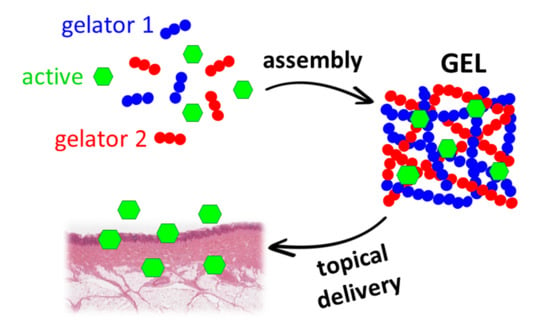
Delivery of Active Peptides by Self-Healing, Biocompatible and Supramolecular Hydrogels
Abstract
:1. Introduction
2. Results
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ning, C.; Zhou, Z.; Tan, G.; Zhu, Y.; Mao, C. Electroactive polymers for tissue regeneration: Developments and perspectives. Prog. Polym. Sci. 2018, 81, 144–162. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.; Haponiuk, J.T.; Thomas, S.; Gopi, S. Biopolymer based nanomaterials in drug delivery systems: A review. Mater. Today Chem. 2018, 9, 43–55. [Google Scholar] [CrossRef]
- Mondal, S.; Das, S.; Nandi, A.K. A review on recent advances in polymer and peptide hydrogels. Soft Matter 2020, 16, 1404–1454. [Google Scholar] [CrossRef] [PubMed]
- Mayr, J.; Saldías, C.; Díaz Díaz, D. Release of small bioactive molecules from physical gels. Chem. Soc. Rev. 2018, 47, 1484–1515. [Google Scholar] [CrossRef]
- Tomasini, C.; Castellucci, N. Peptides and peptidomimetics that behave as low molecular weight gelators. Chem. Soc. Rev. 2013, 42, 156–172. [Google Scholar] [CrossRef]
- Draper, E.R.; Adams, D.J. Low-Molecular-Weight Gels: The State of the Art. Chem 2017, 3, 390. [Google Scholar] [CrossRef] [Green Version]
- Adams, D.J.; Butler, M.F.; Frith, W.J.; Kirkland, M.; Mullen, L.; Sanderson, P. A new method for maintaining homogeneity during liquid–hydrogel transitions using low molecular weight hydrogelators. Soft Matter 2009, 5, 1856–1862. [Google Scholar] [CrossRef]
- Xian, S.; Webber, M.J. Temperature-responsive supramolecular hydrogels. J. Mater. Chem. B 2020, 8, 9197–9211. [Google Scholar] [CrossRef]
- Chen, L.; Pont, G.; Morris, K.; Lotze, G.; Squires, A.; Serpell, L.C.; Adams, D.J. Salt-induced hydrogelation of functionalised-dipeptides at high pH. Chem. Commun. 2011, 47, 12071–12073. [Google Scholar] [CrossRef]
- Castellucci, N.; Falini, G.; Angelici, G.; Tomasini, C. Formation of gels in the presence of metal ions. Amino Acids 2011, 41, 609–620. [Google Scholar] [CrossRef]
- Pramanik, B.; Ahmed, S. Peptide-Based Low Molecular Weight Photosensitive Supramolecular Gelators. Gels 2022, 8, 533. [Google Scholar] [CrossRef]
- Bardelang, D.; Camerel, F.; Margeson, J.C.; Leek, D.M.; Schmutz, M.; Zaman, M.B.; Yu, K.; Soldatov, D.V.; Ziessel, R.; Ratcliffe, C.I.; et al. Unusual sculpting of dipeptide particles by ultrasound induces gelation. J. Am. Chem. Soc. 2008, 130, 3313–3315. [Google Scholar] [CrossRef]
- Poustchi, F.; Amani, H.; Ahmadian, Z.; Niknezhad, S.V.; Mehrabi, S.; Santos, H.A.; Shahbazi, M.A. Combination Therapy of Killing Diseases by Injectable Hydrogels: From Concept to Medical Applications. Adv. Healthc. Mater. 2021, 10, e2001571. [Google Scholar] [CrossRef]
- Tu, Y.; Chen, N.; Li, C.; Liu, H.; Zhu, R.; Chen, S.; Xiao, Q.; Liu, J.; Ramakrishna, S.; He, L. Advances in injectable self-healing biomedical hydrogels. Acta Biomater. 2019, 90, 1–20. [Google Scholar] [CrossRef]
- Valls, A.; Isabel Burguete, M.; Kuret, L.; Altava, B.; Luis, S.V. Open chain pseudopeptides as hydrogelators with reversible and dynamic responsiveness to pH, temperature and sonication as vehicles for controlled drug delivery. J. Mol. Liq. 2022, 348, 118051. [Google Scholar] [CrossRef]
- Giuri, D.; Marshall, L.J.; Dietrich, B.; McDowall, D.; Thomson, L.; Newton, J.Y.; Wilson, C.; Schweins, R.; Adams, D.J. Exploiting and controlling gel-to-crystal transitions in multicomponent supramolecular gels. Chem. Sci. 2021, 12, 9720–9725. [Google Scholar] [CrossRef]
- Okesola, B.O.; Mata, A. Multicomponent self-assembly as a tool to harness new properties from peptides and proteins in material design. Chem. Soc. Rev. 2018, 47, 3721–3736. [Google Scholar] [CrossRef]
- Genio, F.A.F.; Paderes, M.C. Functional Supramolecular Gels Comprised of Bis-Urea Compounds and Cosmetic Solvents. ChemistrySelect 2021, 6, 7906–7911. [Google Scholar] [CrossRef]
- Uzan, S.; Barış, D.; Çolak, M.; Aydın, H.; Hoşgören, H. Organogels as novel carriers for dermal and topical drug delivery vehicles. Tetrahedron 2016, 72, 7517–7525. [Google Scholar] [CrossRef]
- Palomo, M.C.; Prieto, V.M.; Kirilov, P. Colloidal dispersions of gelled lipid nanoparticles (Gln): Concept and potential applications. Gels 2017, 3, 33. [Google Scholar] [CrossRef] [Green Version]
- Nicastro, G.; Black, L.M.; Ravarino, P.; D’Agostino, S.; Faccio, D.; Tomasini, C.; Giuri, D. Controlled Hydrolysis of Odorants Schiff Bases in Low-Molecular-Weight Gels. Int. J. Mol. Sci. 2022, 23, 3105. [Google Scholar] [CrossRef] [PubMed]
- Kornhauser, A.; Coelho, S.G.; Hearing, V.J. Effects of cosmetic formulations containing hydroxyacids on sun-exposed skin: Current applications and future developments. Dermatol. Res. Pract. 2012, 2012, 710893. [Google Scholar] [CrossRef]
- Bernstein, E.F.; Underhill, C.B.; Lakkakorpi, J.; Ditre, C.M.; Uitto, J.; Yu, R.J.; Van Scott, E. Citric acid increases viable epidermal thickness and glycosaminoglycan content of sun-damaged skin. Dermatol. Surg. 1997, 23, 689–694. [Google Scholar] [CrossRef]
- Lukić, M.; Pantelić, I.; Savić, S.D. Towards optimal ph of the skin and topical formulations: From the current state of the art to tailored products. Cosmetics 2021, 8, 69. [Google Scholar] [CrossRef]
- Bartosova, L.; Bajgar, J. Transdermal Drug Delivery In Vitro Using Diffusion Cells. Curr. Med. Chem. 2012, 19, 4671–4677. [Google Scholar] [CrossRef]
- Giuri, D.; Jurković, L.; Fermani, S.; Kralj, D.; Falini, G.; Tomasini, C. Supramolecular Hydrogels with Properties Tunable by Calcium Ions: A Bio-Inspired Chemical System. ACS Appl. Bio Mater. 2019, 2, 5819–5828. [Google Scholar] [CrossRef]
- Di Filippo, M.F.; Giuri, D.; Marchiori, G.; Maglio, M.; Pagani, S.; Fini, M.; Tomasini, C.; Panzavolta, S. Self-assembling of fibers inside an injectable calcium phosphate bone cement: A feasibility study. Mater. Today Chem. 2022, 24, 100991. [Google Scholar] [CrossRef]
- Kogan, A.; Garti, N. Microemulsions as transdermal drug delivery vehicles. Adv. Colloid Interface Sci. 2006, 123–126, 369–385. [Google Scholar] [CrossRef]
- Shakeel, F.; Baboota, S.; Ahuja, A.; Ali, J.; Aqil, M.; Shafiq, S. Nanoemulsions as vehicles for transdermal delivery of aceclofenac. AAPS PharmSciTech 2007, 8, 91–98. [Google Scholar] [CrossRef] [Green Version]
- Giuri, D.; D’Agostino, S.; Ravarino, P.; Faccio, D.; Falini, G.; Tomasini, C. Water Remediation from Pollutant Agents by the Use of an Environmentally Friendly Supramolecular Hydrogel. ChemNanoMat 2022, 8, e202200093. [Google Scholar] [CrossRef]
- D’Souza, S. A Review of In Vitro Drug Release Test Methods for Nano-Sized Dosage Forms. Adv. Pharm. 2014, 2014, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Rizzi, V.; Gubitosa, J.; Fini, P.; Cosma, P. Neurocosmetics in skincare-the fascinating world of skin-brain connection: A review to explore ingredients, commercial products for skin aging, and cosmetic regulation. Cosmetics 2021, 8, 66. [Google Scholar] [CrossRef]
- Schagen, S.K. Topical peptide treatments with effective anti-aging results. Cosmetics 2017, 4, 16. [Google Scholar] [CrossRef] [Green Version]
- Yuan, L.; Alexander, P.B.; Wang, X.-F. Cellular senescence: From anti-cancer weapon to anti-aging target. Sci. China. Life Sci. 2020, 63, 332–342. [Google Scholar] [CrossRef] [Green Version]
- Han, F.; Luo, D.; Qu, W.; Chen, D.; Hong, Y.; Sheng, J.; Yang, X.; Liu, W. Nanoliposomes codelivering bioactive peptides produce enhanced anti-aging effect in human skin. J. Drug Deliv. Sci. Technol. 2020, 57, 101693. [Google Scholar] [CrossRef]
- Yu, L.; Ding, J. Injectable hydrogels as unique biomedical materials. Chem. Soc. Rev. 2008, 37, 1473–1481. [Google Scholar] [CrossRef]
- Yesilyurt, V.; Webber, M.J.; Appel, E.A.; Godwin, C.; Langer, R.; Anderson, D.G. Injectable Self-Healing Glucose-Responsive Hydrogels with pH-Regulated Mechanical Properties. Adv. Mater. 2016, 28, 86–91. [Google Scholar] [CrossRef] [Green Version]
- Yan, C.; Altunbas, A.; Yucel, T.; Nagarkar, R.P.; Schneider, J.P.; Pochan, D.J. Injectable solid hydrogel: Mechanism of shear-thinning and immediate recovery of injectable β-hairpin peptide hydrogels. Soft Matter 2010, 6, 5143–5156. [Google Scholar] [CrossRef] [Green Version]
- Salamanca, C.H.; Barrera-Ocampo, A.; Lasso, J.C.; Camacho, N.; Yarce, C.J. Franz diffusion cell approach for pre-formulation characterisation of ketoprofen semi-solid dosage forms. Pharmaceutics 2018, 10, 148. [Google Scholar] [CrossRef] [Green Version]
- Limpongsa, E.; Umprayn, K. Preparation and evaluation of diltiazem hydrochloride diffusion-controlled transdermal delivery system. AAPS PharmSciTech 2008, 9, 464–470. [Google Scholar] [CrossRef] [Green Version]
- Clément, P.; Laugel, C.; Marty, J.P. Influence of three synthetic membranes on the release of caffeine from concentrated W/O emulsions. J. Control. Release 2000, 66, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Uchida, T.; Kadhum, W.R.; Kanai, S.; Todo, H.; Oshizaka, T.; Sugibayashi, K. Prediction of skin permeation by chemical compounds using the artificial membrane, Strat-MTM. Eur. J. Pharm. Sci. 2015, 67, 113–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbero, A.M.; Frasch, H.F. Pig and guinea pig skin as surrogates for human in vitro penetration studies: A quantitative review. Toxicol. Vitr. 2009, 23, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; He, H. A review of cosmetic skin delivery. J. Cosmet. Dermatol. 2021, 20, 2020–2030. [Google Scholar] [CrossRef] [PubMed]
- Patravale, V.B.; Mandawgade, S.D. Novel cosmetic delivery systems: An application update. Int. J. Cosmet. Sci. 2008, 30, 19–33. [Google Scholar] [CrossRef]
- European Commission, Directorate-General for Health and Consumers. Basic Criteria for the In Vitro Assessment of Dermal Absorption of Cosmetic Ingredients; European Union: Brussels, Belgium, 2010; Volume SCCS/1358. [Google Scholar] [CrossRef]








| Gel | 1 | 2 | Citric Acid | T (°C) | Result | pH |
|---|---|---|---|---|---|---|
| A | 0.65% | 0.35% | - | 23 | Gel | 7.60 |
| B | 0.65% | 0.50% | - | 23 | Gel | 7.30 |
| C | 0.35% | 0.65% | - | 23 | Gel | 6.80 |
| D | 0.35% | 0.65% | 0.38% | 23 | Sol | 5.84 |
| E | 0.35% | 0.65% | 0.38% | 60 | Gel | 5.55 |
| Gel | 1 | 2 | Active Peptide | T (°C) | Result | pH |
|---|---|---|---|---|---|---|
| F | 0.35% | 0.65% | 3 (0.1%) | 40 | Gel | 6.70 |
| G | 0.35% | 0.65% | 4 (0.1%) | 40 | Gel | 6.72 |
| Gel | 1 (mg) | 1 M NaOH (µL) | H2O (mL) | 2 (mg) | PB 0.1 M (mL) | Tot V (mL) |
|---|---|---|---|---|---|---|
| A | 13 | 27 | 1.303 | 7 | 0.670 | 2 |
| B | 13 | 27 | 1.303 | 10 | 0.670 | 2 |
| C | 7 | 15 | 1.315 | 13 | 0.670 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shariati Pour, S.R.; Oddis, S.; Barbalinardo, M.; Ravarino, P.; Cavallini, M.; Fiori, J.; Giuri, D.; Tomasini, C. Delivery of Active Peptides by Self-Healing, Biocompatible and Supramolecular Hydrogels. Molecules 2023, 28, 2528. https://doi.org/10.3390/molecules28062528
Shariati Pour SR, Oddis S, Barbalinardo M, Ravarino P, Cavallini M, Fiori J, Giuri D, Tomasini C. Delivery of Active Peptides by Self-Healing, Biocompatible and Supramolecular Hydrogels. Molecules. 2023; 28(6):2528. https://doi.org/10.3390/molecules28062528
Chicago/Turabian StyleShariati Pour, Seyedeh Rojin, Sara Oddis, Marianna Barbalinardo, Paolo Ravarino, Massimiliano Cavallini, Jessica Fiori, Demetra Giuri, and Claudia Tomasini. 2023. "Delivery of Active Peptides by Self-Healing, Biocompatible and Supramolecular Hydrogels" Molecules 28, no. 6: 2528. https://doi.org/10.3390/molecules28062528
APA StyleShariati Pour, S. R., Oddis, S., Barbalinardo, M., Ravarino, P., Cavallini, M., Fiori, J., Giuri, D., & Tomasini, C. (2023). Delivery of Active Peptides by Self-Healing, Biocompatible and Supramolecular Hydrogels. Molecules, 28(6), 2528. https://doi.org/10.3390/molecules28062528