fibre on quality and storage stability of reduced-fat set-type yogurt. Int. J. Food Sci. Technol. 2016, 52, 180–187. [Google Scholar] [CrossRef]







| Food Product | Objectives | Results | Ref. |
|---|---|---|---|
| Powdered milk | Evaluate the efficacy of MO leaves as powdered milk to use as a supplementary food for malnutrition. | An increase in weight was observed in the children regularly supplemented with MO powdered milk for two months, compared to the control group. | [43] |
| Butter | Study the use of MO leaf extract for the stabilization of butter at refrigeration temperature. | The addition of the extract (600 ppm) did not impact the butter composition and improved the antioxidant properties of the product without impairing the overall acceptability. | [44] |
| Improve the nutritional value of buttermilk using MO dry leaves to prevent and correct malnutrition. | The fortified buttermilk did not present any significant differences in pH or acidity but presented an increase in protein and ash content and also in vitamin C and vitamin B. The addition of 2% MO leaves did not alter the overall acceptability. | [45] | |
| Cheese | Improve the nutritional value of cape gooseberry Petit Suisse cheese using MO leaf powder. | The addition of 2% MO to the formulation increased its nutritional value (increased ash, protein, fat, and fibre contents) but decreased the sensory acceptance. | [46] |
| Improve the quality and shelf life of cream cheese using ethanolic MO leaf extract. | The addition of MO (up to 4%) increased the protein, ash and total phenolic contents, and also the antioxidant activity of the cheese. It also enhanced the growth of probiotic strains. An improvement was observed in both flavour and taste during storage. | [47] | |
| Ice cream | Study the use of ice cream enriched with MO leaf powder as an alternative to sugar-sweetened ice cream. | The MO-enriched ice creams (0.05% and 0.5%) presented an increase in the total phenolic and flavonoid contents, and in the inhibition of α-amylase and α-glucosidase enzymes, and improved antioxidant properties. These ice creams reduced the glycaemic index in vitro but presented a reduction in the overall acceptability. | [48] |
| Evaluate the influence of MO leaves-enriched ice cream on the redox and chlorogenic systems of rats. | The addition of MO to ice cream (0.59–2.35%) reduced the rat’s body weight gain, the glycaemic index and the lipid profile (triglycerides and cholesterol), inhibited the brain cholinergic enzymes (AChE and BChE) and increased the brain antioxidant enzyme activities (SOD and CAT). The overall acceptability of MO-enriched ice cream was lower than the control (commercial ice cream). | [49] | |
| Yoghurt | Evaluate the effects of MO leaf extract on the fermentation, bioactive properties and quality characteristics of yoghurt. | The addition of the extract accelerated yoghurt fermentation by promoting the growth of lactic acid bacteria and increased the viscosity and free radical-scavenging during storage. Changes in the colour of the yoghurt were observed but the overall acceptability was not significantly influenced by the addition of 0.5% MO extract. | [50] |
| Produce a yoghurt supplemented with MO leaf powder. | The fortified yoghurt presented a decrease in the syneresis and moisture, and an increase in total solids, protein and ash contents. The best results were obtained by combining 1% MO leaf powder and mango flavour. | [51] | |
| Develop a fortified yogurt with MO leaves as a carrier of probiotics and micronutrients. | The supplementation with MO (around 30%) increased the viability of the probiotic strain (L. rhamnosus GR-1) but reduced the overall acceptability of the product. | [52] |
| TPC (mgGAE/gdried extract) | Antioxidant Capacity (IC50—mgextract/L) | Antibacterial Activity (dhalo—mm) | ||
|---|---|---|---|---|
| DPPH | ABTS | E. coli | S. aureus | |
| 54.5 ± 16.8 | 133.4 ± 12.3 | 60.0 ± 9.9 | ND | ND |
| Compound | RT (min) | Calibration Curves | R2 | IDL (mg/L) | IQL (mg/L) | Standard Concentration (mgcompound/gextract) |
|---|---|---|---|---|---|---|
| Caffeic acid | 29.23 | A = 5.56 × 105 C − 1.56 × 106 | 0.9992 | 4.03 | 13.43 | 0.16 |
| Catechin | 24.38 | A = 1.57 × 105 C − 7.93 × 105 | 0.9861 | 41.50 | 138.34 | 19.83 |
| Chlorogenic acid | 26.62 | A = 1.91 × 105 C − 1.16 × 105 | 0.9999 | 2.84 | 9.48 | 1.04 |
| Epicatechin | 30.34 | A = 4.15 × 105 C − 1.56 × 106 | 0.9983 | 5.82 | 19.39 | 0.67 |
| Gallic acid | 11.28 | A = 1.21 × 105 C + 1.33 × 106 | 0.9978 | 27.96 | 93.20 | 0.13 |
| Kaempferol | 52.79 | A = 7.34 × 105 C + 2.41 × 105 | 0.9993 | 1.49 | 4.97 | 0.02 |
| Quercetin | 49.34 | A = 7.37 × 105 C − 2.68 × 105 | 0.9994 | 1.37 | 4.58 | 0.06 |
| Yoghurt | t0 | t3 | ||
|---|---|---|---|---|
| K (mPa·sn) | n | K (mPa·sn) | n | |
| NC | 9322 | 0.451 | 12135 | 0.366 |
| PC | 6729 | 0.486 | 8275 | 0.431 |
| ME | 8984 | 0.469 | 6036 | 0.478 |
| ME2 | 9096 | 0.438 | 8545 | 0.431 |
| MP | 8020 | 0.476 | 3745 | 0.548 |
| Yoghurt | TPC (mg/L) | |||
|---|---|---|---|---|
| t0 | t1 | t2 | t3 | |
| NC | 0.54 ± 0.01 a,A | 0.54 ± 0.01 a,A | 0.38 ± 0.01 a,B | 0.20 ± 0.02 a,C |
| PC | 0.66 ± 0.02 a,A | 0.61 ± 0.01 a,A | 0.44 ± 0.02 a,B | 0.32 ± 0.03 a,C |
| ME | 0.88 ± 0.08 b,A | 0.65 ± 0.03 a,B | 0.60 ± 0.06 b,B | 0.45 ± 0.12 b,C |
| ME2 | 0.93 ± 0.05 b,A | 0.79 ± 0.03 b,B | 0.71 ± 0.04 b,c,B | 0.57 ± 0.04 b,c,C |
| MP | 0.88 ± 0.06 b,A | 0.85 ± 0.03 b,A | 0.85 ± 0.03 c,A | 0.63 ± 0.01 c,B |
| Yoghurt | E. coli | S. aureus | ||
|---|---|---|---|---|
| t0 | t2 | t0 | t2 | |
| NC | 10.7 ± 0.5 | ND | 14.3 ± 0.9 A | 9.0 ± 1.6 B |
| PC | 11.0 ± 1.4 A | 8.3 ± 0.9 B | 17.7 ± 3.7 A | 10.0 ± 0.8 B |
| ME | 11.7 ± 0.9 | ND | 15.3 ± 1.7 A | 9.0 ± 0.8 B |
| ME2 | 12.3 ± 0.9 A | 8.7 ± 0.5 B | 16.3 ± 0.9 A | 11.0 ± 0.8 B |
| MP | 11.7 ± 0.9 A | 7.5 ± 0.5 B | 15.3 ± 0.5 A | 10.7 ± 1.2 B |
| Formulation | Additives |
|---|---|
| NC | yoghurt with no additives (negative control) |
| PC | yoghurt with 1.0 g/L * of sorbic acid (positive control) |
| ME | yoghurt with 1.0 g/L of M. oleifera extract |
| ME2 | yoghurt with 2.0 g/L of M. oleifera extract |
| MP | yoghurt with 2.9 g/L * of M. oleifera leaf powder |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, S.M.; Leitão, A.; Alves, A.; Santos, L. Incorporation of Moringa oleifera Leaf Extract in Yoghurts to Mitigate Children’s Malnutrition in Developing Countries. Molecules 2023, 28, 2526. https://doi.org/10.3390/molecules28062526
Gomes SM, Leitão A, Alves A, Santos L. Incorporation of Moringa oleifera Leaf Extract in Yoghurts to Mitigate Children’s Malnutrition in Developing Countries. Molecules. 2023; 28(6):2526. https://doi.org/10.3390/molecules28062526
Chicago/Turabian StyleGomes, Sandra M., Anabela Leitão, Arminda Alves, and Lúcia Santos. 2023. "Incorporation of Moringa oleifera Leaf Extract in Yoghurts to Mitigate Children’s Malnutrition in Developing Countries" Molecules 28, no. 6: 2526. https://doi.org/10.3390/molecules28062526
APA StyleGomes, S. M., Leitão, A., Alves, A., & Santos, L. (2023). Incorporation of Moringa oleifera Leaf Extract in Yoghurts to Mitigate Children’s Malnutrition in Developing Countries. Molecules, 28(6), 2526. https://doi.org/10.3390/molecules28062526