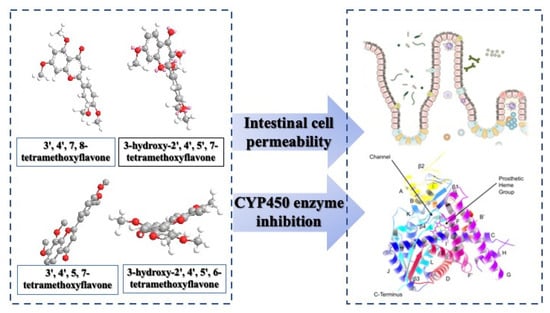
Hydroxylated Tetramethoxyflavone Affects Intestinal Cell Permeability and Inhibits Cytochrome P450 Enzymes
Abstract
:1. Introduction
2. Results and Discussions
2.1. Analysis of TMFs in the Caco-2 Cell by LC-MS
2.2. Transport of TMFs across Caco-2 Cell Monolayer
2.2.1. The Transport Efficiency of TMFs
2.2.2. The Apparent Permeability Coefficient of TMFs
2.3. CYP Inhibitory Effect of TMFs In Vitro
2.3.1. IC50 of CYP Affected by TMFs
2.3.2. CYP Inhibitory Effect of TMF Structure
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Analysis and Characterization of TMFs by LC-MS
Method Validation
3.3. Caco-2 Cell Cultures
3.4. Transport Experiments
3.5. Absorption Experiments
3.6. CYP Inhibition Assay
3.7. IC50 Determination of CYP
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Maqbool, Z.; Khalid, W.; Atiq, H.T.; Koraqi, H.; Javaid, Z.; Alhag, S.K.; Al-Shuraym, L.A.; Bader, D.M.D.; Almarzuq, M.; Afifi, M.; et al. Citrus Waste as Source of Bioactive Compounds: Extraction and Utilization in Health and Food Industry. Molecules 2023, 28, 1636. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Tao, Y.; Huang, Y.; Zogona, D.; Wu, T.; Liu, R.; Pan, S.; Xu, X. Aged Pericarpium Citri Reticulatae ‘Chachi’ Attenuates Oxidative Damage Induced by tert-Butyl Hydroperoxide (t-BHP) in HepG2 Cells. Foods 2022, 11, 273. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Mei, X.; Liu, Z.; Li, J.; Zhang, X.; Lang, S.; Dai, L.; Zhang, J. Drug Metabolite Cluster-Based Data-Mining Method for Comprehensive Metabolism Study of 5-hydroxy-6,7,3′,4′-tetramethoxyflavone in Rats. Molecules 2019, 24, 3278. [Google Scholar] [CrossRef]
- Xu, Y.; Lv, X.; Yang, G.; Zhan, J.; Li, M.; Long, T.; Ho, C.T.; Li, S. Simultaneous separation of six pure polymethoxyflavones from sweet orange peel extract by high performance counter current chromatography. Food Chem. 2019, 292, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.; Wei, W.; Zhang, J.; Wang, H.; Bai, Y.; Guo, D. A Scientometric Study to a Critical Review on Promising Anticancer and Neuroprotective Compounds: Citrus Flavonoids. Antioxidants 2023, 12, 669. [Google Scholar] [CrossRef]
- Mushtaq, Z.; Aslam, M.; Imran, M.; Abdelgawad, M.A.; Saeed, F.; Khursheed, T.; Umar, M.; Al Abdulmonem, W.; Al Ghorab, A.H.; Alsagaby, S.A.; et al. Polymethoxyflavones: An updated review on pharmacological properties and underlying molecular mechanisms. Int. J. Food Propert. 2023, 26, 866–893. [Google Scholar] [CrossRef]
- Yang, J.; Liu, H.; Li, L.; Liu, H.; Shi, W.; Wu, L. The chondroprotective role of TMF in PGE2-induced apoptosis associating with endoplasmic reticulum stress. Evid.-Based Complem. Altern. Med. 2015, 2015, 297423. [Google Scholar] [CrossRef]
- Ahmed, O.M.; AbouZid, S.F.; Ahmed, N.A.; Zaky, M.Y.; Liu, H. An Up-to-Date Review on Citrus Flavonoids: Chemistry and Benefits in Health and Diseases. Curr. Pharm. Des. 2021, 27, 513–530. [Google Scholar] [CrossRef]
- Wu, L.; Liu, H.; Li, L.; Liu, H.; Yang, K.; Liu, Z.; Huang, H. 5,7,3′,4′-Tetramethoxyflavone exhibits chondroprotective activity by targeting beta-catenin signaling in vivo and in vitro. Biochem. Biophys. Res. Commun. 2014, 452, 682–688. [Google Scholar] [CrossRef]
- Peng, F.; Huang, X.; Shi, W.; Xiao, Y.; Jin, Q.; Li, L.; Xu, D.; Wu, L. 5,7,3′,4′-tetramethoxyflavone ameliorates cholesterol dysregulation by mediating SIRT1/FOXO3a/ABCA1 signaling in osteoarthritis chondrocytes. Future Med. Chem. 2021, 13, 2153–2166. [Google Scholar] [CrossRef]
- Wu, L.; Liu, H.; Li, L.; Xu, D.; Gao, Y.; Guan, Y.; Chen, Q. 5,7,3′,4′-Tetramethoxyflavone protects chondrocytes from ER stress-induced apoptosis through regulation of the IRE1alpha pathway. Connect. Tissue Res. 2018, 59, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Xia, X.; Zhong, H.; Shen, J.; Li, S. Protective Effect of Tangeretin and 5-Hydroxy-6,7,8,3′,4′-Pentamethoxyflavone on Collagen-Induced Arthritis by Inhibiting Autophagy via Activation of the ROS-AKT/mTOR Signaling Pathway. J. Agric. Food Chem. 2021, 69, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.S.; Yam, M.F. Mechanism of vasorelaxation induced by 3′-hydroxy-5,6,7,4′-tetramethoxyflavone in the rats aortic ring assay. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2018, 391, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Zarrabi, A.; Saberifar, S.; Hashemi, F.; Hushmandi, K.; Hashemi, F.; Moghadam, E.R.; Mohammadinejad, R.; Najafi, M.; Garg, M. Nobiletin in Cancer Therapy: How This Plant Derived-Natural Compound Targets Various Oncogene and Onco-Suppressor Pathways. Biomedicines 2020, 8, 110. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Lin, C.C.; Yang, Y.; Yuan, L.; Wang, P.; Wen, X.; Pan, M.H.; Zhao, H.; Ho, C.T.; Li, S. Nobiletin Prevents Trimethylamine Oxide-Induced Vascular Inflammation via Inhibition of the NF-κB/MAPK Pathways. J. Agric. Food Chem. 2019, 67, 6169–6176. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.S.; Wu, J.C.; Ho, C.T.; Pan, M.H. Disease chemopreventive effects and molecular mechanisms of hydroxylated polymethoxyflavones. Biofactors 2015, 41, 301–313. [Google Scholar] [CrossRef]
- Fontana, G.; Bruno, M.; Sottile, F.; Badalamenti, N. The Chemistry and the Anti-Inflammatory Activity of Polymethoxyflavonoids from Citrus Genus. Antioxidants 2022, 12, 23. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Ma, J.; Li, M.; Zhang, Y.; Jiang, B.; Zhao, X.; Huai, C.; Shen, L.; Zhang, N.; He, L.; et al. Cytochrome P450 enzymes and drug metabolism in humans. Int. J. Mol. Sci. 2021, 22, 12808. [Google Scholar] [CrossRef]
- Manikandan, P.; Nagini, S. Cytochrome P450 structure, function and clinical significance: A review. Curr. Cancer Drug Targets 2018, 19, 38–54. [Google Scholar] [CrossRef]
- Fang, Y.; Xia, M.; Liang, F.; Cao, W.; Pan, S.; Xu, X. Establishment and use of human mouth epidermal carcinoma (KB) cells overexpressing P-glycoprotein to characterize structure requirements for flavonoids transported by the efflux transporter. J. Agric. Food Chem. 2019, 67, 2350–2360. [Google Scholar] [CrossRef]
- You, Q.; Li, D.; Ding, H.; Chen, H.; Hu, Y.; Liu, Y. Pharmacokinetics and Metabolites of 12 Bioactive Polymethoxyflavones in Rat Plasma. J. Agric. Food Chem. 2021, 69, 12705–12716. [Google Scholar] [CrossRef]
- Iftikhar, M.; Iftikhar, A.; Zhang, H.; Gong, L.; Wang, J. Transport, metabolism and remedial potential of functional food extracts (FFEs) in Caco-2 cells monolayer: A review. Food Res. Int. 2020, 136, 109240. [Google Scholar] [CrossRef] [PubMed]
- Naseem, A.; Pal, A.; Gowan, S.; Asad, Y.; Donovan, A.; Temesszentandrási-Ambrus, C.; Kis, E.; Gaborik, Z.; Bhalay, G.; Raynaud, F. Intracellular Metabolomics Identifies Efflux Transporter Inhibitors in a Routine Caco-2 Cell Permeability Assay-Biological Implications. Cells 2022, 11, 3286. [Google Scholar] [CrossRef] [PubMed]
- Berben, P.; Bauer-Brandl, A.; Brandl, M.; Faller, B.; Flaten, G.E.; Jacobsen, A.C.; Brouwers, J.; Augustijns, P. Drug permeability profiling using cell-free permeation tools: Overview and applications. Eur. J. Pharm. Sci. 2018, 119, 219–233. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Zhiti, A.; Barba-Bon, A.; Hennig, A.; Nau, W.M. Real-Time Parallel Artificial Membrane Permeability Assay Based on Supramolecular Fluorescent Artificial Receptors. Front. Chem. 2020, 8, 597927. [Google Scholar] [CrossRef] [PubMed]
- Tsuchitani, T.; Akiyoshi, T.; Imaoka, A.; Ohtani, H. Mechanistic bottom-up estimation of passive drug absorption from the gastrointestinal tract: Comparison among primary cultured human intestinal cells, Caco-2 cells, artificial membrane, and animal scale-up. Int. J. Clin. Pharmacol. Ther. 2022, 60, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Pham-The, H.; Cabrera-Pérez, M.Á.; Nam, N.H.; Castillo-Garit, J.A.; Rasulev, B.; Le-Thi-Thu, H.; Casañola-Martin, G.M. In Silico Assessment of ADME Properties: Advances in Caco-2 Cell Monolayer Permeability Modeling. Curr. Top. Med. Chem. 2018, 18, 2209–2229. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Pan, M.H.; Wang, Z.; Lambros, T.; Ho, C.T. Biological activity, metabolism and separation of polymethoxyflavonoids from citrus peels. Tree For. Sci. Biotechnol. 2008, 2, 36–51. [Google Scholar]
- Li, S.; Pan, M.H.; Lo, C.Y.; Tan, D.; Wang, Y.; Shahidi, F.; Ho, C.T. Chemistry and health effects of polymethoxyflavones and hydroxylated polymethoxyflavones. J. Funct. Foods 2009, 1, 2–12. [Google Scholar] [CrossRef]
- Verma, D.; Mitra, D.; Paul, M.; Chaudhary, P.; Kamboj, A.; Thatoi, H.; Janmeda, P.; Jain, D.; Panneerselvam, P.; Shrivastav, R.; et al. Potential inhibitors of SARS-CoV-2 (COVID 19) proteases PLpro and Mpro/3CLpro: Molecular docking and simulation studies of three pertinent medicinal plant natural components. Curr. Res. Pharmacol. Drug Discov. 2021, 2, 100038. [Google Scholar] [CrossRef]
- Nagayoshi, H.; Murayama, N.; Takenaka, S.; Kim, V.; Kim, D.; Komori, M.; Yamazaki, H.; Guengerich, F.P.; Shimada, T. Roles of cytochrome P450 2A6 in the oxidation of flavone, 4′-hydroxyflavone, and 4′-, 3′-, and 2′-methoxyflavones by human liver microsomes. Xenobiotica 2021, 51, 995–1009. [Google Scholar] [CrossRef] [PubMed]
- Brahmi, Z.; Niwa, H.; Yamasato, M.; Shigeto, S.; Kusakari, Y.; Sugaya, K.; Onose, J.; Abe, N. Effective cytochrome P450 (CYP) inhibitor isolated from thyme (Thymus saturoides) purchased from a Japanese market. Biosci. Biotechnol. Biochem. 2011, 75, 2237–2239. [Google Scholar] [CrossRef] [PubMed]
- Surichan, S.; Androutsopoulos, V.P.; Sifakis, S.; Koutala, E.; Tsatsakis, A.; Arroo, R.R.; Boarder, M.R. Bioactivation of the citrus flavonoid nobiletin by CYP1 enzymes in MCF7 breast adenocarcinoma cells. Food Chem. Toxicol. 2012, 50, 3320–3328. [Google Scholar] [CrossRef]
- Koolaji, N.; Shammugasamy, B.; Schindeler, A.; Dong, Q.; Dehghani, F.; Valtchev, P. Citrus Peel Flavonoids as Potential Cancer Prevention Agents. Curr. Dev. Nutr. 2020, 4, nzaa025. [Google Scholar] [CrossRef] [PubMed]
- Kashiwabuchi, Y.; Nishimura, Y.; Kurata, N.; Iwase, M.; Kiuchi, Y.; Nobe, K. Inhibition of CYP3A-mediated Midazolam Metabolism by Kaempferia parviflora. Food Saf. 2022, 10, 32–41. [Google Scholar] [CrossRef]
- Weiss, J.; Gattuso, G.; Barreca, D.; Haefeli, W.E. Nobiletin, sinensetin, and tangeretin are the main perpetrators in clementines provoking food-drug interactions in vitro. Food Chem. 2020, 30, 126578. [Google Scholar] [CrossRef]
- Bojić, M.; Kondža, M.; Rimac, H.; Benković, G.; Maleš, Ž. The Effect of Flavonoid Aglycones on the CYP1A2, CYP2A6, CYP2C8 and CYP2D6 Enzymes Activity. Molecules 2019, 24, 3174. [Google Scholar] [CrossRef]
- Shrestha, R.; Kim, J.H.; Nam, W.; Lee, H.S.; Lee, J.M.; Lee, S. Selective inhibition of CYP2C8 by fisetin and its methylated metabolite, geraldol, in human liver microsomes. Drug Metab. Pharmacokinet. 2018, 33, 111–117. [Google Scholar] [CrossRef]
- Deodhar, M.; Al Rihani, S.B.; Arwood, M.J.; Darakjian, L.; Dow, P.; Turgeon, J.; Michaud, V. Mechanisms of CYP450 Inhibition: Understanding Drug-Drug Interactions Due to Mechanism-Based Inhibition in Clinical Practice. Pharmaceutics 2020, 12, 846. [Google Scholar] [CrossRef]
- Lu, C.; Fu, K.; Cao, K.; Wei, J.; Zhou, J.; Zhao, D.; Li, N.; Lu, Y.; Chen, X.; Zhang, Y. Permeability and transport mechanism of trihexyphenidyl hydrochloride in Caco-2 cell monolayer model with a validated UPLC-MS method. J. Pharm. Biomed. Anal. 2020, 178, 112924. [Google Scholar] [CrossRef]
- Inada, A.; Sawao, A.; Takahashi, K.; Oshima, T. Enhanced water dispersibility and Caco-2 cell monolayer permeability of quercetin by complexation with casein hydrolysate. J. Food Sci. 2022, 87, 1174–1183. [Google Scholar] [CrossRef] [PubMed]
- Lanevskij, K.; Didziapetris, R. Physicochemical QSAR Analysis of Passive Permeability Across Caco-2 Monolayers. J. Pharm. Sci. 2019, 108, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Jan, K.C.; Chang, Y.W.; Hwang, L.S.; Ho, C.T. Tissue distribution and cytochrome P450 inhibition of sesaminol and its tetrahydrofuranoid metabolites. J. Agric. Food Chem. 2012, 60, 8616–8623. [Google Scholar] [CrossRef] [PubMed]
- Fraser, A.W.; Lewis, J.R. Eupatorin, a constituent of Merrillia caloxylon. Planta Med. 1974, 13, 1561–1564. [Google Scholar] [CrossRef]
- Azuma, T.; Tanaka, Y.; Kikuzaki, H. Phenolic glycosides from Kaempferia parviflora. Phytochemistry 2008, 69, 2743–2748. [Google Scholar] [CrossRef] [PubMed]
- Chaipech, S.; Morikawa, T.; Ninomiya, K.; Yoshikawa, M.; Pongpiriyadacha, Y.; Hayakawa, T.; Muraoka, O. Structures of two new phenolic glycosides, kaempferiaosides A and B, and hepatoprotective constituents from the rhizomes of Kaempferia parviflora. Chem. Pharm. Bull. 2012, 60, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, M.; Iinuma, M.; Ohara, M.; Tanaka, T.; Iwamasa, M. Chemotaxonomy of the Genus Citrus Based on Polymethoxyflavones. Chem. Pharm. Bull. 1991, 39, 945–949. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Li, N.; Che, Y.Y.; Zhang, Y.; Liang, S.X.; Zhao, M.B.; Jiang, Y.; Tu, P.F. Characterization of seventy polymethoxylated flavonoids (PMFs) in the leaves of Murraya paniculata by on-line high-performance liquid chromatography coupled to photodiode array detection and electrospray tandem mass spectrometry. J. Pharm. Biomed. Anal. 2011, 56, 950–961. [Google Scholar] [CrossRef]
- Tuchinda, P.; Reutrakul, V.; Claeson, P.; Pongprayoon, U.; Sematongz, T.; Santisuk, T.; Taylor, W.C. Anti-inflammatory cyclohexenyl chalcone derivatives in Boesenbergia pandurata. Phytochemistry 2002, 59, 169–173. [Google Scholar] [CrossRef]
- Jaipetch, T.; Reutrakul, V.; Tuntiwachwuttikul, P.; Santisuk, T. Flavonoids in the black rhizomes of Boesenbergia panduta. Phytochemistry 1983, 22, 625–626. [Google Scholar] [CrossRef]
- Asamenew, G.; Kim, H.W.; Lee, M.K. Characterization of phenolic compounds from normal ginger (Zingiber officinale Rosc.) and black ginger (Kaempferia parviflora Wall.) using UPLC–DAD–QToF–MS. Eur. Food Res. Technol. 2019, 245, 653–665. [Google Scholar] [CrossRef]
- Rajudin, E.; Ahmad, F.; Sirat, H.M.; Arbain, D.; Aboul-Enein, H.Y. Chemical constituents from tiger’s betel, Piper porphyrophyllum N.E.Br. (Fam. Piperaceae). Nat. Prod. Res. 2010, 24, 387–390. [Google Scholar] [CrossRef]
- Kim, Y.D.; Ko, W.J.; Koh, K.S.; Jeon, Y.J.; Kim, S.H. Composition of Flavonoids and Antioxidative Activity from Juice of Jeju Native Citrus Fruits during Maturation. Korean J. Nutr. 2009, 42, 278–290. [Google Scholar] [CrossRef]
- Lu, W.C.; Sheen, J.F.; Hwang, L.S.; Wei, G.J. Identification of 5,7,3′,4′-tetramethoxyflavone metabolites in rat urine by the isotope-labeling method and ultrahigh-performance liquid chromatography-electrospray ionization-mass spectrometry. J. Agric. Food Chem. 2012, 60, 8123–8128. [Google Scholar] [CrossRef] [PubMed]
- Fourie, T.G.; du Preez, I.C.; Roux, D.G. 3′,4′,7,8-tetrahydroxyflavonoids from the heartwood of Acacia nigrescens and their conversion products. Phytochemistry 1972, 11, 1763–1770. [Google Scholar] [CrossRef]
- Li, S.M.; Yang, J.L.; Liu, Y.P.; Fu, Y.H. Studies on non-alkaloid constituents from Alstonia mairei. Chin. Tradit. Herb. Drugs 2015, 24, 2683–2688. [Google Scholar]
- Ballesteros, J.F.; Sanz, M.J.; Ubeda, A.; Miranda, M.A.; Iborra, S.; Payá, M.; Alcaraz, M.J. Synthesis and pharmacological evaluation of 2′-hydroxychalcones and flavones as inhibitors of inflammatory mediators generation. J. Med. Chem. 1995, 38, 2794–2797. [Google Scholar] [CrossRef]
- Weng, Z.; Patel, A.B.; Panagiotidou, S.; Theoharides, T.C. The novel flavone tetramethoxyluteolin is a potent inhibitor of human mast cells. J. Allergy Clin. Immunol. 2015, 135, 1044–1052.e5. [Google Scholar] [CrossRef]
- Bawazeer, M.A.; Theoharides, T.C. IL-33 stimulates human mast cell release of CCL5 and CCL2 via MAPK and NF-κB, inhibited by methoxyluteolin. Eur. J. Pharmacol. 2019, 865, 172760. [Google Scholar] [CrossRef]
- Patel, A.B.; Theoharides, T.C. Methoxyluteolin Inhibits Neuropeptide-stimulated Proinflammatory Mediator Release via mTOR Activation from Human Mast Cells. J. Pharmacol. Exp. Ther. 2017, 361, 462–471. [Google Scholar] [CrossRef]
- Taracanova, A.; Alevizos, M.; Karagkouni, A.; Weng, Z.; Norwitz, E.; Conti, P.; Leeman, S.E.; Theoharides, T.C. SP and IL-33 together markedly enhance TNF synthesis and secretion from human mast cells mediated by the interaction of their receptors. Proc. Natl. Acad. Sci. USA 2017, 114, E4002–E4009. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Tsilioni, I. Tetramethoxyluteolin for the Treatment of Neurodegenerative Diseases. Curr. Top. Med. Chem. 2018, 18, 1872–1882. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C.; Stewart, J.M.; Tsilioni, I. Tolerability and benefit of a tetramethoxyluteolin-containing skin lotion. Int. J. Immunopathol. Pharmacol. 2017, 30, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Chen, Z.; Shi, W.; Zhang, R.; Li, L.; Liu, H.; Wu, L. TMF inhibits miR-29a/Wnt/β-catenin signaling through upregulating Foxo3a activity in osteoarthritis chondrocytes. Drug Des. Devel Ther. 2019, 19, 2009–2019. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Li, L.; Shi, W.; Liu, H.; Huang, X.; Liu, Z.; Wu, L. TMF protects chondrocytes from ER stress-induced apoptosis by down-regulating GSK-3β. Biomed. Pharmacother. 2017, 89, 1262–1268. [Google Scholar] [CrossRef]
- Patel, A.B.; Tsilioni, I.; Weng, Z.; Theoharides, T.C. TNF stimulates IL-6, CXCL8 and VEGF secretion from human keratinocytes via activation of mTOR, inhibited by tetramethoxyluteolin. Exp. Dermatol. 2018, 27, 135–143. [Google Scholar] [CrossRef]
- Sae-Wong, C.; Matsuda, H.; Tewtrakul, S.; Tansakul, P.; Nakamura, S.; Nomura, Y.; Yoshikawa, M. Suppressive effects of methoxyflavonoids isolated from Kaempferia parviflora on inducible nitric oxide synthase (iNOS) expression in RAW 264.7 cells. J. Ethnopharmacol. 2011, 136, 488–495. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Tsilioni, I. Amyotrophic Lateral Sclerosis, Neuroinflammation, and Cromolyn. Clin. Ther. 2020, 42, 546–549. [Google Scholar] [CrossRef]
- Nakao, K.; Murata, K.; Deguchiz, T.; Itoh, K.; Fujita, T.; Higashino, M.; Yoshioka, Y.; Matsumura, S.; Tanaka, R.; Shinada, T.; et al. Xanthine oxidase inhibitory activities and crystal structures of methoxyflavones from Kaempferia parviflora rhizome. Biol. Pharm. Bull. 2011, 34, 1143–1146. [Google Scholar] [CrossRef]
- Murata, K.; Hayashi, H.; Matsumura, S.; Matsuda, H. Suppression of benign prostate hyperplasia by Kaempferia parviflora rhizome. Pharmacogn. Res. 2013, 5, 309–314. [Google Scholar] [CrossRef]




| TMF | LC-MS Parent Ion Selection | Fragment Ions, Positively Charged | Chemical Formula | Molecular Weight/ Exact Mass |
|---|---|---|---|---|
| 78-TMF | 343.12 [M + H]+ | 327.09, 312.06, 299.09, 164.08 | C19H18O6 | 342.35/342.11 |
| 57-TMF | 343.12 [M + H]+ | 327.09, 312.06, 299.09, 167.03 | C19H18O6 | 342.35/342.11 |
| 3H7-TMF | 359.1 [M + H]+ | 344.09, 329.06, 299.09, 168.08, 151.04 | C19H18O7 | 358.35/358.11 |
| 3H6-TMF | 359.1 [M + H]+ | 344.09, 329.06, 301.07, 273.08, 151.04 | C19H18O7 | 358.35/358.11 |
| Time (h) | Transport Efficiency (%) * | |||
|---|---|---|---|---|
| 78-TMF | 57-TMF | 3H7-TMF | 3H6-TMF | |
| 1 | 30.49 ± 0.05 c** | 22.48 ± 3.90 c | 37.82 ± 0.07 c | 25.42 ± 1.92 c |
| 2 | 33.98 ± 0.75 b | 33.03 ± 0.46 b | 41.96 ± 1.14 b | 33.27 ± 2.59 b |
| 4 | 37.58 ± 2.11 a | 34.00 ± 1.81 ab | 46.29 ± 2.16 a | 39.43 ± 3.50 a |
| IC50(μM) * | CYP1A2 | CYP2D6 | CYP2C9 | CYP2C19 | CYP3A4 | |
|---|---|---|---|---|---|---|
| TMF | ||||||
| 78-TMF | 0.79 ± 0.12 c | ND | 1.49 ±0.16 c | 1.85 ±0.14 b | 108 ± 6.31 a | |
| 57-TMF | 35.6 ± 12.31 a | ND | 62.5 ± 2.12 a | ND | 3.56 ± 1.23 b | |
| 3H7-TMF | 15.5 ±1.95 b | 2.28 ± 0.46 b | 18.3 ± 8.93 b | 14.8 ± 3.15 a | 0.15 ± 0.07 c | |
| 3H6-TMF | 20.6 ± 0.98 a | 95.5 ± 3.21 a | 8.03 ±3.75 b | 10.2 ± 2.47 a | 0.44 ± 0.12 c | |
| Ketoconazole | ND | 2.35 ± 0.46 b | 1.62 ± 0.74 c | 1.31 ± 0.47 b | 0.022 ± 0.013 d | |
| Furafylline | 0.72 ± 0.24 c | ND | ND | ND | ND | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jan, K.-C.; Gavahian, M. Hydroxylated Tetramethoxyflavone Affects Intestinal Cell Permeability and Inhibits Cytochrome P450 Enzymes. Molecules 2024, 29, 322. https://doi.org/10.3390/molecules29020322
Jan K-C, Gavahian M. Hydroxylated Tetramethoxyflavone Affects Intestinal Cell Permeability and Inhibits Cytochrome P450 Enzymes. Molecules. 2024; 29(2):322. https://doi.org/10.3390/molecules29020322
Chicago/Turabian StyleJan, Kuo-Ching, and Mohsen Gavahian. 2024. "Hydroxylated Tetramethoxyflavone Affects Intestinal Cell Permeability and Inhibits Cytochrome P450 Enzymes" Molecules 29, no. 2: 322. https://doi.org/10.3390/molecules29020322
APA StyleJan, K. -C., & Gavahian, M. (2024). Hydroxylated Tetramethoxyflavone Affects Intestinal Cell Permeability and Inhibits Cytochrome P450 Enzymes. Molecules, 29(2), 322. https://doi.org/10.3390/molecules29020322