Development of Anthocyanin-Rich Gel Beads from Colored Rice for Encapsulation and In Vitro Gastrointestinal Digestion
Abstract
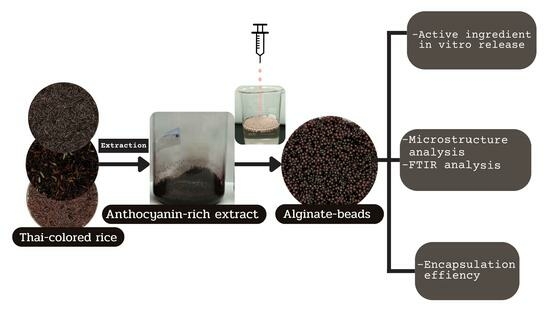
:1. Introduction
2. Results and Discussion
2.1. Anthocyanin Content of Colored Rice Samples
2.2. Gel Beads: Conventional and Oil-Wax Gel Beads
2.3. Particle Size, Visible Observation, and SEM Analysis of Gel Beads
| Beads Type | Mean Diameter (mm) | Anthocyanin as C3G 1 Content (µg/g) |
|---|---|---|
| Gel bead (no oil) | 1.48 ± 0.14 d | 29.07 ± 2.89 d |
| Gel bead + oil | 2.85 ± 0.21 b | 45.78 ± 1.70 a |
| Gel bead + oil + 1% beeswax | 2.83 ± 0.28 b | 40.13 ± 3.19 bc |
| Gel bead + oil + 2% beeswax | 3.05 ± 0.21 a | 41.09 ± 3.04 bc |
| Gel bead + oil + 3% beeswax | 3.08 ± 0.18 a | 42.83 ± 0.95 ab |
| Gel bead + oil + 1% carnauba wax | 3.00 ± 0.21 a | 37.99 ± 0.56 c |
| Gel bead + oil + 2% carnauba wax | 2.51 ± 0.39 c | 31.91 ± 2.08 d |
| Gel bead + oil + 3% carnauba wax | 3.11 ± 0.37 a | 32.16 ± 0.61 d |

2.4. Fourier Transform Infrared Spectroscopy Analysis

2.5. Encapsulation Efficiency Percentage

2.6. Release of Anthocyanin Content

2.7. Release of Phenolic Content

2.8. Release of Antioxidant

3. Materials and Methods
3.1. Chemical
3.2. Rice Samples and Processing
3.3. Anthocyanin Analysis Using HPLC
3.4. Preparation of Anthocyanin-Rich Extract
3.5. Encapsulation of Alginate Gel Beads
3.5.1. Conventional Calcium Alginate Gel Beads
3.5.2. Oil-Wax-Incorporated Gel Beads
3.5.3. Oil-Wax Gel Beads
3.6. Anthocyanin-Rich Gel Bead Characterization
3.6.1. Particle Size of the Gel Beads Study
3.6.2. Fourier Transform Infrared Absorption Study
3.6.3. Scanning Electron Microscopy Study
3.6.4. Determination of Percentage of Encapsulation Efficiency
3.6.5. Active Ingredient Release Determination
3.6.6. Total Phenolic Content Determination
3.6.7. Antioxidant Activity Based on Ferric-Reducing Antioxidant Power Determination
3.7. Data Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xia, D.; Zhou, H.; Wang, Y.; Li, P.; Fu, P.; Wu, B.; He, Y. How rice organs are colored: The genetic basis of anthocyanin biosynthesis in rice. Crop J. 2021, 9, 598–608. [Google Scholar] [CrossRef]
- Semmarath, W.; Mapoung, S.; Umsumarng, S.; Arjsri, P.; Srisawad, K.; Thippraphan, P.; Yodkeeree, S.; Dejkriengkraikul, P. Cyanidin-3-O-glucoside and Peonidin-3-O-glucoside-rich fraction of black rice germ and bran suppresses inflammatory responses from SARS-CoV-2 spike glycoprotein S1-induction in vitro in A549 lung cells and THP-1 macrophages via inhibition of the NLRP3 inflammasome pathway. Nutrients 2022, 14, 2738. [Google Scholar] [PubMed]
- Oliveira, H.; Fernandes, A.; Brás, N.F.; Mateus, N.; de Freitas, V.; Fernandes, I. Anthocyanins as antidiabetic agents—In Vitro and in silico approaches of preventive and therapeutic effects. Molecules 2020, 25, 3813. [Google Scholar] [CrossRef] [PubMed]
- de Arruda Nascimento, E.; de Lima Coutinho, L.; da Silva, C.J.; de Lima, V.; Dos Santos Aguiar, J. In vitro anticancer properties of anthocyanins: A systematic review. Biochim. Biophys. Acta Rev. Cancer. 2022, 1877, 188748. [Google Scholar] [CrossRef] [PubMed]
- Enaru, B.; Drețcanu, G.; Pop, T.D.; Stǎnilǎ, A.; Diaconeasa, Z. Anthocyanins: Factors affecting their stability and degradation. Antioxidants 2021, 10, 1967. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.W.; Wang, C.K.; Huang, X.Y.; Hu, D.G. Anthocyanin stability and degradation in plants. Plant Signal. Behav. 2021, 16, 1987767. [Google Scholar] [CrossRef] [PubMed]
- Norkaew, O.; Thitisut, P.; Mahatheeranont, S.; Pawin, B.; Sookwong, P.; Yodpitak, S.; Lungkaphin, A. Effect of wall materials on some physicochemical properties and release characteristics of encapsulated black rice anthocyanin microcapsules. Food Chem. 2019, 294, 493–502. [Google Scholar] [CrossRef]
- Klojdová, I.; Milota, T.; Smetanová, J.; Stathopoulos, C. Encapsulation: A Strategy to Deliver Therapeutics and Bioactive Compounds? Pharmaceuticals 2023, 16, 362. [Google Scholar] [CrossRef]
- Frent, O.D.; Vicas, L.G.; Duteanu, N.; Morgovan, C.M.; Jurca, T.; Pallag, A.; Muresan, M.E.; Filip, S.M.; Lucaciu, R.-L.; Marian, E. Sodium alginate—Natural microencapsulation material of polymeric microparticles. Int. J. Mol. Sci. 2022, 23, 12108. [Google Scholar] [CrossRef]
- Soradech, S.; Petchtubtim, I.; Thongdon-A, J.; Muangman, T. Development of wax-incorporated emulsion gel beads for the encapsulation and intragastric floating delivery of the active antioxidant from Tamarindus indica L. Molecules 2016, 21, 380. [Google Scholar] [CrossRef]
- Bulatao, R.M.; Samin, J.P.A.; Salazar, J.R.; Monserate, J.J. Encapsulation of anthocyanins from black rice (Oryza sativa L.) bran extract using chitosan-alginate nanoparticles. J. Food Res. 2017, 6, 40. [Google Scholar] [CrossRef]
- Arriola, N.D.A.; de Medeiros, P.M.; Prudencio, E.S.; Müller, C.M.O.; Amboni, R.D.M. Encapsulation of aqueous leaf extract of Stevia rebaudiana Bertoni with sodium alginate and its impact on phenolic content. Food Biosci. 2016, 13, 32–40. [Google Scholar] [CrossRef]
- Martău, G.A.; Mihai, M.; Vodnar, D.C. The use of chitosan, alginate, and pectin in the biomedical and food sector—Biocompatibility, bioadhesiveness, and biodegradability. Polymers 2009, 11, 1837. [Google Scholar] [CrossRef] [PubMed]
- Seke, F.; Manhivi, V.E.; Slabbert, R.M.; Sultanbawa, Y.; Sivakumar, D. In vitro release of anthocyanins from microencapsulated natal plum (Carissa macrocarpa) phenolic extract in alginate/psyllium mucilage beads. Food 2022, 11, 2550. [Google Scholar] [CrossRef] [PubMed]
- Ching, S.H.; Bansal, N.; Bhandari, B. Alginate gel particles—A review of production techniques and physical properties. Crit. Rev. Food Sci. Nutr. 2017, 57, 1133–1152. [Google Scholar] [CrossRef] [PubMed]
- Tarone, A.G.; Cazarin, C.B.B.; Junior, M.R.M. Anthocyanins: New techniques and challenges in microencapsulation. Food Res. Int. 2020, 133, 109092. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.; Brás, N.F.; Mateus, N.; de Freitas, V. Understanding the molecular mechanism of anthocyanin binding to pectin. Langmuir 2014, 30, 8516–8527. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Mascaraque, L.G.; Martínez-Sanz, M.; Hogan, S.A.; López-Rubio, A.; Brodkorb, A. Nano- and microstructural evolution of alginate beads in simulated gastrointestinal fluids. Impact of M/G ratio, molecular weight and pH. Carbohydr. Polym. 2019, 223, 115121. [Google Scholar] [CrossRef]
- Sriamornsak, P.; Nunthanid, J. Calcium pectinate gel beads for controlled release drug delivery: I. preparation and in vitro release studies. Int. J. Pharm. 1998, 160, 207–212. [Google Scholar] [CrossRef]
- Sriamornsak, P.; Thirawong, N.; Puttipipatkhachorn, S. Emulsion gel beads of calcium pectinate capable of floating on the gastric fluid: Effect of some additives, hardening agent or coating on release behavior of metronidazole. Eur. J. Pharm. Sci. 2005, 24, 363–373. [Google Scholar] [CrossRef]
- Daemi, H.; Barikani, M. Synthesis and characterization of calcium alginate nanoparticles, sodium homopolymannuronate salt and its calcium nanoparticles. Sci. Iran. 2012, 19, 2023–2028. [Google Scholar] [CrossRef]
- Sriamornsak, P.; Asavapichayont, P.; Nunthanid, J.; Luangtana-Anan, M.; Limmatvapirat, S.; Piriyaprasarth, S. Wax-incorporated emulsion gel beads of calcium pectinate for intragastric floating drug delivery. AAPS PharmSciTech. 2008, 9, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Gavini, V.; Reddy, B.P.; Konijeti, S.R.; Kiran, K.P. Formulation & in vitro evaluation of wax incorporated floating beads of silymarin. Int. J. Pharmtech Res. 2014, 6, 1824–1832. [Google Scholar]
- Pérez-Gregorio, R.M.; García-Falcón, M.S.; Simal-Gándara, J.; Rodrigues, S.A.; Almeida, D.P.F. Identification and quantification of flavonoids in traditional cultivars of red and white onions at harvest. J. Food Compos. Anal. 2010, 23, 592–598. [Google Scholar] [CrossRef]
- Kusuktham, B.; Prasertgul, J.; Srinun, P. Morphology and property of calcium silicate encapsulated with alginate beads. Silicon 2014, 6, 191–197. [Google Scholar] [CrossRef]
- Talens, P.; Krochta, J.M. Plasticizing effects of beeswax and carnauba wax on tensile and water vapor permeability properties of whey protein films. J. Food Sci. 2006, 70, 239–243. [Google Scholar] [CrossRef]
- Homayoonfal, M.; Mousavi, M.; Kiani, H.; Askari, G.; Desobry, S.; Arab-Tehrany, E. Modifying the stability and surface characteristic of anthocyanin compounds Incorporated in the nanoliposome by chitosan biopolymer. Pharmaceutics 2022, 14, 1622. [Google Scholar] [CrossRef]
- Gundev, P.; Chauhan, K.; Sachdev, D.; Swer, T.L. Formulation and characterization of butylated hydroxytoluene (BHT) microspheres using natural beeswax as encapsulating material. J. Food Process. Preserv. 2022, 46, e16458. [Google Scholar] [CrossRef]
- Penagos, I.A.; Murillo Moreno, J.S.; Dewettinck, K.; Van Bockstaele, F. Carnauba wax and beeswax as structuring agents for water-in-oleogel emulsions without added emulsifiers. Foods 2023, 12, 1850. [Google Scholar] [CrossRef]
- Peanparkdee, M.; Iwamoto, S.; Yamauchi, R. Preparation and release behavior of gelatin-based capsules of antioxidants from ethanolic extracts of Thai riceberry bran. Food Bioproc. Technol. 2017, 10, 1737–1748. [Google Scholar] [CrossRef]
- Bermúdez-Soto, M.J.; Tomás-Barberán, F.A.; García-Conesa, M.T. Stability of polyphenols in chokeberry (Aronia melanocarpa) subjected to in vitro gastric and pancreatic digestion. Food Chem. 2007, 102, 865–874. [Google Scholar] [CrossRef]
- Porntip, P.; Oran, K.; Sirichai, A. Alginate-based encapsulation of polyphenols from Clitoria ternatea petal flower extract enhances stability and biological activity under simulated gastrointestinal conditions. Food Hydrocoll. 2016, 61, 772–779. [Google Scholar]
- Kay, C.D.; Kroon, P.A.; Cassidy, A. The bioactivity of dietary anthocyanins is likely to be mediated by their degradation products. Mol. Nutr. Food Res. 2009, 53, S92–S101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Kou, X.; Fugal, K.; McLaughlin, J. Comparison of HPLC methods for determination of anthocyanins and anthocyanidins in bilberry extracts. J. Agric. Food Chem. 2004, 52, 688–691. [Google Scholar] [CrossRef] [PubMed]
- Flores, F.P.; Singh, R.K.; Kerr, W.L.; Pegg, R.B.; Kong, F. Total phenolics content and antioxidant capacities of microencapsulated blueberry anthocyanins during in vitro digestion. Food Chem. 2014, 153, 272–278. [Google Scholar] [CrossRef]
- Ceymann, M.; Arrigoni, E.; Schärer, H.; Nising, A.B.; Hurrell, R.F. Identification of apples rich in health-promoting flavan-3-ols and phenolic acids by measuring the polyphenol profile. J. Food Compost. Anal. 2012, 26, 128–135. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soiklom, S.; Siri-anusornsak, W.; Petchpoung, K.; Kansandee, W. Development of Anthocyanin-Rich Gel Beads from Colored Rice for Encapsulation and In Vitro Gastrointestinal Digestion. Molecules 2024, 29, 270. https://doi.org/10.3390/molecules29010270
Soiklom S, Siri-anusornsak W, Petchpoung K, Kansandee W. Development of Anthocyanin-Rich Gel Beads from Colored Rice for Encapsulation and In Vitro Gastrointestinal Digestion. Molecules. 2024; 29(1):270. https://doi.org/10.3390/molecules29010270
Chicago/Turabian StyleSoiklom, Siriwan, Wipada Siri-anusornsak, Krittaya Petchpoung, and Wiratchanee Kansandee. 2024. "Development of Anthocyanin-Rich Gel Beads from Colored Rice for Encapsulation and In Vitro Gastrointestinal Digestion" Molecules 29, no. 1: 270. https://doi.org/10.3390/molecules29010270
APA StyleSoiklom, S., Siri-anusornsak, W., Petchpoung, K., & Kansandee, W. (2024). Development of Anthocyanin-Rich Gel Beads from Colored Rice for Encapsulation and In Vitro Gastrointestinal Digestion. Molecules, 29(1), 270. https://doi.org/10.3390/molecules29010270