Bathochromic Shift of Fluorescence Peak in Dipyrrolo[1,2-a:2′,1′-c]quinoxaline by Introducing Each of Electron-Donating and Electron-Withdrawing Substituent
Abstract
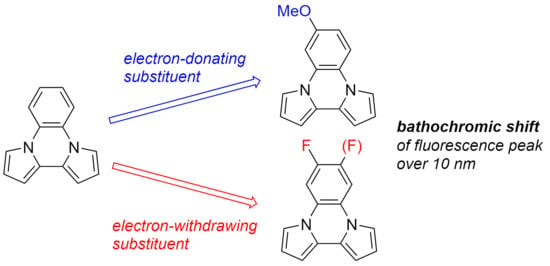
:1. Introduction
2. Results and Discussions
3. Materials and Methods
3.1. General Information
3.1.1. Preparation of 1,2-Dibromo-4-methoxybenzene [29]
3.1.2. Synthesis of Substituted Dipyrrolo [1,2-a:2′,1′-c]quinoxaline (1b–d)
3.1.3. Synthesis of 6,7-Difluoro-3,10-diphenyldipyrrolo [1,2-a:2′,1′-c]quinoxaline (3d)
3.2. Measurement of Absorption and Fluorescence Spectra
3.3. DFT Calculation Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Wang, H.; Aydıner, B.; Seferoglu, Z.; Bureš, F.; Liu, J. Development and Application of Non-Conventional Luminophores with Aggregation Based Emission. Dyes Pigment. 2022, 205, 110354. [Google Scholar] [CrossRef]
- Wong, M.Y.; Zysman-Colman, E. Purely Organic Thermally Activated Delayed Fluorescence Materials for Organic Light-Emitting Diodes. Adv. Mater. 2017, 29, 1605444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, G.-X.; Liu, D.-H.; Jiang, S.-M.; Yang, Z.-H.; Chen, Z.-J.; Qiu, W.-D.; Gan, Y.-Y.; Liu, K.-K.; Li, D.-L.; Su, S.-J. Novel Polycyclic Fused Amide Derivatives; Properties and Application for Sky-Blue Electroluminescent Devices. Molecules 2022, 27, 5181. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Sun, Y.; Fang, X.; Li, J.; Wu, Q.; Bu, W.; Guo, X.; Wang, H.; Jiao, L.; Hao, E.D. Aromatic-Ring-Fused BOPPY Fluorophores: Synthesis, Spectral, Redox Properties, and Bioimaging Application. Inorg. Chem. 2022, 61, 16718–16729. [Google Scholar] [CrossRef] [PubMed]
- Bakholdina, A.; Lukin, A.; Bakulina, O.; Guranova, N.; Krasavin, M. Dual Use of Propargylamine Building Blocks in the Construction of Polyheterocyclic Scaffolds. Tetrahedron Lett. 2020, 61, 151970. [Google Scholar] [CrossRef]
- Yagishita, F.; Tanigawa, J.; Nii, C.; Tabata, A.; Nagamune, H.; Takanari, H.; Imada, Y.; Kawamura, Y. Fluorescent Imidazo [1,5-a]pyridinium Salt for a Potential Cancer Therapy Agent. ACS Med. Chem. Lett. 2019, 10, 1110–1114. [Google Scholar] [CrossRef]
- Huang, C.-C.; Xue, M.-M.; Wu, F.-P.; Yuan, Y.; Liao, L.-S.; Fung, M.-K. Deep-Blue and Hybrid-White Organic Light Emitting Diodes Based on a Twisting Carbazole-benzofuro [2,3-b]pyrazine Fluorescent Emitter. Molecules 2019, 24, 353. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Chen, L.; Ma, S.; Luo, H.; Cao, J.; Chen, R.; Duan, Z.; Mathey, F. Synthesis of 1,3-Azaphospholes with Pyrrolo [1,2-a]quinoline Skeleton and Their Optical Applications. Org. Lett. 2018, 20, 4103–4106. [Google Scholar] [CrossRef]
- Yagishita, F.; Nii, C.; Tezuka, Y.; Tabata, A.; Nagamune, H.; Uemura, N.; Yoshida, Y.; Mino, T.; Sakamoto, M.; Kawamura, Y. Fluorescent N-Heteroarenes Having Large Stokes Shift and Water Solubility Suitable for Bioimaging. Asian J. Org. Chem. 2018, 7, 1614. [Google Scholar] [CrossRef]
- Cisse, L.; Djande, A.; Capo-Chichi, M.; Khonté, A.; Bakhoum, J.-P.; Delattre, F.; Yoda, J.; Saba, A.; Tine, A.; Aaron, J.-J. Quantitative Study of the Substituent Effects on the Electronic Absorption and Fluorescence Spectra of Coumarines. J. Phys. Org. Chem. 2020, 33, e4014. [Google Scholar] [CrossRef]
- Chavan, S.N.; Toche, R.B.; Chavan, S.M. Substituent Effect on Absorption and Fluorescence Properties of Thieno [3,2-c]pyridine Derivatives. J. Fluoresc. 2017, 27, 443–450. [Google Scholar] [CrossRef]
- Jiu, T.; Li, Y.; Liu, H.; Ye, J.; Liu, X.; Jiang, L.; Yuan, M.; Li, J.; Li, C.; Wang, S.; et al. Brightly Full-Color Emissions of Oligo(p-phenylenevinylene)s: Substituent Effects on Photophysical Properties. Tetrahedron 2007, 63, 3168–3172. [Google Scholar] [CrossRef]
- Miura, Y.; Kobayashi, K.; Yoshioka, N. V-Shaped Fluorophores with a 1-Methyl-4,5-bis(arylethynyl)imidazole Skeleton Displaying Solid-State Fluorescence, Acid Responsiveness, and Remarkable Fluorescence Solvatochromism. New J. Chem. 2021, 45, 898–905. [Google Scholar] [CrossRef]
- Vázquez, J.L.; Velazco-Cabral, I.; Flores-Álamo, M.; Turlakov, G.; Rodríguez, G.; Moggio, I.; Arias, E.; Peña-Cabrera, E.; Vázquez, M.A. Synthesis of Polysubstituted Symmetrical BODIPYs via Fischer Carbene Complexes: Theoretical, Photophysical and Electrochemical Evaluation. Chem. Eur. J. 2022, 28, e202202446. [Google Scholar] [CrossRef]
- Takamuki, Y.; Maki, S.; Niwa, H.; Ikeda, H.; Hirano, T. Substituent Effects on the Spectroscopic Properties of Solvatochromic 2-Phenylimidazo [1,2-a]pyrazine-3(7H)-ones: An Effective Control for the Colorimetric Sensor Properties. Tetrahedron 2005, 61, 10073–10080. [Google Scholar] [CrossRef]
- Chen, C.; Huang, R.; Batsanov, A.S.; Pander, P.; Hsu, Y.-T.; Chi, Z.; Dias, F.B.; Bryce, M.R. Temperature Phosphorescence and Thermally Activated Delayed Fluorescence. Angew. Chem. Int. Ed. 2018, 57, 16407–16411. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Wang, K.; Zhuang, G.; Xie, Z.; Zhang, C.; Cao, F.; Pan, G.; Chen, H.; Zou, B.; Ma, Y. Multicolored-Fluorescence Switching of ICT-Type Organic Solids with Clear Color Difference: Mechanically Controlled Excited State. Chem. Eur. J. 2015, 21, 2474–2479. [Google Scholar] [CrossRef]
- Du, Y.; Wang, H.; Zhao, S.; Fan, J.; Huang, S.; Hao, Y. Design of an ICT-Based Fluorescent Probe with Excellent Sensitivity for Visualizing GSH Levels in Live Cells. Chem. Pap. 2022, 76, 4571–4579. [Google Scholar] [CrossRef]
- Abeywickrama, C.S. Large Stokes Shift Benzothiazolium Cyanine Dyes with Improved Intramolecular Charge Transfer (ICT) for Cell Imaging Applications. Chem. Commun. 2022, 58, 9855–9869. [Google Scholar] [CrossRef]
- Yu, F.; Li, P.; Song, P.; Wang, B.; Zhao, J.; Han, K. An ICT-Based Strategy to a Colorimetric and Ratiometric Fluorescence Probe for Hydrogen Sulfide in Living Cells. Chem. Commun. 2012, 48, 2852–2854. [Google Scholar] [CrossRef]
- Matsumoto, S.; Qu, S.; Kobayashi, T.; Kanehiro, M.; Akazome, M.; Ogura, K. Novel Formation of Dipyrrolo- and Diindolo [1,2-a:2′,1′-c]quinoxaline Derivatives and Their Optical Properties. Heterocycles 2010, 80, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, S.; Bathmunkh, E.; Akazome, M.; Takata, Y.; Tamano, M. Novel Formation of Diimidazo [1,2-a:2′,1′-c]quinoxaline Derivatives and Their Optical Properties. Org. Biomol. Chem. 2011, 9, 5941–5944. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, S.; Abe, H.; Akazome, M. Fluorescence of Diimidazo [1,2-a:2′,1′-c]quinoxalinium Salts under Various Conditions. J. Org. Chem. 2013, 78, 2397–2404. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, S.; Sakamoto, K.; Akazome, M. Systematic Investigation of Fluorescence Properties of Symmetric and Asymmetric Diazolo [1,2-a:2′,1′-c]quinoxaline Derivatives. Heterocycles 2015, 91, 795–814. [Google Scholar] [CrossRef]
- Pérez-Bolívar, C.; Takizawa, S.; Nishimura, G.; Montes, V.A.; Anzenbacher, P., Jr. High-Efficiency Tris(8-hydroxyquinoline)aluminum (Alq3) Complexes for Organic White-Light-Emitting Diodes and Solid-State Lighting. Chem. Eur. J. 2011, 17, 9076–9082. [Google Scholar] [CrossRef]
- Antilla, J.C.; Klapars, A.; Buchwald, S.L. The Copper-Catalyzed N-Arylation of Indoles. J. Am. Chem. Soc. 2002, 124, 11684–11688. [Google Scholar] [CrossRef]
- Deprez, N.R.; Kalyani, D.; Krause, A.; Sanford, M.S. Room Temperature Palladium-Catalyzed 2-Arylation of Indoles. J. Am. Chem. Soc. 2006, 128, 4972–4973. [Google Scholar] [CrossRef]
- Valeur, B. Molecular Fluorescence. Principles and Applications; Wiley-VCH: Weinheim, Germany, 2002; p. 54. [Google Scholar]
- Andersh, B.; Murphy, D.; Olson, R. Hydrochloric Acid Catalysis of N-Bromosuccinimide (NBS) Mediated Nuclear Aromatic Brominations in Acetone. Synth. Commun. 2000, 30, 2091–2098. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; Revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]






| Entry | Compound | λabs (nm) [ε (M−1 cm−1)] 1 | λem (nm) 2 [ΦF] 3 | Δλ (nm) 4 [Δν (cm−1) 5] | |||
|---|---|---|---|---|---|---|---|
| In THF | In CH3CN | In THF | In CH3CN | In THF | In CH3CN | ||
| 1 | 1a | 321 [10,600] | 320 [10,700] | 416 [0.43] | 434 [0.17] | 95 [7114] | 114 [8209] |
| 2 | 1b | 367 [4800] | 369 [4900] | 435 [0.01] | 434 [0.02] | 68 [4259] | 65 [4059] |
| 3 | 1c | 322 [11,200] | 322 [15,600] | 434 [0.22] | 453 [0.12] | 112 [8014] | 131 [8981] |
| 4 | 1d | 325 [9500] | 324 [8100] | 449 [0.25] | 458 [0.21] | 124 [8498] | 134 [9030] |
| 5 | 3d | 377 [10,600] | 375 [8700] | 466 [0.35] | 473 [0.32] | 89 [5066] | 98 [5525] |
| Entry | Compound | HOMO Energy (eV) | Difference of HOMO Energy against 1a (eV) | LUMO Energy (eV) | Difference of LUMO Energy against 1a (eV) | Energy Gap between HOMO and LUMO (eV) | λem (nm) in THF |
|---|---|---|---|---|---|---|---|
| 1 | 1a | −6.5830 | - | 6.8151 | - | 6.8151 | 416 |
| 2 | 1b | −6.5122 | 0.0708 | 0.2653 | 0.0332 | 6.7775 | 435 |
| 4 | 1c | −6.7092 | −0.1262 | −0.0063 | −0.2384 | 6.7029 | 434 |
| 6 | 1d | −6.8233 | −0.2403 | −0.1973 | −0.4294 | 6.6260 | 449 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsumoto, S.; Takamori, M.; Akazome, M. Bathochromic Shift of Fluorescence Peak in Dipyrrolo[1,2-a:2′,1′-c]quinoxaline by Introducing Each of Electron-Donating and Electron-Withdrawing Substituent. Molecules 2023, 28, 2896. https://doi.org/10.3390/molecules28072896
Matsumoto S, Takamori M, Akazome M. Bathochromic Shift of Fluorescence Peak in Dipyrrolo[1,2-a:2′,1′-c]quinoxaline by Introducing Each of Electron-Donating and Electron-Withdrawing Substituent. Molecules. 2023; 28(7):2896. https://doi.org/10.3390/molecules28072896
Chicago/Turabian StyleMatsumoto, Shoji, Makoto Takamori, and Motohiro Akazome. 2023. "Bathochromic Shift of Fluorescence Peak in Dipyrrolo[1,2-a:2′,1′-c]quinoxaline by Introducing Each of Electron-Donating and Electron-Withdrawing Substituent" Molecules 28, no. 7: 2896. https://doi.org/10.3390/molecules28072896
APA StyleMatsumoto, S., Takamori, M., & Akazome, M. (2023). Bathochromic Shift of Fluorescence Peak in Dipyrrolo[1,2-a:2′,1′-c]quinoxaline by Introducing Each of Electron-Donating and Electron-Withdrawing Substituent. Molecules, 28(7), 2896. https://doi.org/10.3390/molecules28072896