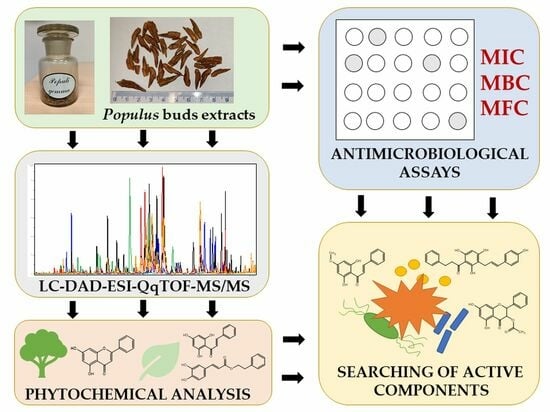
Phytochemical Profiles and Antimicrobial Activity of Selected Populus spp. Bud Extracts
Abstract
:1. Introduction
2. Results and Discussion
2.1. LC-UV-ESI-qTOF-MS/MS Profile of Extracts
2.2. Antimicrobial Properties of Extracts
2.2.1. Activity against Gram-Negative Strains
2.2.2. Activity against Gram-Positive Strains
2.2.3. Activity against Candida spp.
2.2.4. Activity against Helicobacter pylori
3. Materials and Methods
3.1. Populus Buds and Chemicals
3.2. Preparation of Populus Bud Extracts
3.3. UHPLC-DAD-MS/MS Profiling of Populus Bud Extracts
3.4. Determination of Antimicrobial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Białobok, S.; Bugała, W.; Hejnowicz, A.; Jakuszewski, T.; Jankiewicz, L.J.; Obmiński, Z.; Siwecki, R.; Srodoń, A.; Surmiński, J.; Suszka, B.; et al. Nasze Drzewa Leśne: Topole Populus L., 1st ed.; Białobok, S., Ed.; Państwowe Wydawnictwo Naukowe: Warszawa/Poznań, Poland, 1973. [Google Scholar]
- Korbik, M. A review of systematics of Populus L. [article in Polish: Przegląd systematyki rodzaju Populus L.]. Rocz. Pol. Tow. Dendrol. 2020, 68, 77–90. [Google Scholar]
- Stevens, P.F. Angiosperm Phylogeny Website. Available online: https://www.mobot.org/mobot/research/apweb/ (accessed on 7 September 2023).
- Collective Work. Variability of European Black Poplar (Populus nigra L.) in the Danube Basin; Tomović, Z., Vasić, I., Eds.; Public Enterprise Vojvodinašume: Novi Sad, Serbia, 2014. [Google Scholar]
- WFO. World Flora Online. 2023. Available online: https://www.worldfloraonline.org/taxon/wfo-0000928194 (accessed on 7 September 2023).
- Antoniadou, K.; Herz, C.; Le, N.P.K.; Mittermeier-Kleßinger, V.K.; Förster, N.; Zander, M.; Ulrichs, C.; Mewis, I.; Hofmann, T.; Dawid, C.; et al. Identification of salicylates in willow bark (Salix cortex) for targeting peripheral inflammation. Int. J. Mol. Sci. 2021, 22, 11138. [Google Scholar] [CrossRef] [PubMed]
- Willow Bark (Salicis cortex) Monograph. In European Pharmacopoeia, 11th ed.; Council of Europe: Strasbourg, France, 2023; pp. 1778–1779.
- Oketch-Rabah, H.A.; Marles, R.J.; Jordan, S.A.; Low Dog, T. United States Pharmacopeia safety review of willow bark. Planta Med. 2019, 85, 1192–1202. [Google Scholar] [CrossRef]
- Autor, E.; Cornejo, A.; Bimbela, F.; Maisterra, M.; Gandía, L.M.; Martínez-Merino, V. Extraction of phenolic compounds from Populus Salicaceae bark. Biomolecules 2022, 12, 539. [Google Scholar] [CrossRef] [PubMed]
- Abreu, I.N.; Ahnlund, M.; Moritz, T.; Albrectsen, B.R. UHPLC-ESI/TOFMS Determination of Salicylate-like Phenolic Gycosides in Populus tremula Leaves. J. Chem. Ecol. 2011, 37, 857–870. [Google Scholar] [CrossRef]
- Stanciauskaite, M.; Marksa, M.; Babickaite, L.; Majiene, D.; Ramanauskiene, K. Comparison of ethanolic and aqueous Populus balsamifera L. Bud extracts by different extraction methods: Chemical composition, antioxidant and antibacterial activities. Pharmaceuticals 2021, 14, 1018. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.P.; Zheng, H.Q.; Liu, G.; Hu, F.L. Development and validation of HPLC method for determination of salicin in poplar buds: Application for screening of counterfeit propolis. Food Chem. 2011, 127, 345–350. [Google Scholar] [CrossRef]
- Hameed, F.A. Chapter 69—Gout. In Integrative Medicine, 4th ed.; Rakel, D., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 689–696.e2. ISBN 978-0-323-35868-2. [Google Scholar]
- Populi folium Monograph. In Farmakopea Polska, 12th ed.; PTFarm: Warszawa, Poland, 2022; p. 4683.
- Shikov, A.N.; Narkevich, I.A.; Flisyuk, E.V.; Luzhanin, V.G.; Pozharitskaya, O.N. Medicinal plants from the 14th edition of the Russian Pharmacopoeia, recent updates. J. Ethnopharmacol. 2021, 268, 113685. [Google Scholar] [CrossRef]
- Okińczyc, P.; Szumny, A.; Szperlik, J.; Kulma, A.; Franiczek, R.; Żbikowska, B.; Krzyżanowska, B.; Sroka, Z. Profile of polyphenolic and essential oil composition of polish propolis, black poplar and aspens buds. Molecules 2018, 23, 1262. [Google Scholar] [CrossRef]
- Isidorov, V.A.; Szczepaniak, L.; Bakier, S. Rapid GC/MS determination of botanical precursors of Eurasian propolis. Food Chem. 2014, 142, 101–106. [Google Scholar] [CrossRef]
- Trudić, B.; Anđelković, B.; Orlović, S.; Tešević, V.; Pilipović, A.; Cvetković, M.; Stanković, J. HPLC/MS-TOF analysis of surface resins from three poplar clones grown in Serbia. South-East Eur. 2016, 2, 129–133. [Google Scholar] [CrossRef]
- Isidorov, V.A.; Bakier, S.; Pirożnikow, E.; Zambrzycka, M.; Święcicka, I. Selective behaviour of honeybees in acquiring European propolis plant precursors. J. Chem. Ecol. 2016, 42, 475–485. [Google Scholar] [CrossRef]
- Okińczyc, P. Phytochemical Characteristics and Biological Activity of Different Kinds of Propolis and Their Plant Sources. Ph.D. Thesis, Uniwersytet Medyczny we Wrocławiu, Wrocław, Poland, 2017. [Google Scholar]
- Vardar-Ünlü, G.; Silici, S.; Ünlü, M. Composition and in vitro antimicrobial activity of Populus buds and poplar-type propolis. World J. Microbiol. Biotechnol. 2008, 24, 1011–1017. [Google Scholar] [CrossRef]
- Isidorov, V.A. Alchemy of bees. In Bees and Bee Products in Chemist Eyes, Original Title: Alchemia Pszczół. Pszczoły i Produkty Pszczele Oczami Chemika, 1st ed.; Sądecki Bartnik: Stróże, Poland, 2007. (In Polish) [Google Scholar]
- Isidorov, V.A.; Brzozowska, M.; Czyżewska, U.; Glinka, L. Gas chromatographic investigation of phenylpropenoid glycerides from aspen (Populus tremula L.) buds. J. Chromatogr. A 2008, 1198–1199, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Isidorov, V.; Szczepaniak, L.; Wróblewska, A.; Pirożnikow, E.; Vetchinnikova, L. Gas chromatographic-mass spectrometric examination of chemical composition of two Eurasian birch (Betula L.) bud exudates and its taxonomical implication. Biochem. Syst. Ecol. 2014, 52, 41–48. [Google Scholar] [CrossRef]
- English, S.; Greenaway, W.; Whatley, F.R. Analysis of phenolics of Populus trichocarpa bud exudate by GC-MS. Phytochemistry 1991, 30, 531–533. [Google Scholar] [CrossRef]
- Greenaway, W.; English, S.; May, J.; Whatley, F.R. Analysis of phenolics of bud exudates of Populus cathayana and Populus szechuanica by GC-MS. Z. Für Naturforsch.-Sect. C J. Biosci. 1992, 47, 308–312. [Google Scholar] [CrossRef]
- Greenaway, W.; English, S.; Whatley, F.R. Variation in bud exudate composition of Populus nigra assessed by gas ahromatography-mass spectrometry. Z. Für Naturforsch. C-Sect. C J. Biosci. 1990, 45, 931–936. [Google Scholar] [CrossRef]
- Kuś, P.M.; Okińczyc, P.; Jakovljević, M.; Jokić, S.; Jerković, I. Development of supercritical CO2 extraction of bioactive phytochemicals from black poplar (Populus nigra L.) buds followed by GC–MS and UHPLC-DAD-qTOF-MS. J. Pharm. Biomed. Anal. 2018, 158, 15–27. [Google Scholar] [CrossRef]
- Rubiolo, P.; Casetta, C.; Cagliero, C.; Brevard, H.; Sgorbini, B.; Bicchi, C. Populus nigra L. bud absolute: A case study for a strategy of analysis of natural complex substances. Anal. Bioanal. Chem. 2013, 405, 1223–1235. [Google Scholar] [CrossRef]
- Isidorov, V.A.; Vinogorova, V.T. GC-MS analysis of compounds extracted from buds of Populus balsamifera and Populus nigra. Z. Für Naturforsch.-Sect. C J. Biosci. 2003, 58, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Greenaway, W.; May, J.; Scaysbrook, T.; Whatley, F.R. Compositions of bud and leaf exudates of some Populus species compared. Z. Für Naturforsch.-Sect. C J. Biosci. 1992, 47, 329–334. [Google Scholar] [CrossRef]
- Greenaway, W.; Wollenweber, E.; Whatley, F.R. Esters of caffeic acid with aliphatic alcohols in bud exudate of Populus nigra. Z. Für Naturforsch.-Sect. C J. Biosci. 1988, 43, 795–798. [Google Scholar] [CrossRef]
- Okińczyc, P.; Widelski, J.; Ciochoń, M.; Paluch, E.; Bozhadze, A.; Jokhadze, M.; Mtvarelishvili, G.; Korona-Głowniak, I.; Krzyżanowska, B.; Kuś, P.M. Phytochemical profile, plant precursors and some properties of Georgian propolis. Molecules 2022, 27, 7714. [Google Scholar] [CrossRef]
- Asakawa, Y.; Takemoto, T.; Wollenweber, E.; Aratani, T. Lasiocarpin A, B and C, three novel phenolic triglycerides from Populus lasiocarpa. Phytochem. 1977, 16, 1791–1795. [Google Scholar] [CrossRef]
- Eftekhari, A. Chemical and Biological Study on Constituents of Populus tremuloides Buds. Ph.D. Thesis, Universite du Quebec, Quebec, QC, Canada, 2009. [Google Scholar]
- Bélanger, A.; Grenier, A.; Simard, F.; Gendreau, I.; Pichette, A.; Legault, J.; Pouliot, R. Dihydrochalcone derivatives from Populus balsamifera L. buds for the treatment of psoriasis. Int. J. Mol. Sci. 2020, 21, 256. [Google Scholar] [CrossRef] [PubMed]
- Stanciauskaite, M.; Marksa, M.; Liaudanskas, M.; Ivanauskas, L.; Ivaskiene, M.; Ramanauskiene, K. Extracts of poplar buds (Populus balsamifera L., Populus nigra L.) and lithuanian propolis: Comparison of their composition and biological activities. Plants 2021, 10, 828. [Google Scholar] [CrossRef] [PubMed]
- Kakoullis, L.; Papachristodoulou, E.; Chra, P.; Panos, G. Mechanisms of antibiotic resistance in important antibiotic solutions. Antibiotics 2021, 10, 415. [Google Scholar] [CrossRef]
- Zgurskaya, H.I.; López, C.A.; Gnanakaran, S. Permeability barrier of Gram-negative cell envelopes and approaches to bypass it. ACS Infect. Dis. 2016, 1, 512–522. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Mirzoeva, O.K.; Grishanin, R.N.; Calder, P.C. Antimicrobial action of propolis and some of its components: The effects on growth, membrane potential and motility of bacteria. Microbiol. Res. 1997, 152, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Pal, A.; Darokar, M.P. A polyphenolic flavonoid glabridin: Oxidative stress response in multidrug-resistant Staphylococcus aureus. Free Radic. Biol. Med. 2015, 87, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Widelski, J.; Okińczyc, P.; Paluch, E.; Mroczek, T.; Szperlik, J.; Żuk, M.; Sroka, Z.; Sakipova, Z.; Chinou, I.; Skalicka-Woźniak, K.; et al. The antimicrobial properties of poplar and aspen–poplar propolises and their active components against selected microorganisms, including Helicobacter Pylori. Pathog. 2022, 11, 191. [Google Scholar] [CrossRef] [PubMed]
- Simard, F.; Gauthier, C.; Legault, J.; Lavoie, S.; Mshvildadze, V.; Pichette, A. Structure elucidation of anti-methicillin resistant Staphylococcus aureus (MRSA) flavonoids from balsam poplar buds. Bioorganic Med. Chem. 2016, 24, 4188–4198. [Google Scholar] [CrossRef] [PubMed]
- Kaleta, J. The Physicochemical Analysis of Propolis and the Possibility of Its Standardization. Ph.D. Thesis, Jagiellonian University, Kraków, Poland, 2007. [Google Scholar]
- Widelski, J.; Okińczyc, P.; Suśniak, K.; Malm, A.; Bozhadze, A.; Jokhadze, M.; Korona-Głowniak, I. Correlation between chemical profile of Georgian propolis extracts and their activity against Helicobacter pylori. Molecules 2023, 28, 1374. [Google Scholar] [CrossRef] [PubMed]
- Ivyna de Araújo Rêgo, R.; Guedes Silvestre, G.F.; Ferreira de Melo, D.; Albino, S.L.; Pimentel, M.M.; Silva Costa Cruz, S.B.; Silva Wurzba, S.D.; Rodrigues, W.F.; Goulart de Lima Damasceno, B.P.; Cançado Castellano, L.R. Flavonoids-rich plant extracts against Helicobacter pylori infection as prevention to gastric cancer. Front. Pharmacol. 2022, 13, 951125. [Google Scholar] [CrossRef]
- Krzyżek, P.; Migdał, P.; Paluch, E.; Karwańska, M.; Wieliczko, A.; Gościniak, G. Myricetin as an antivirulence compound interfering with a morphological transformation into coccoid forms and potentiating activity of antibiotics against Helicobacter pylori. Int. J. Mol. Sci. 2021, 22, 2695. [Google Scholar] [CrossRef]
- González, A.; Salillas, S.; Velázquez-Campoy, A.; Espinosa Angarica, V.; Fillat, M.F.; Sancho, J.; Lanas, Á. Identifying potential novel drugs against Helicobacter pylori by targeting the essential response regulator HsrA. Sci. Rep. 2019, 9, 11294. [Google Scholar] [CrossRef]
- Song, M.Y.; Lee, D.Y.; Han, Y.M.; Kim, E.H. Anti-inflammatory effect of Korean propolis on Helicobacter pylori-infected gastric mucosal injury mice model. Nutrients 2022, 14, 4644. [Google Scholar] [CrossRef]
- The European Committee on Antimicrobial Susceptibility Testing—EUCAST. Available online: https://www.eucast.org/ (accessed on 7 September 2023).
- Korona-Głowniak, I.; Głowniak-Lipa, A.; Ludwiczuk, A.; Baj, T.; Malm, A. The in vitro activity of essential oils against Helicobacter pylori growth and urease activity. Molecules 2020, 25, 586. [Google Scholar] [CrossRef]


| No. | Component | RT (min) | UVmax (nm) | [M−H]− | Base MS/MS Peak | Secondary MS/MS Peaks m/z (A (%)) | [M−H]− (Formula) | Error (mDa) | Error (ppm) | RDB |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Unidentified | 0.84 | ND/- | 181.0721 | - | - | C6H13O6 | −0.3 | −1.8 | 0.0 |
| 2 | Unidentified | 0.87 | ND/- | 195.0515 | - | - | C6H11O7 | −0.4 | −2.3 | 1.0 |
| 3 | Unidentified | 0.89 | ND/- | 341.1090 | 113.1273 | - | C12H21O11 | −0.1 | −0.2 | 2.0 |
| 4 | Unidentified | 1.22 | 290 | 191.0201 | 111.1597 | - | C6H7O7 | −0.4 | −2.0 | 3.0 |
| 5 | C Leonuriside A | 4.31 | 268 | 331.1029 | 123.1365 | - | C14H19O9 | 0.5 | 1.5 | 11.0 |
| 6 | A Chlorogenic acid | 6.55 | *324 | 353.0876 | 191.1526 | 135.1 (62.42), 179.2 (50.00) | C16H17O9 | 0.2 | 0.5 | 8.0 |
| 7 | B Caffeoylglucose isomer I | 8.24 | 328 | 341.0884 | 161.1265 | 133.1 (20.50), 135.1 (6.55), 179.1 (4.77) | C15H17O9 | −0.6 | −1.6 | 7.0 |
| 8 | B Caffeoylglucose isomer II | 9.19 | 326 | 341.0889 | 161.0962 | 179.1 (56.51), 135.1 (55.31), 177.2 (32.50), 221.2 (31.58) | C15H17O9 | −1.1 | −3.1 | 7.0 |
| 9 | A Vanilline | 9.36 | 273, 304sh | 151.0397 | 108.1554 | - | C8H7O3 | 0.3 | 2.1 | 5.0 |
| 10 | C Salicyl alcohol dihexoside | 10.02 | 265 | 447.1508 | 269.2836 | - | C19H27O12 | 0.0 | 0.0 | 6.0 |
| 11 | B Caffeoylglucose isomer III | 10.24 | 321 | 341.0880 | 135.1517 | 179.2 (91.78), 161.1 (62.10), 221.2 (28.24), 177.1 (23.94) | C15H17O9 | −0.2 | −0.5 | 7.0 |
| 12 | Cp-Coumaric acid hexoside isomer I | 10.54 | 314 | 325.0936 | 145.1268 | 117.1 (22.35) | C15H17O8 | −0.7 | −2.3 | 7.0 |
| 13 | B Catechin or Epicatechin | 10.87 | 279 | 289.0724 | 123.1394 | 109.1 (87.26), 221.3 (35.68), 137.3 (36.96), 203.2 (30.66) | C15H13O6 | −0.6 | −2.2 | 9.0 |
| 14 | A Caffeic acid | 11.46 | 323 | 179.0346 | 135.0449 | 107.0 (8) | C9H7O4 | 0.4 | 2.0 | 6.0 |
| 15 | B di-Caffeoylglycerol | 11.91 | 323 | 415.1240 | 161.1272 | 415.4 (15.58), 179.1 (6.67), 133.2 (6.42) | C18H23O11 | 0.6 | 1.5 | 7.0 |
| 16 | D Feruloyl or isoferuloyl hexoside isomer I | 12.14 | 328 | 355.1041 | 175.1718 | 160.1 (90.68) | C16H19O9 | −0.7 | −1.8 | 7.0 |
| 17 | B Methoxybenzaldehyde | 12.64 | *279 | 135.0451 | 92.3923 | - | C8H7O2 | 0.0 | 0.0 | 5.0 |
| 18 | B Caffeoylglycerol | 13.06 | *320 | 253.0711 | 161.0743 | 133.2 (92.59), 135.1 (40.05) | C12H13O6 | 0.6 | 2.5 | 6.0 |
| 19 | C p-Coumaric acid hexoside isomer II | 13.11 | *314 | 325.0931 | 145.1625 | 119.2 (72.18), 163.1 (50.79), 205.1 (33.66) | C15H17O8 | −0.2 | −0.7 | 7.0 |
| 20 | D Feruloyl or isoferuloyl hexoside isomer II | 13.36 | *325 | 355.1037 | 134.1126 | 160.2 (95.34), 193.2 (87.81), 191.1 (66.31), 235.2 (62.90) | C16H19O9 | −0.3 | −0.8 | 7.0 |
| 21 | A p-Coumaric acid | 14.42 | 309 | 163.0401 | 119.1668 | 93.1 (10.59) | C9H7O3 | 0.0 | −0.1 | 6.0 |
| 22 | C 3,4,5-Trimethoxy-cinnamic acid | 14.42 | ND/- | 237.0777 | 117.1037 | 145.1 (84.14) | C12H13O5 | −0.8 | −3.4 | 6.0 |
| 23 | B Salicortin | 14.93 | 273 | 423.1303 | 123.1625 | 155.2 (61.54), 121.2 (49.91), 111.3 (45.59) | C20H23O10 | −0.6 | −1.4 | 9.0 |
| 24 | C 7-O-caffeoylsalirepin | 15.18 | 324 | 463.1247 | 179.1438 | 161.2 (42.81), 135.2 (24.90) | C22H23O11 | −0.1 | −0.2 | 11.0 |
| 25 | A Ferulic acid | 15.20 | 321 | 193.0509 | 134.1322 | - | C10H9O4 | −0.3 | −1.5 | 6.0 |
| 26 | B Caffeic acid dihydroxypentyl or isopentyl ester isomer I | 15.22 | ND/- | 281.1031 | 161.1330 | 135.1 (48.99), 133.3 (56.95), 179.2 (8.42) | C14H17O6 | −0.1 | −0.2 | 6.0 |
| 27 | A Isoferulic acid | 15.71 | 322 | 193.0504 | 134.1696 | - | C10H9O4 | 0.3 | 1.4 | 6.0 |
| 28 | C Taxifolin (Dihydroquercetin) isomer I | 16.00 | 289 | 303.0517 | 125.0824 | 153.2 (21.31) | C15H11O7 | −0.7 | −2.3 | 10.0 |
| 29 | D Caffeic acid derivate | 16.01 | 326 | 439.1613 | 161.1313 | 439.4 (17.06), 179.1 (11.23), 133.2 (5.41), 135.1 (3.72) | C21H27O10 | −0.3 | −0.7 | 8.0 |
| 30 | D Caffeic acid derivate | 16.23 | 326 | 439.1612 | 161.1249 | 439.4 (19.80), 179.15 (10.97), 135.2 (5.13), 133.2 (4.22) | C21H27O10 | −0.3 | −0.6 | 8.0 |
| 31 | D Caffeic acid derivate | 16.46 | 326 | 439.1612 | 161.1236 | 439.5 (28.26), 179.2 (11.69), 133.2 (3.78) | C21H27O10 | −0.2 | −0.6 | 8.0 |
| 32 | D Caffeic acid derivate | 16.65 | 326 | 439.1601 | 161.1282 | 439.5 (41.10), 179.1 (6.95), 133.2 (3.52) | C21H27O10 | 0.9 | 0.2 | 8.0 |
| 33 | Unidentified | 16.67 | ND | 295.0828 | 161.1384 | 133.3 (55.74), 135.1 (37.87), 159.3 (11.28), 137.1 (8.71), 179.2 (6.48) | C14H15O7 | −0.4 | −1.5 | 7.0 |
| 34 | B Populoside isomer I | 16.68 | 326 | 447.1297 | 161.1201 | 323.3 (29.72), 179.1 (16.49), 123.1 (9.47), 135.1 (6.61), 203.2 (3.00) | C22H23O10 | −0.1 | −0.2 | 11.0 |
| 35 | C Azelaic acid (Nonanedioic acid) | 17.07 | ND/- | 187.0976 | - | - | C9H15O4 | −0.1 | −0.3 | 2.0 |
| 36 | D p-Coumaric derivate | 17.13 | 311 | 425.1459 | 145.1490 | 163.1 (45.05), 307.3 (31.45), 117.1 (21.30), 265.2 (19.12), 205.2 (18.31), 119.1 (15.12), 161.1 (6.99), 235.2 (5.94) | C20H25O10 | −0.6 | −1.3 | 8.0 |
| 37 | B Populoside isomer II | 17.33 | *326 | 447.1295 | 161.1565 | 179.1 (11.93), 123.1 (7.17), 121.1 (6.73), 135.1 (6.01), 133.3 (7.23), 323.3 (6.06), 447.3 (2.49) | C22H23O10 | 0.1 | 0.3 | 11.0 |
| 38 | C Eriodictyol (Dihydroluteolin) isomer | 17.37 | 288 | 287.0560 | 139.2901 | 137.2 (24.06) | C15H11O6 | 0.1 | 0.5 | 10.0 |
| 39 | Unidentified | 17.40 | *293 | 451.1249 | 121.1191 | 283.2 (46.50), 163.1 (12.29), 175.1 (4.64), 193.1 (3.37), 135.1 (3.40), 145.1 (2.41), 181.1 (2.31) | C21H23O11 | −0.3 | −0.7 | 10.0 |
| 40 | C Pinobanksin- or Naringenin 7-O-hexoside | 17.57 | 286 | 433.1145 | 271.2293 | 165.1 (73.88), 433.4 (42.77), 253.2 (16.18), 243.2 (14.45), 225.2 (10.81), 313.3 (6.69), 227.2 (3.49), 197.2 (2.75), 151.2 (3.19), 241.2 (2.87) | C21H21O10 | −0.5 | −1.2 | 11.0 |
| 41 | Unidentified | 17.69 | *283 | 451.1247 | 138.2292 | 121.1 (5.43), 413.1 (4.41), 163.1 (4.39), 151.1 (4.07), 181.2 (3.36), 405.3 (2.60), 193.2 (2.04) | C21H23O11 | −0.1 | −0.3 | 10.0 |
| 42 | C Vanilloyl-methyl-ketone | 17.79 | *286 | 193.0504 | 133.1891 | - | C10H9O4 | 0.2 | 1.0 | 6.0 |
| 43 | C Taxifolin (Dihydroquercetin) isomer II | 17.84 | 289 | 303.0519 | 151.1068 | 303.1 (17.51) | C15H11O7 | −0.9 | −2.8 | 10.0 |
| 44 | B Salireposide | 18.27 | ND/- | 405.1192 | 242.2316 | 151.1 (78.89), 107.1 (27.34) | C20H21O9 | −0.1 | −0.3 | 10.0 |
| 45 | Unidentified | 18.42 | ND/- | 193.0872 | - | - | C11H13O3 | −0.2 | −1.0 | 5.0 |
| 46 | Unidentified | 18.46 | ND/- | 465.1397 | 123.1096 | 155.2 (46.57) | C22H25O11 | 0.5 | 1.2 | 10.0 |
| 47 | Unidentified | 18.48 | ND/- | 511.1463 | 155.1086 | 123.1 (98.45), 111.1 (94.09), 137.1 (46.54), 109.1 (22.50), 405.4 (21.74), 121.1 (14.37) | C23H27O13 | −0.5 | −1.1 | 10.0 |
| 48 | B Eriodictyol (Dihydroluteolin) | 18.83 | 291 | 287.0564 | 125.1152 | 177.2 (56.00), 152.4 (32.51), 107.4 (12.25), 259.3 (11.25), 213.2 (9.01) | C15H11O6 | −0.2 | −0.8 | 10.0 |
| 49 | B Isograndidentatin A | 18.88 | 314 | 423.1651 | 145.1113 | 163.1 (12.51), 119.1 (6.64), 117.2 (5.33), 423.4 (6.05) | C21H27O9 | 1.0 | 2.3 | 8.0 |
| 50 | B Grandidentatin | 19.30 | 312 | 431.1349 | 145.1447 | 163.1 (13.99), 123.1 (12.54), 307.4 (10.10), 119.1 (5.65), 121.1 (4.50), 187.1 (3.86) | C22H23O9 | −0.1 | −0.3 | 11.0 |
| 51 | B Caffeic acid dimethyl ether | 19.48 | 324 | 207.0664 | - | - | C11H11O4 | −0.1 | −0.5 | 6.0 |
| 52 | D Caffeic acid derivate | 19.72 | 321 | 481.1716 | 161.1269 | 179.2 (38.23), 481.4 (14.79), 135.1 (6.74), 421.5 (6.68), 439.6 (5.39) | C23H29O11 | −0.1 | −0.1 | 9.0 |
| 53 | C Taxifolin 3′-methyl ether (Dihydroisorhamnetin) | 20.29 | ND/- | 317.0673 | 152.1004 | 125.1 (38.44), 179.2 (16.01), 192.2 (11.21) | C16H13O7 | −0.6 | −2.0 | 10.0 |
| 54 | B Populoside isomer III | 20.91 | 325 | 447.1301 | 179.1367 | 135.1 (26.66), 161.1 (24.41) | C22H23O10 | −0.4 | −0.9 | 11.0 |
| 55 | B Apigenin 7-O-glucoside (Apigetrin) | 21.10 | 264, 309sh | 431.0983 | 268.2682 | 431.3 (23.37), 240.1 (9.85), 211.2 (9.64) | C21H19O10 | 0.0 | 0.1 | 12.0 |
| 56 | B Diosmetin 7-O-rutinoside (Diosmin) | 21.21 | ND/- | 607.1675 | 111.1002 | 155.2 (88.44), 123.1 (57.43), 161.1 (28.94), 137.1 (28.43), 109.1 (20.03), 423.5 (18.42), 405.4 (15.17), 299.3 (12.25), 113.1 (7.23), 561.5 (8.28), 101.3 (6.73), 143.1 (4.42), 93.2 (3.19), 317.5 (4.12), 165.2 (2.96), 449.3 (2.56), 159.2 (2.39) | C28H31O15 | −0.6 | −1.0 | 13.0 |
| 57 | Unidentified | 22.15 | ND/- | 385.1508 | 223.2634 | 208.2 (10.07), 152.1 (6.81), 205.2 (2.06) | C18H25O9 | −0.4 | −1.1 | 6.0 |
| 58 | D Caffeic acid derivate | 22.22 | 328 | 489.1407 | 161.1159 | 179.2 (15.01), 123.1 (12.65), 133.2 (8.52), 135.1 (6.63), 489.3 (4.33) | C24H25O11 | −0.5 | −0.9 | 12.0 |
| 59 | B Caffeic ethyl ester | 22.65 | 322 | 207.0664 | 133.3012 | 135.1162 (48.30), 161.0890 (16.11) | C11H11O4 | −0.1 | −0.5 | 6.0 |
| 60 | C Aromadendrin (Dihydrokaempferol) | 23.08 | 288 | 287.0566 | 135.1329 | 151.1078 (15.43) | C15H11O6 | −0.5 | −1.8 | 10.0 |
| 61 | B Pinobanksin 5-methyl ether | 23.40 | 287 | 285.0777 | 252.0429 | 224.0 (55.83), 138.0 (38.07), 241.0 (31.50), 165.0 (14.95), 239.1 (12.13), 195.0 (12.02), 151.0 (11.81), 213.1 (11.34), 267.1 (11.02), 285.1 (9.31), 136.0 (8.53), 107.0 (6.81) | C16H13O5 | −0.8 | −2.9 | 10.0 |
| 62 | C Kaempferol 3-methyl ether (Isokaempferide) | 23.66 | 288 | 299.0553 | 227.1837 | 255.2 (69.84), 284.2 (9.97), 299.1 (7.83) | C16H11O6 | 0.8 | 2.6 | 12.0 |
| 63 | Unidentified | 23.83 | ND/- | 589.1563 | 122.2014 | 139.1 (89.97), 155.1 (75.54), 111.1 (52.83), 387.4 (44.27), 137.2 (32.78), 109.1 (26.60), 233.3 (22.16), 215.3 (20.13), 135.1 (16.17), 205.2 (6.94), 543.5 (8.81), 165.1 (4.14), 163.2 (3.15), 405.3 (2.79), 265.3 (2.13) | C28H29O14 | 0.0 | −0.1 | 14.0 |
| 64 | B di-Caffeoylglycerol | 24.58 | 320 | 415.1033 | 253.2248 | 161.1 (84.50), 179.1 (65.63), 135.1 (55.89) | C21H19O9 | 0.1 | 0.3 | 12.0 |
| 65 | A Quercetin | 25.10 | 364, 270sh, 265 | 301.0353 | 151.0034 | 121.0 (29.41), 107.0 (22.18), 149.0 (14.01), 178.9 (13.92), 301.0 (7.58), 245.0 (6.32), 273.0 (5.48), 163.0 (4.87), 211.0 (3.84) | C15H9O7 | 0.1 | 0.3 | 11.0 |
| 66 | D Caffeic acid derivate | 25.33 | ND/- | 445.1510 | 161.1296 | 445.5 (18.12), 179.2 (7.44), 135.2 (4.83) | C23H25O9 | −0.6 | −1.3 | 11.0 |
| 67 | A Luteolin | 25.40 | 370 | 285.0412 | 133.1356 | 285.2 (83.77), 151.0 (33.21), 199.1 (15.09), 107.1 (12.83) | C15H9O6 | −0.8 | −2.7 | 11.0 |
| 68 | B Quercetin 3-methyl ether | 26.85 | 255, 355 | 315.0497 | 271.0253 | 300.0 (71.14), 255.0 (42.89), 243.0 (22.59), 227.0 (2.55) | C16H11O7 | 0.2 | 0.5 | 11.0 |
| 69 | A Pinobanksin (Dihydrogalangin) | 27.25 | 291 | 271.0615 | 197.0617 | 253.0 (89.28), 161.1 (67.51), 271.1 (56.26), 125.02 (53.39), 151.0 (30.14), 225.1 (24.71), 107.0 (23.97), 209.1 (16.07), 185.1 (15.86), 115.1 (15.08), 157.1 (14.43), 181.1 (14.14), 215.1 (11.83) | C15H11O5 | −0.3 | −1.1 | 10.0 |
| 70 | C Boropinic acid (Caffeic acid 3-methyl, 4-(3-methyl−2-buten−1-yl) ether) | 28.52 | ND/- | 261.1133 | 145.1340 | 117.2 (48.10), 119.1 (14.84), 115.1 (3.56), 113.1 (3.07) | C15H17O4 | −0.1 | −0.2 | 7.0 |
| 71 | A Naringenin (Dihydroapigenin) | 28.60 | 290 | 271.0612 | 119.1344 | 151.0 (43.37), 107.1 (21.94), 187.2 (10.00) | C15H11O5 | 0.0 | 0.1 | 10.0 |
| 72 | B Chrysin 5-methyl ether | 28.80 | ND/- | 267.0662 | 224.1747 | 180.2 (92.97), 252.2 (26.27), 195.2 (15.00) | C16H11O4 | 0.1 | 0.3 | 11.0 |
| 73 | B Eriodictyol 3’-methyl ether (Homoeriodictyol) or Eriodictyol 4’-methyl ether (Hesperetin) | 28.83 | 292 | 301.0723 | 152.0994 | 176.1 (55.71), 283.2 (57.41), 125.1 (50.48), 301.2 (51.90), 227.4 (33.57), 268.2 (25.56), 107.2 (17.38) | C16H13O6 | −0.6 | −1.9 | 10.0 |
| 74 | B 1-Caffeoyl−3-p-coumaroylglycerol | 28.96 | 312 | 399.1085 | 163.1721 | 161.1 (48.44), 119.1 (48.96), 253.2 (46.08), 179.2 (25.62), 145.2 (24.73), 235.1 (20.40), 161.2 (10.73), 237.2 (8.31), 399.2 (5.30) | C21H19O8 | 0.0 | 0.1 | 12.0 |
| 75 | C Flavonoid | 29.55 | 286 | 269.0822 | 150.0692 | 184.2 (88.87), 165.1 (80.74), 122.1 (55.22), 254.2 (50.90), 227.2 (38.24), 269.3 (20.13) | C16H13O4 | −0.3 | −1.0 | 10.0 |
| 76 | D Caffeic acid derivate | 29.63 | 326 | 277.1084 | 135.1237 | 179.1 (11.92) | C15H17O5 | −0.3 | −0.9 | 7.0 |
| 77 | B Caffeic acid propyl or isopropyl ester | 29.88 | 323 | 221.0824 | 133.7267 | 161.1 (22.49) | C12H13O4 | −0.5 | −2.2 | 6.0 |
| 78 | C Aromadendrin 7-methyl ether | 30.36 | 287 | 301.0722 | 164.1585 | 151.1 (72.31), 136.1 (49.48), 134.3 (49.49), 108.1 (29.20), 242.2 (17.13), 286.2 (15.52), 214.6 (17.83) | C16H13O6 | −0.5 | −1.5 | 10.0 |
| 79 | C Naringenin chalcone | 30.45 | 290 | 271.0617 | 125.1004 | 145.1 (23.55), 117.4 (8.59), 151.1 (6.04), 107.1 (3.82) | C15H11O5 | −0.5 | −1.7 | 10.0 |
| 80 | A Apigenin | 30.51 | 267, 336 | 269.0457 | 117.0349 | 269.0 (52.06), 151.0 (39.01), 149.0 (25.91), 227.0 (12.66), 107.0 (11.48), 225.0 (10.59), 201.1 (7.44), 183.0 (6.40), 181.1 (5.14), 121.0 (4.92), 197.1 (2.28) | C15H9O5 | −0.2 | −0.7 | 11.0 |
| 81 | A Kaempferol | 31.19 | 264, 365 | 285.0405 | 285.0400 | 239.0 (8.81), 187.0 (8.20), 185.0 (8.14), 229.0 (7.99), 159.0 (6.63) | C15H9O6 | −0.1 | −0.3 | 11.0 |
| 82 | B Caffeic acid hydroxyphenylethyl ester | 31.47 | *324 | 299.0922 | 135.1402 | 179.2 (24.97), 161.1 (5.41) | C17H15O5 | 0.3 | 1.0 | 10.0 |
| 83 | A Quercetin 3’-methyl ether (Isorhamnetin) | 31.77 | *253, 357 | 315.0509 | 300.1989 | 151.1 (26.66), 271.4 (11.37), 164.1 (7.61), 283.1 (6.12), 148.1 (5.64), 315.2 (5.60), 255.2 (4.65), 216.2 (3.38), 108.2 (2.95), 244.2 (2.60), 136.2 (2.55) | C16H11O7 | 0.1 | 0.3 | 11.0 |
| 84 | B Quercetin methyl ether isomer I | 32.28 | 254, 367 | 315.0511 | 300.1857 | 151.1 (26.12), 271.3 (11.15), 164.1 (7.58), 283.1 (5.81), 216.3 (4.63) | C16H11O7 | 0.0 | −0.1 | 11.0 |
| 85 | B Luteolin 5-methyl ether | 33.03 | 265, 349 | 299.0549 | 255.0300 | 227.03 (59.96), 284.0 (15.07), 211.0 (6.11) | C16H11O6 | −0.2 | −0.7 | 11.0 |
| 86 | C Syringenin (sinapyl alcohol) | 33.24 | *296 | 209.0826 | 165.1925 | 125.1 (95.68), 123.2 (53.31), 124.3 (23.62) | C11H13O4 | −0.7 | −3.2 | 5.0 |
| 87 | B Caffeic acid butenyl or isobutenyl ester | 33.73 | ND/- | 233.0818 | 133.3938 | - | C13H13O4 | 0.1 | 0.5 | 7.0 |
| 88 | B Quercetin dimethyl ether isomer I | 33.74 | 256, 354 | 329.0669 | 271.1688 | 299.2 (99.34), 243.2 (90.63), 285.4 (51.12), 257.2 (31.51), 314.2 (29.44), 227.2 (5.23), 215.2 (3.74), 199.2 (3.06), 255.1 (2.88) | C17H13O7 | −0.2 | −0.6 | 11.0 |
| 89 | D p-Coumaric acid derivate | 33.81 | ND | 311.0923 | 119.1298 | 163.2 (30.72), 135.1 (7.31), 145.1 (3.83) | C18H15O5 | 0.2 | 0.7 | 11.0 |
| 90 | B 1,3-di-p-Coumaroylglycerol | 33.98 | 312 | 383.1143 | 163.1491 | 119.1 (69.49), 145.1 (61.09), 117.2 (8.68), 219.2 (7.20), 237.2 (6.59), 383.4 (2.42) | C21H19O7 | −0.7 | −1.8 | 12.0 |
| 91 | B Galangin 5-methyl ether | 34.41 | *260, 350 | 283.0612 | 211.1796 | 239.2 (58.94), 283.3 (5.07), 268.2 (4.79) | C16H11O5 | 0.0 | −0.1 | 11.0 |
| 92 | B 1,2-di-p-Coumaroylglycerol II | 34.46 | 315 | 383.1137 | 163.1447 | 119.1 (78.80), 145.1 (70.92) | C21H19O7 | −0.1 | −0.2 | 12.0 |
| 93 | B Pinobanksin 5-methyl ether 3-acetate | 34.69 | 288 | 327.0878 | 224.1781 | 267.2 (67.46), 252.2 (62.85), 285.2 (45.11), 239.5 (36.67) | C18H15O6 | −0.4 | −1.1 | 11.0 |
| 94 | Bm-Coumaric acid (3-Hydroxycinnamic acid) | 35.01 | 311 | 163.0400 | 119.1298 | 163.2 (30.72), 135.1 (7.31), 145.1 (3.83) | C9H7O3 | 0.1 | 0.4 | 6.0 |
| 95 | B Pinobanksin 3-hydroxybutanoate isomer I | 35.14 | *292 | 357.0977 | 253.2321 | 271.2 (7.29), 197.2 (4.96), 209.3 (3.60) | C19H17O7 | 0.3 | 0.8 | 11.0 |
| 96 | B 2-Acetyl−1,3-di-caffeoylglycerol | 35.23 | 326 | 457.1143 | 179.1554 | 161.1 (75.83), 235.2 (53.60), 135.1 (48.32), 295.3 (40.83), 457.3 (5.85), 397.3 (5.27) | C23H21O10 | −0.3 | −0.7 | 13.0 |
| 97 | B 1-Acetyl−2,3-di-caffeoylglycerol | 35.73 | 325 | 457.1135 | ||||||
| 98 | D Caffeic acid derivate | 35.81 | *326 | 291.1248 | 135.1307 | 179.1 (21.22), 269.1 (4.90) | C16H19O5 | −1.0 | −3.3 | 7.0 |
| 99 | B Quercetin methyl ether isomer II | 36.15 | ND/- | 315.0883 | 164.0962 | 136.1 (51.83), 285.2 (39.16), 315.2 (22.41), 300.3 (14.01), 271.3 (12.30), 273.2 (10.71), 258.2 (7.41) | C17H15O6 | −0.8 | −2.7 | 10.0 |
| 100 | A Quercetin 7-methyl ether (Rhamnetin) | 36.53 | 256, 353 | 315.0509 | 165.1079 | 121.1 (39.04), 300.2 (27.72), 151.1 (9.49), 272.2119 (6.69), 244.2 (4.72), 256.3 (3.45) | C16H11O7 | 0.1 | 0.4 | 11.0 |
| 101 | B Kaempferol methyl ether isomer I | 36.68 | ND/- | 299.0563 | 284.1907 | 299.2 (7.35), 256.1 (5.21), 133.2 (5.23), 151.1 (2.37), 227.3 (2.53) | C16H11O6 | −0.2 | −0.7 | 11.0 |
| 102 | B Caffeic acid butyl or isobutyl ester isomer I | 37.33 | 325 | 235.0978 | 133.5359 | 161.1 (41.79) | C13H15O4 | −0.2 | −1.0 | 6.0 |
| 103 | B Pinobanksin 3-hydroxybutanoate isomer II | 37.55 | 293 | 357.0983 | 253.223 | 197.2 (4.80), 271.2 (4.93), 209.4 (2.89), 225.2 (2.52) | C19H17O7 | −0.3 | −0.8 | 11.0 |
| 104 | Unidentified | 37.74 | 288, 308sh | 205.0877 | 117.388 | 145.2 (23.35) | C12H13O3 | −0.7 | −3.3 | 6.0 |
| 105 | B Caffeic acid butyl or isobutyl ester isomer II | 37.96 | 325 | 235.0976 | 161.1424 | 135.1 (93.59) | C13H15O4 | −0.1 | −0.2 | 6.0 |
| 106 | C 2’,4’,6’-Trihydroxypentanophenone | 38.89 | 286 | 209.0827 | 152.0951 | 124.1 (84.90), 194.2 (11.41), 148.1 (9.02), 111.1 (8.47), 96.2 (6.71), 179.1 (4.46) | C11H13O4 | −0.7 | 3.4 | 5.0 |
| 107 | B Quercetin dimethyl ether isomer II | 39.00 | 261, 357 | 329.0669 | 299.1970 | 271.2 (30.28), 314.2 (21.06), 285.2 (2.46) | C17H13O7 | −0.3 | −0.8 | 11.0 |
| 108 | B Caffeic acid 2-methyl−2-butenyl ester | 39.19 | 325 | 247.0979 | 135.1258 | 161.1 (36.02), 179.1 (11.25) | C14H15O4 | −0.4 | −1.5 | 7.0 |
| 109 | B Quercetin dimethyl ether isomer III | 39.41 | ND | 329.0670 | 299.1828 | 271.2 (39.73), 314.2 (27.41), 285.2 (12.61), 329.3 (2.26) | C17H13O7 | −0.3 | −0.9 | 11.0 |
| 110 | B Caffeic acid 3-methyl−2-butenyl ester (basic prenyl ester) | 40.68 | 324 | 247.0979 | 134.2235 | 106.1 (6.32) | C14H15O4 | −0.4 | −1.7 | 7.0 |
| 111 | B Caffeic acid 3-methyl−3-butenyl ester (prenyl ester isomer I) | 41.16 | 325 | 247.0977 | 134.2234 | 106.2 (5.64) | C14H15O4 | −0.1 | −0.4 | 7.0 |
| 112 | B Sakuranetin dihydrochalcone | 41.56 | 285 | 287.0921 | 166.1295 | 181.2 (73.27), 152.1 (44.21), 124.1 (30.43), 226.2 (11.95), 193.1 (10.26), 254.2 (9.25), 178.2 (8.01), 139.1 (7.01), 93.1 (6.92), 189.2 (6.18), 150.2 (3.68), 269.3 (3.49) | C16H15O5 | 0.4 | 1.5 | 9.0 |
| 113 | Unidentified | 41.66 | 286 | 251.1648 | - | - | C15H23O3 | 0.5 | 1.8 | 4.0 |
| 114 | B 2-Acetyl−1-caffeoyl−3-p-coumaroylglycerol | 41.79 | 315 | 441.1197 | 163.1479 | 179.1 (85.75), 161.1 (42.10), 135.1 (40.85), 145.2 (39.56), 119.1 (35.73), 235.2 (27.59), 295.3 (14.64), 219.2 (7.31), 173.2 (6.88), 381.4 (7.79), 217.2 (4.50), 441.3 (4.75), 189.2 (3.80), 277.3 (2.86) | C23H21O9 | −0.6 | −1.3 | 13.0 |
| 115 | A Chrysin | 42.12 | 267, 312sh | 253.0505 | 253.0507 | 143.0 (41.53), 145.0 (21.10), 209.1 (14.10), 107.0 (13.33), 181.1 (8.16), 185.1 (6.19) | C15H9O4 | −0.7 | −2.8 | 11.0 |
| 116 | B Caffeic acid benzyl ester | 42.55 | 324 | 269.0818 | 134.1302 | 161.0 (22.96), 137.0 (4.03) | C16H13O4 | −0.3 | −1.1 | 10.0 |
| 117 | B 2-Acetyl−3-caffeoyl−2-feruloylglycerol | 42.59 | 314 | 471.1290 | 193.1743 | 179.1 (91.94), 135.1 (38.51), 161.1 (37.37), 175.1 (35.75), 235.2 (23.96), 295.2 (15.68), 149.1 (9.17), 411.3 (9.38), 173.2 (7.01), 249.2 (6.57), 471.5 (7.85), 217.1 (5.91), 367.2 (4.13), 189.2 (3.32), 117.2 (3.04), 277.3 (2.88) | C24H23O10 | 0.7 | 1.5 | 13.0 |
| 118 | D Flavonoid | 42.60 | ND/- | 285.0772 | 119.1332 | 165.28 (29.97), 150.4 (14.74), 121.1 (5.58), 122.1 (5.43), 269.3 (5.59), 97.1 (3.07), 136.2 (2.95), 177.2 (2.52) | C16H13O5 | −0.3 | −1.1 | 10.0 |
| 119 | Unidentified | 42.71 | 313 | 217.0869 | 117.1863 | 145.2 (2.80) | C13H13O3 | 0.1 | 0.4 | 7.0 |
| 120 | A Pinocembrin | 43.07 | 289 | 255.0666 | 171.0464 | 151.0 (80.69), 255.1 (75.17), 213.1 (74.89), 145.1 (70.09), 107.0 (52.59), 185.1 (34.69), 169.1 (24.91), 211.1 (23.68), 164.0 (17.93), 187.1 (16.78), 136.0 (16.34) | C15H11O4 | −0.2 | −0.8 | 10.0 |
| 121 | A Pinocembrin chalcone | 43.30 | 342 | 255.0668 | 171.2600 | 151.1 (61.32), 107.3 (40.48), 145.1 (29.50), 255.2 (25.04), 169.2 (23.80), 213.1 (21.71), 211.2 (14.01), 164.1 (9.13), 136.3 (7.29), 187.2 (6.32), 143.2 (4.35), 193.3 (3.34) | C15H11O4 | −0.5 | −2.0 | 10.0 |
| 122 | A Naringenin 7-methyl ether (Sakuranetin) | 43.32 | 289 | 285.0768 | 119.1265 | 165.1 (18.08) | C16H13O5 | 0.3 | 0.7 | 10.0 |
| 123 | Unidentified | 43.88 | ND | 223.0985 | 179.2917 | 139.1 (70.78), 137.1 (40.96), 115.2 (8.88) | C12H15O4 | −0.9 | −3.9 | 5.0 |
| 124 | A Naringenin 4’-methyl ether (Isosakuranetin) | 44.31 | 290 | 285.0773 | 124.1060 | 139.1 (64.17), 145.1 (42.28), 148.1 (8.73), 165.1 (4.71) | C16H13O5 | −0.4 | −1.6 | 10.0 |
| 125 | A Galangin | 44.82 | 265, 357 | 269.0454 | 269.0454 | 169.1 (12.64), 171.0 (10.87), 213.0 (10.73), 143.0 (8.90), 223.0 (8.03,) 195.0 (7.34) | C15H9O5 | −0.2 | −0.8 | 11.0 |
| 126 | B Isosakuranetin dihydrochalcone | 44.91 | 291 | 287.0925 | 243.2789 | 166.1 (70.19), 152.1 (32.79), 119.1 (27.87), 188.2 (24.60), 203.2 (23.97), 186.2 (20.81), 122.1 (18.36), 228.2 (16.99), 125.1 (14.66), 287.2 (14.92), 254.2 (13.89), 201.21 (11.46), 135.1 (8.29), 269.2 (7.27), 107.2 (6.87), 213.2 (6.61), 161.2 (5.14), 138.2 (4.19), 146.2 (3.57) | C16H15O5 | 0.0 | 0.1 | 9.0 |
| 127 | A Pinocembrin dihydrochalcone | 45.45 | 287 | 257.0820 | 213.2040 | 173.2 (66.35), 151.1 (33.34), 171.2 (31.95), 156.2 (24.29), 122.1 (19.76), 257.2 (12.86), 169.3 (13.48), 239.3 (11.24) | C15H13O4 | −0.1 | −0.4 | 9.0 |
| 128 | A Apigenin 3’-methyl ether (Acacetin) or A Apigenin 7-methyl ether (Genkwanin) | 45.45 | 267, 338 | 283.0619 | 268.2004 | 240.2 (6.84), 151.1 (4.25) | C16H11O5 | −0.7 | −2.3 | 11.0 |
| 129 | B Caffeic acid pentyl or isopentyl ester | 46.52 | 324 | 249.1138 | 161.1050 | - | C14H17O4 | −0.6 | −2.3 | 6.0 |
| 130 | A Caffeic acid phenethyl ester (CAPE) | 46.82 | 326 | 283.0981 | 135.1231 | 161.1 (46.24), 179.1 (20.40) | C17H15O4 | −0.6 | −2.0 | 10.0 |
| 131 | A Kaempferol 3’-methyl ether (Kaempferide) | 46.89 | 267, 364 | 299.0564 | 165.1098 | 163.1 (76.38), 256.2 (73.45), 243.2 (69.45), 284.2 (70.50), 271.2 (64.61), 151.0 (53.68), 228.2 (49.64), 178.1 (39.93), 212.2 (32.76), 240.2 (23.93) | C16H11O6 | −0.3 | −0.9 | 11.0 |
| 132 | B Pinobanksin 3-acetate | 47.27 | 295 | 313.0725 | 253.051 | 197.1 (5.86), 271.1 (5.36), 209.1 (4.75), 143.0 (3.17) | C17H13O6 | −0.7 | −2.3 | 16.0 |
| 133 | B Kaempferol methyl ether isomer II | 47.52 | 264, 360 | 299.0561 | 284.2051 | 151.1 (32.52), 164.1 (9.83), 107.2 (6.51), 132.1 (5.38), 299.2 (3.39), 228.2 (3.31) | C16H11O6 | 0.1 | 0.2 | 11.0 |
| 134 | B Tetramethyl flavonoid | 47.84 | ND/- | 329.0669 | 299.1782 | 271.2 (41.49), 314.2 (14.48) | C17H13O7 | −0.2 | −0.6 | 11.0 |
| 135 | B Methoxychrysin | 47.87 | 265 | 283.0614 | 211.0405 | 239.0 (65.55), 268.0 (8.80) | C16H11O5 | −0.2 | −0.6 | 11.0 |
| 136 | C 2’,6’-Dihydroxy−4’-methoxypentanophenone | 48.00 | 287 | 223.0983 | 152.0864 | 124.1 (77.51), 193.1 (13.04), 125.1 (11.95), 175.1 (6.65), 208.2 (5.84), 96.2 (6.22), 223.2 (3.86), 191.2 (3.24), 205.3 (2.47), 162.2 (2.47) | C12H15O4 | −0.7 | −3.0 | 5.0 |
| 137 | Unidentified | 48.12 | 310 | 219.1033 | 117.1531 | 145.1 (48.72), 119.1 (7.85) | C13H15O3 | −0.7 | −3.1 | 6.0 |
| 138 | Unidentified | 48.93 | 310 | 219.1028 | 117.3711 | 145.1 (32.70) | C13H15O3 | −0.2 | −0.8 | 6.0 |
| 139 | B Kaempferol 3,4’-dimethyl ether (Ermanin) | 49.93 | 350, 267 | 313.0719 | 283.2122 | 255.2 (24.32), 253.2 (17.11), 298.2 (10.64) | C17H13O6 | −0.1 | −0.3 | 11.0 |
| 140 | Bp-Coumaric acid 3-methyl−3-butenyl ester | 50.27 | 310 | 231.1028 | 117.1725 | 119.1 (90.59), 145.1 (49.02), 163.1 (4.99) | C14H15O3 | −0.1 | −0.4 | 7.0 |
| 141 | B 2-Acetyl−1,3-di-p-coumaroylglycerol | 50.51 | 312 | 425.1242 | 163.0403 | 145.0 (53.67), 119.0 (49.02), 219.1 (11.88), 215.1 (6.36), 237.1 (5.21), 171.1 (5.05), 117.0 (4.31) | C23H21O8 | 0.0 | 0.1 | 13.0 |
| 142 | B 1-Acetyl−2-p-coumaroyl−3-feruloylglycerol | 51.48 | 315 | 455.1347 | 163.1173 | 193.2 (78.06), 134.2 (46.98), 145.1 (41.86), 175.1 (42.27), 119.1 (40.73) | C24H23O9 | 0.1 | 0.2 | 13.0 |
| 143 | B 1-Acetyl−2,3-di-p-coumaroylglycerol | 51.68 | 311 | 425.1244 | 163.1361 | 145.1 (64.46), 119.1 (57.20), 219.2 (13.02), 171.3 (7.70) | C23H21O8 | −0.2 | −0.4 | 13.0 |
| 144 | Bp-Coumaric acid 3-methyl−2-butenyl or 2-methyl−2-butenyl | 51.75 | 311 | 231.1027 | 117.2347 | - | C14H15O3 | 0.0 | 0.0 | 7.0 |
| 145 | Bp-Coumaric acid 3-methyl−2-butenyl or 2-methyl−2-butenyl | 52.43 | 311 | 231.1029 | 117.2403 | - | C14H15O3 | −0.2 | −0.9 | 7.0 |
| 146 | Unidentified | 53.16 | ND/- | 311.2237 | 157.1924 | 153.3 (41.78), 187.2 (5.50), 135.3 (5.35), 113.3 (4.75) | C18H31O4 | −0.9 | −3.0 | 3.0 |
| 147 | Bp-Coumaric acid benzyl ester | 53.48 | 316 | 253.0869 | 117.2666 | 145.1 (12.89), 121.3 (3.15) | C16H13O3 | 0.1 | 0.3 | 10.0 |
| 148 | Unidentified | 54.60 | 299, 329 | 433.0921 | 243.2264 | 271.2 (41.07), 415.4 (26.05), 161.1 (19.62), 253.3 (11.06), 125.1 (7.62), 135.1 (6.88), 152.1 (5.62), 180.1 (4.85), 165.1 (4.69), 227.3 (4.97), 199.2 (3.56), 371.4 (3.43), 225.3 (3.13), 280.2 (2.54) | C24H17O8 | 0.8 | 1.7 | 16.0 |
| 149 | B Ferulic acid benzyl ester | 54.92 | 283.0979 | 133.1788 | 147.3 (16.46), 119.2 (8.42) | C17H15O4 | −0.3 | −1.0 | 10.0 | |
| 150 | B Caffeic acid phenylpropenyl ester | 55.71 | 325 | 295.0978 | 134.1210 | - | C18H15O4 | −0.2 | −0.7 | 11.0 |
| 151 | B Caffeic acid phenylpropyl ester | 55.85 | 326 | 297.1139 | 161.1417 | 135.1 (44.14), 297.3 (15.52), 179.2 (11.00), 137.2 (4.01) | C18H17O4 | −0.7 | −2.2 | 10.0 |
| 152 | B Pinobanksin 3-propanoate | 57.82 | 294 | 327.0878 | 253.2179 | 197.2 (5.41), 209.2 (3.72), 271.3 (2.71), 143.2 (2.09) | C18H15O6 | −0.4 | −1.2 | 11.0 |
| 153 | B Caffeic acid hexyl or isohexyl ester isomer I | 57.99 | ND/- | 263.1298 | 134.5851 | 161.1 (73.06), 135.1 (51.49), 179.1 (10.43), 263.3 (10.02) | C15H19O4 | −0.9 | −3.3 | 6.0 |
| 154 | Bp-Coumaric acid phenethyl ester | 58.08 | 310 | 267.1031 | 119.1235 | 145.1 (76.86), 117.2 (81.82), 163.1 (11.95) | C17H15O3 | −0.5 | −1.8 | 10.0 |
| 155 | Unidentified | 58.78 | ND/- | 233.1192 | 152.0855 | 124.1 (81.75) | C14H17O3 | −0.8 | −3.6 | 6.0 |
| 156 | B Caffeic acid hexyl or isohexyl ester isomer II | 59.51 | ND/- | 263.1294 | 161.1533 | 135.1 (70.52), 263.3 (14.21) | C15H19O4 | −0.5 | −1.9 | 6.0 |
| 157 | Unidentified | 59.68 | ND/- | 403.1187 | 293.2895 | 109.1 (43.64), 171.2 (26.99), 189.1 (17.54), 255.3 (19.17), 189.2 (16.73), 385.4 (16.30), 403.4 (14.83), 265.4 (10.04), 187.2 (7.72), 211.2 (7.08), 213.2 (6.59), 251.2 (5.91), 145.2 (5.09), 317.3 (4.94), 249.3 (4.31), 231.2 (3.90), 359.4 (4.00), 202.2 (3.44), 299.6 (3.66) | C24H19O6 | 0.1 | 0.1 | 15.0 |
| 158 | B Pinostrobin chalcone | 60.28 | 343 | 269.0827 | 122.0703 | 165.1 (83.49), 253.4 (86.88), 177.2 (49.29), 226.2 (47.58), 171.1 (35.51), 150.1 (31.31), 163.1 (21.30), 269.2 (16.42), 136.1 (13.47), 198.2 (14.25) | C16H13O4 | −0.3 | −0.8 | 10.0 |
| 159 | Unidentified | 61.08 | ND/- | 403.1194 | 281.2707 | 135.1 (32.65), 255.3 (34.47), 237.2 (29.91), 267.3 (26.49), 109.1 (17.43), 171.3 (14.49), 177.1 (12.64), 211.2 (12.58), 403.5 (10.85), 293.3 (7.11), 163.2 (4.33), 239.2 (3.69), 295.2 (3.70), 151.1 (3.50), 213.3 (3.82), 169.1 (2.89), 187.2 (2.60), 195.2 (2.49), 145.2 (2.40), 190.1 (2.25), 299.2 (2.19) | C24H19O6 | −0.1 | −1.7 | 15.0 |
| 160 | C 2’,6’-Dihydroxy−4’,4-dimethoxy dihydrochalcone (Calomelanone) | 61.32 | 285 | 301.1091 | 152.1075 | 124.1 (55.29), 253.2 (54.38), 165.4 (23.22), 268.3 (20.76), 301.3 (12.14), 180.1 (8.43), 119.1 (7.49), 283.2 (8.06), 188.1 (6.14), 193.2 (6.40), 203.3 (3.19) | C17H17O5 | −0.9 | −3.1 | 9.0 |
| 161 | B 2’,6’-Dihydroxy−4’-methoxy dihydrochalcone | 61.90 | 286 | 271.0979 | 152.0937 | 124.1 (60.13), 210.2 (27.77), 238.3 (25.34), 173.2 (13.05), 165.1 (10.13), 271.2 (7.97), 253.2 (6.31) | C16H15O4 | −0.3 | −1.1 | 9.0 |
| 162 | A Tectochrysin (Chrysin 7-methyl ether) [M+H]! | 62.70 | 267, 310sh | 269.0815 | 269.2764 | 226.2 (59.49), 254.2 (23.65), 167.1 (8.30), 270.5 (6.16), 186.3 (4.73), 129.1 (2.37), 209.2 (2.14) | C16H13O4 | −0.7 | −2.5 | 11.0 |
| 163 | B Pinobanksin 3-butenoate or isobutenoate | 62.91 | ND/- | 339.0880 | 253.2128 | 197.2 (5.11), 209.1 (3.28) | C19H15O6 | −0.5 | −1.6 | 12.0 |
| 164 | A Pinostrobin (Pinocembrin 7-methyl ether) [M+H]! | 63.20 | 289 | 271.0969 | 167.1288 | 131.1 (33.29), 103.2 (24.27), 269.3 (11.76), 226.3 (8.78), 271.2 (4.49), 270.5 (3.31), 254.3 (2.97), 186.3 (2.31), 165.2 (2.29) | C16H15O4 | −0.4 | −1.5 | 10.0 |
| 165 | Unidentified | 63.92 | *351 | 551.1708 | 267.2518 | 283.2 (46.28), 255.3 (28.92), 551.6 (5.49), 281.2 (3.07), 135.1 (2.44), 429.5 (2.48) | C33H27O8 | 0.4 | 0.6 | 20.0 |
| 166 | Bp-Coumaric acid cinnamyl ester | 63.94 | 313 | 279.1029 | 117.3253 | - | C18H15O3 | −0.3 | −1.0 | 11.0 |
| 167 | Unidentified | 64.26 | *310 | 281.1193 | 117.5723 | 145.2 (61.96), 121.1 (2.83), 281.3 (2.54) | C18H17O3 | −0.1 | −3.6 | 10.0 |
| 168 | B Caffeic acid heptyl or isoheptyl ester | 64.41 | ND/- | 277.1453 | 161.1393 | 135.1 (62.26), 277.3 (19.86), 179.2 (12.63) | C16H21O4 | −0.7 | −2.6 | 6.0 |
| 169 | B Pinobanksin 3-butanoate or isobutanoate | 64.74 | 293 | 341.1037 | 253.2173 | 197.2 (4.89), 209.2 (3.17) | C19H17O6 | −0.6 | −1.8 | 11.0 |
| 170 | Unidentified | 64.81 | ND/- | 387.1239 | 387.4150 | 171.2 (61.15), 173.1 (47.69), 283.2 (42.97), 197.2 (32.37), 343.8 (33.65), 215.2 (14.04), 255.2 (12.57), 301.4 (13.69), 211.3 (10.84), 169.2 (9.51), 239.4 (7.86), 145.2 (6.57), 156.2 (5.92), 281.2 (4.77), 359.4 (5.34), 183.3 (3.92), 147.2 (3.44), 226.3 (3.24), 213.2 (2.60), 259.3 (2.47) | C24H19O5 | −0.2 | −0.4 | 15.0 |
| 171 | C Balsacone A/B/E/F isomer I | 65.06 | 266,289 | 419.1510 | 419.4067 | 375.4 (53.98), 283.3 (28.14), 257.2 (17.67), 173.2 (13.80), 389.4 (12.76), 203.5 (13.11), 213.2 (8.97), 298.3 (8.44), 401.3 (7.43), 152.1 (5.63), 254.2 (5.53), 311.3 (5.42), 171.2 (4.79), 333.4 (5.16) | C25H23O6 | −1.0 | −2.4 | 14.0 |
| 172 | Unidentified | 65.21 | 262, 347 | 387.1240 | 387.4117 | 281.2 (97.94), 267.2 (78.24), 171.2 (60.37), 119.1 (46.94), 283.3 (43.02), 237.2 (36.62), 173.2 (27.63), 197.3 (28.18), 177.1 (23.64), 343.4 (27.07), 293.4 (21.25), 252.4 (17.83), 163.1 (12.94), 255.2 (12.36), 145.2 (11.09), 169.2 (10.43), 156.2 (10.70), 148.3 (11.59), 211.2 (9.84), 239.2 (9.08), 301.4 (9.81) | C24H19O5 | −0.2 | −0.6 | 15.0 |
| 173 | C Balsacone A/B/E/F isomer II | 65.37 | 266,289 | 419.1502 | 299.3067 | 313.3 (99.88), 419.5 (60.65), 119.1 (37.21), 375.4 (37.37), 178.2 (23.02), 269.4 (15.55), 325.4 (12.80), 203.2 (10.30), 152.1 (8.19), 192.2 (7.87), 137.1 (6.24), 213.6 (7.04), 254.4 (5.10), 285.4 (5.04), 257.3 (4.35), 93.1 (3.44), 145.2 (3.48), 265.3 (3.48), 173.2 (3.50), 287.3 (3.31), 243.2 (2.63), 295.3 (2.53), 163.2 (2.29) | C25H23O6 | −0.2 | −0.5 | 14.0 |
| 174 | Unidentified | 65.39 | ND | 469.1875 | 341.4908 | 469.5 (96.13), 257.2 (38.88), 357.4 (25.58), 383.8 (26.07), 311.3 (19.05), 438.4 (18.53), 328.3 (14.76), 339.3 (13.56), 327.8 (24.60), 125.1 (7.25), 297.3 (7.74), 215.2 (7.16), 242.2 (5.92), 223.3 (5.67), 353.3 (3.21) | C26H29O8 | −0.7 | −1.5 | 12.0 |
| 175 | B Pinobanksin 3-pentenoate or isopentenoate isomer I | 65.40 | 292 | 353.1039 | 253.2231 | 197.2305 (4.88), 209.1898 (2.96) | C20H17O6 | −0.9 | −2.5 | 12.0 |
| 176 | C Balsacone C or Balsacone D | 65.72 | 266,289 | 389.1402 | 283.3324 | 269.3 (90.93), 119.1 (47.02), 345.4 (58.63), 389.5 (54.23), 173.1 (25.16), 239.4 (23.64), 178.1 (17.40), 213.2 (13.48), 295.2 (12.39), 257.2 (9.36), 171.2 (9.05), 152.1 (9.06), 281.4 (11.13), 267.2 (7.85), 235.2 (7.28), 265.2 (7.13), 145.1 (6.59), 191.3 (6.98) | C24H21O5 | −0.7 | −1.9 | 14.0 |
| 177 | Unidentified | 65.74 | 290 | 469.1877 | 469.4952 | 437.5 (32.16), 343.3 (24.07), 353.4 (16.39), 341.3 (15.58), 223.3 (11.52), 385.4 (11.04), 325.4 (10.76), 393.5 (9.40), 257.3 (8.54), 297.4 (7.75), 215.3 (7.33), 357.4 (7.13), 311.6 (8.16), 125.1 (4.21), 280.6 (5.98), 189.2 (3.63), 367.4 (4.22) | C26H29O8 | −0.9 | −1.9 | 12.0 |
| 178 | B Pinobanksin 3-pentenoate or isopentenoate isomer II | 65.74 | 282 | 353.1035 | 253.2266 | 271.2 (26.83), 197.3 (5.55), 209.6 (3.51), 225.3 (2.59) | C20H17O6 | −0.5 | −1.9 | 12.0 |
| 179 | Unidentified | 65.76 | ND/- | 387.1238 | 267.3253 | 119.1 (58.67), 281.2 (57.66), 177.2 (26.82), 387.8 (34.42), 163.1 (16.67), 293.2 (14.40), 283.2 (9.40), 239.2 (7.44), 345.3 (6.47), 237.3 (6.12), 173.2 (4.93), 225.2 (4.67), 255.7 (5.16), 197.2 (3.28) | C24H19O5 | 0.0 | −0.1 | 15.0 |
| 180 | Unidentified | 66.01 | ND/- | 417.1336 | 297.2710 | 119.1 (73.94), 311.3 (69.24), 417.3 (40.26), 163.1 (20.53), 177.1 (20.29), 323.3 (12.23), 293.3 (8.90), 283.2 (7.45), 267.3 (6.89), 282.7 (12.85), 285.3 (2.95) | C25H21O6 | 0.8 | 1.9 | 15.0 |
| 181 | Unidentified | 66.10 | ND/- | 413.1972 | 134.2314 | 161.1 (98.90), 179.1 (23.69), 137.1 (11.08), 395.3 (6.95), 251.3 (7.21), 325.7 (4.12) | C24H29O6 | −0.2 | −0.5 | 10.0 |
| 182 | Unidentified | 66.24 | ND/- | 399.2180 | 134.1583 | 178.4 (38.54), 399.5 (21.28), 161.2 (4.22) | C24H31O5 | −0.3 | −0.7 | 9.0 |
| 183 | Unidentified | 66.57 | ND/- | 417.1349 | 135.1393 | 295.3 (28.85), 109.1 (20.68), 281.3 (13.63), 269.2 (11.44), 252.9 (5.46), 307.3 (2.47), 267.2 (2.40), 238.3 (2.36) | C25H21O6 | −0.5 | −1.2 | 15.0 |
| 184 | Unidentified | 66.60 | ND/- | 399.2176 | 134.2299 | 179.1 (15.44), 399.5 (14.91), 137.1 (10.24), 139.1 (2.82), 121.1 (2.64) | C24H31O5 | 0.1 | 0.3 | 9.0 |
| 185 | B Pinobanksin 3-benzoate | 66.80 | 64.81 | 375.0878 | 253.2202 | 197.1 (4.84), 225.2 (3.56), 121.2 (3.04), 209.2 (2.85) | C22H15O6 | −0.4 | −1.0 | 15.0 |
| 186 | Unidentified | 67.12 | ND/- | 377.1396 | 258.2083 | 377.4 (78.69), 344.4 (17.20), 271.4 (13.77), 359.5 (12.78), 230.3 (12.47), 165.1 (8.77), 316.4 (9.27), 362.4 (8.55), 138.1 (4.54), 245.3 (5.43), 269.2 (3.55), 173.3 (2.37), 243.2 (2.03) | C23H21O5 | −0.2 | −0.5 | 13.0 |
| 187 | B Pinobanksin derivate | 67.49 | 291 | 389.1037 | 253.2235 | 271.2 (48.46), 197.2 (5.15), 225.2 (3.00) | C23H17O6 | −0.7 | −1.7 | 15.0 |
| 188 | Unidentified | 67.63 | ND | 295.2290 | 277.4654 | 171.2 (70.40), 295.5 (10.03) | C18H31O3 | −1.2 | −4.0 | 3.0 |
| 189 | B Pinobanksin 3-pentanoate or isopentenoate isomer I | 67.76 | 293 | 355.1192 | 253.2167 | 197.2 (4.62), 271.2 (3.55), 209.2 (2.17) | C20H19O6 | −0.5 | −1.5 | 11.0 |
| 190 | B Pinobanksin 3-pentanoate or isopentenoate isomer II | 67.91 | 293 | 355.1194 | 197.2 (4.47), 209.2 (2.52) | C20H19O6 | −0.6 | −1.8 | 11.0 | |
| 191 | C Caffeic acid monoterpene (geranyl) ester | 68.07 | 326 | 315.1604 | 134.2007 | 137.1 (5.27), 179.2 (2.20), 106.1 (1.86) | C19H23O4 | −0.2 | −0.8 | 8.0 |
| 192 | Unidentified | 68.20 | ND/- | 401.1403 | 371.3482 | 401.4 (51.83), 297.2 (18.94), 267.2 (11.76), 385.6 (7.59), 254.3 (6.17), 171.2 (4.55), 295.2 (4.52), 282.3 (4.10), 197.2 (2.99), 226.4 (3.29), 343.2 (2.32) | C25H21O5 | −0.8 | −2.0 | 15.0 |
| 193 | Unidentified | 68.49 | ND/- | 403.1557 | 373.3656 | 403.4 (80.28), 269.2 (13.96), 385.9 (18.04), 297.4 (10.90), 370.5 (9.85), 355.3 (7.55), 342.4 (5.91), 271.3 (5.43), 173.2 (3.54), 309.3 (3.05), 241.2 (2.33) | C25H23O5 | −0.6 | −1.6 | 14.0 |
| 194 | B Pinobanksin 3-hexenoate or isohexenoate | 68.54 | ND/- | 367.1189 | 253.2181 | 271.2 (31.89), 197.3 (5.77), 209.5 (3.20), 225.3 (2.91) | C21H19O6 | −0.2 | −0.4 | 12.0 |
| 195 | Unidentified | 68.70 | ND/- | 397.2018 | 145.1398 | 118.4 (56.33), 163.2 (26.66), 251.4 (16.67), 121.1 (4.44) | C24H29O5 | 0.2 | 0.6 | 10.0 |
| 196 | C Ricinoleic acid or 8-(3-octyloxiran−2-yl)octanoic acid | 68.84 | ND/- | 297.2435 | 297.4100 | 171.2 (28.17) | C18H33O3 | 0.0 | 0.0 | 2.0 |
| 197 | C Balsacone L | 68.86 | *264, 344 | 519.1804 | 267.2076 | 269.2 (30.61), 519.8 (15.48), 399.5 (11.70), 251.3 (8.02), 471.7 (7.67), 413.3 (4.76), 119.1 (3.28), 279.2 (3.01), 293.6 (3.09) | C33H27O6 | 0.9 | 1.8 | 20.0 |
| 198 | B Pinobanksin 3-cinnamate | 68.89 | 278 | 401.1033 | 253.2046 | 197.1602 (4.77), 225.2060 (2.94) | C24H17O6 | −0.2 | −0.6 | 16.0 |
| 199 | Unidentified | 69.01 | ND/- | 401.1390 | 119.1599 | 279.2 (73.08), 281.4 (29.74), 254.2 (23.23), 295.2 (21.52), 401.4 (22.55), 93.1 (8.97), 175.3 (9.77), 297.3 (9.24), 267.3 (8.38), 358.3 (7.40), 386.3 (7.06), 307.2 (5.82), 238.2 (4.23), 171.2 (3.75), 269.3 (3.18), 161.12 (2.92), 163.3 (3.07) | C25H21O5 | 0.4 | 1.1 | 15.0 |
| 200 | B Pinobanksin 3-hydroxycinnamate | 69.16 | 285 | 403.1197 | 253.2276 | 271.2 (4.98), 197.2 (4.05), 225.3 (2.92), 149.1 (2.44) | C24H19O6 | −1.0 | −2.5 | 15.0 |
| 201 | C Balsacone J or Balsacone P | 69.16 | ND/- | 521.1971 | 401.4243 | 521.6 (62.86), 415.4 (54.23), 119.1 (40.59), 295.3 (40.43), 281.2 (23.40), 307.3 (12.76), 309.3 (11.57), 269.2 (9.67), 389.4 (9.17), 399.4 (8.49), 283.2 (5.57), 427.5 (6.46), 519.5 (5.15), 321.4 (4.44), 477.4 (3.18), 345.3 (2.93), 267.4 (3.17) | C33H29O6 | −0.1 | −0.3 | 19.0 |
| 202 | B Metoxycinnamic acid cinnamyl ester isomer I | 69.27 | 282 | 293.2125 | 293.4701 | 185.2 (57.87), 125.2 (49.45), 141.2 (18.74), 197.3 (15.90), 97.2 (11.61) | C18H29O3 | −0.3 | −0.9 | 4.0 |
| 203 | Unidentified | 69.29 | *272 | 403.1557 | 119.1396 | 281.3 (83.80), 283.3 (38.72), 297.3 (35.80), 403.5 (18.39), 93.1 (12.52), 309.3 (11.57), 269.2 (8.11), 178.1 (7.02), 279.3 (6.06), 164.3 (6.49), 263.6 (6.04), 173.2 (3.81), 271.3 (2.39), 295.3 (2.31) | −0.6 | −1.4 | 14.0 | |
| 204 | B Pinobanksin 3-hexanoate or isohexanoate isomer I | 69.57 | 294 | 369.1347 | 253.2138 | 271.2 (4.95), 197.2 (3.43), 225.1 (2.37), 115.2 (1.95) | C21H21O6 | −0.3 | −0.8 | 11.0 |
| 205 | B Pinobanksin 3-heptenoate or isoheptenoate isomer I | 69.72 | ND/- | 381.1351 | 253.2257 | 197.2 (4.11), 271.3 (4.03), 225.2 (1.96) | C22H21O6 | −0.7 | −1.9 | 12.0 |
| 206 | B Metoxycinnamic acid cinnamyl ester isomer II | 69.82 | 282 | 293.2120 | 293.3632 | 185.2 (59.65), 125.2 (51.93) | C18H29O3 | 0.3 | 0.9 | 4.0 |
| 207 | B Pinobanksin 3-hexanoate or isohexanoate isomer II | 69.87 | ND/- | 369.1347 | 253.2245 | 197.2 (4.52), 271.2 (3.90), 225.3 (2.22), 209.2 (1.98), 115.2 (1.93) | C21H21O6 | −0.3 | −0.8 | 11.0 |
| 208 | C Iryantherin D or Balsacone K | 70.20 | ND/- | 551.2078 | 299.2895 | 251.3 (21.30), 551.6 (22.85), 445.4 (7.98), 287.2 (5.17), 419.5 (4.72), 311.3 (4.26), 257.2 (2.60) | C34H31O7 | 0.2 | 0.3 | 19.0 |
| 209 | Unidentified | 70.26 | ND/- | 343.2855 | 283.3972 | 211.3 (96.37), 197.3 (72.36), 253.4 (30.83), 279. 5 (19.71) | C20H39O4 | −0.1 | −0.3 | 1.0 |
| 210 | Unidentified | 70.64 | ND/- | 295.2286 | 295.4295 | 141.2 (39.76), 125.2 (19.27) | C18H31O3 | −0.7 | −2.5 | 3.0 |
| 211 | Unidentified | 70.78 | ND/- | 489.3585 | 489.6854 | 427.6 (28.38), 445.6 (8.05), 471.6 (2.35) | C30H49O5 | 0.1 | 0.1 | 6.0 |
| 212 | B Pinobanksin 3-phenylpentenoate or phenylisopentenoate ester | 70.97 | *291 | 429.1344 | 253.2249 | 271.2 (57.79), 197.2 (3.17), 225.4 (3.81) | C26H21O6 | 0.0 | −0.1 | 16.0 |
| 213 | Unidentified | 71.37 | ND/- | 505.3391 | 283.4780 | - | C26H49O9 | −0.9 | −1.8 | 4.0 |
| 214 | C 2-Hydroxyethyl palmitate or 12-Hydroxystearic acid | 71.52 | ND/- | 299.2595 | 299.4604 | 253.6 (14.77), 281.3 (7.78), 113.2 (6.13) | C18H35O3 | −0.3 | −1.0 | 1.0 |
| 215 | Unidentified | 71.84 | ND/- | 491.3590 | 311.5273 | - | C26H51O8 | −0.1 | −0.1 | 1.0 |
| 216 | Unidentified | 72.43 | ND/- | 473.2337 | 473.5999 | 229.1 (15.75), 320.4 (17.64), 216.2 (8.35), 280.2 (5.94), 267.3 (3.38), 292.3 (2.68), 188.2 (2.50) | C30H33O5 | −0.3 | −0.6 | 14.0 |
| 217 | Unidentified | 72.48 | ND/- | 477.2644 | 255.2330 | 477.6 (4.09), 475.5 (3.45), 211.6 (3.10), 151.1 (1.97) | C30H37O5 | 0.2 | 0.5 | 12.0 |
| 218 | Unidentified | 73.07 | ND/- | 519.3542 | 297.4605 | - | C27H51O9 | −0.3 | −0.6 | 2.0 |
| 219 | Unidentified | 73.85 | ND/- | 371.3171 | 311.4860 | 225.4 (63.83), 239.4 (61.36) | C22H43O4 | −0.5 | −1.3 | 1.0 |
| 220 | Unidentified | 74.37 | ND/- | 533.3707 | 311.5549 | - | C28H53O9 | −1.2 | −2.2 | 2.0 |
| 221 | Unidentified | 74.70 | ND/- | 533.3703 | 311.3955 | - | C28H53O9 | −0.8 | −1.5 | 2.0 |
| 222 | Unidentified | 76.33 | ND/- | 447.3329 | - | - | C24H47O7 | −0.2 | −0.3 | 1.0 |
| 223 | Unidentified | 77.88 | ND/- | 561.4021 | 339.6137 | 211.3 (30.50) | C30H57O9 | −1.3 | −2.4 | 2.0 |
| Extract | B. cereus | B. subtilis | E. faecalis | M. luteus | S. aureus | S. epidermidis | E. coli | K. pneumoniae | P. mirabilis | P. aeruginosa | S. Typhimurium | H. pylori | C. glabrata | C. albicans | C. parapsilosis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P.BA.EtOH | 62.5/1000 S | 125/250 C | 500/500 C | 31.3/31.3 C | 125/125 C | 125/250 C | >1000/>1000 | >1000/>1000 N | >1000/>1000 N | >1000/>1000 | >1000/>1000 N | 62.5/62.5 C | 125/125 C | 125/250 C | 125/500 C |
| P.BA.W/E | >1000/Nd N | 125/125 C | 125/>1000 N | 62.5/62.5 C | 125/125 C | 125/500 C | >1000/Nd N | >1000/Nd N | >1000/Nd N | >1000/N.d | >1000/Nd N | 125/>1000 N | 125/125 C | 125/125 C | 125/125 C |
| P.CA.EtOH | 125/>1000 N | 125/500 C | 250/1000 C | 125/125 C | 125/500 C | 125/500 C | >1000/>1000 N | >1000/>1000 N | >1000/>1000 N | >1000/>1000 | >1000/>1000 N | 62.5/62.5 C | 125/250 C | 250/500 C | 62.5/250 C |
| P.CA.W/E | 1000/1000 C | 250/500 C | 500/1000 C | 125/250 C | 125/250 C | 250/250 C | >1000/N.d N | >1000/Nd N | >1000/Nd N | >1000/Nd | >1000/N.d N | 500/>1000 N | 250/250 C | 250/250 C | 250/500 C |
| P.DE.EtOH | 500/>1000 N | 125/125 C | 500/>1000 N | 125/125 C | 125/250 C | 250/1000 C | >1000/>1000 N | >1000/>1000 N | >1000/>1000 N | >1000/>1000 | >1000/>1000 N | 62.5/62.5 C | 125/250 C | 250/250 C | 250/500 C |
| P.DE.W/E | >1000/Nd N | 125/125 C | 500/1000 C | 62.5/250 C | 125/250 C | 500/500 C | >1000/Nd N | >1000/Nd N | >1000/Nd N | >1000/Nd | >1000/Nd N | 62.5/62.5 C | 250/250 C | 250/500 C | 250/500 C |
| P.DE × P.N.EtOH | 125/>1000 N | 125/500 C | 500/500 C | 125/125 C | 125/250 C | 250/500 C | >1000/>1000 N | >1000/>1000 N | >1000/>1000 N | >1000/>1000 | >1000/>1000 N | 62.5/62.5 C | 125/500 C | 250/250 C | 125/250 C |
| P.DE × P.N.W/E | >1000/Nd N | 125/125 C | 500/1000 C | 62.5/250 C | 125/250 C | 500/500 C | >1000/Nd N | >1000/Nd N | >1000/Nd N | >1000/Nd | >1000/Nd N | 500/>1000 N | 250/250 C | 250/500 C | 250/500 C |
| P.ER.EtOH | 31.3/>1000 N | 62.5/125 C | 250/500 C | 62.5/125 C | 125/125 C | 125/125 C | >1000/>1000 N | >1000/>1000 N | >1000/>1000 N | >1000/>1000 | >1000/>1000 N | 62.5/62.5 C | 125/250 C | 250/250 C | 250/500 C |
| P.ER.W/E | >1000/Nd N | 62.5/125 C | 250/>1000 N | 62.5/125 C | 125/125 C | 125/500 C | >1000/Nd N | >1000/Nd N | >1000/Nd N | >1000/Nd | >1000/Nd N | 250/>1000 N | 125/125 C | 250/250 C | 250/250 C |
| P. × KOM.EtOH | 62.5/>1000 N | 62.5/125 C | 125/250 C | 62.5/62.5 C | 62.5/125 C | 125/125 C | >1000/>1000 N | >1000/>1000 N | >1000/>1000 N | >1000/>1000 | >1000/>1000 N | 62.5/62.5 C | 125/125 C | 125/250 C | 125/500 C |
| P. × KOM.W/E | 125/>1000 N | 125/125 C | 250/250 C | 62.5/62.5 C | 62.5/125 C | 125/250 C | >1000/Nd N | >1000/Nd N | >1000/Nd N | >1000/Nd | >1000/Nd N | 250/250 C C | 125/250 C | 250/250 C | 125/250 C |
| P.LAU.EtOH | 62.5/>1000 N | 125/125 C | 500/>1000 N | 125/125 C | 125/250 C | 250/>1000 S | >1000/>1000 N | >1000/>1000 N | >1000/>1000 N | >1000/>1000 | >1000/>1000 N | 62.5/62.5 C | 250/250 C | 250/1000 C | 250/1000 C |
| P.LAU.W/E | >1000/Nd N | 500/1000 C | 1000/>1000 N | 250/500 C | 500/1000 C | 1000/>1000 N | >1000/Nd N | >1000/Nd N | >1000/Nd N | >1000/Nd | >1000/Nd N | 250/1000 C | >1000/Nd N | >1000/Nd N | >1000/Nd N |
| P.LAS.EtOH | 500/>1000 N | 250/1000 C | 1000/>1000 N | 250/250 C | 250/1000 C | 500/>1000 N | >1000/>1000 N | >1000/>1000 N | >1000/>1000 N | >1000/>1000 | >1000/>1000 N | 250/500 C | >1000/>1000 N | >1000/>1000 N | >1000/>1000 N |
| P.LAS.W/E | >1000/Nd N | 500/1000 C | 1000/>1000 N | 250/500 C | 500/1000 C | 1000>1000 N | >1000/Nd N | >1000/Nd N | >1000/Nd N | >1000/Nd N | >1000/Nd N | >1000/Nd N | >1000/Nd N | >1000/Nd N | >1000/Nd N |
| P.M.HB.ETOH | 31.3/>1000 N | 62.5/125 C | 125/250 C | 31.3/31.3 C | 62.5/125 C | 125/250 C | >1000/>1000 N | >1000/>1000 N | >1000/>1000 N | >1000/>1000 | >1000/>1000 N | 31.3/31.3 C | 125/125 C | 125/125 C | 125/250 C |
| P.M.HB.W/E | 62.5/1000 S | 62.5/62.5 C | 250/250 C | 62.5/62.5 C | 62.5/250 C | 125/125 C | >1000/Nd N | >1000/Nd N | >1000/Nd N | >1000/Nd | >1000/Nd N | 250/250 C | 250/250 C | 250/250 C | 125/250 C |
| P.M × P.B.ETOH | 125/>1000 N | 62.5/250 C | 250/500 C | 62.5/62.5 C | 125/250 C | 125/250 C | >1000/>1000 N | >1000/>1000 N | >1000/>1000 N | >1000/>1000 | >1000/>1000 N | 31.3/31.3 C | 125/125 C | 125/125 C | 125/250 C |
| P.M × P.B.W/E | 1000/>1000 N | 125/125 C | 250/1000 C | 62.5/250 C | 62.5/125 C | 125/250 C | >1000/Nd N | >1000/Nd N | >1000/Nd N | >1000/Nd | >1000/Nd N | 250/1000 C | 125/125 C | 125/125 C | 125/125 C |
| P.M × P.TRI.ETOH | 62.5/1000 S | 62.5/125 C | 31.3/250 S | 15.6/15.6 C | 62.5/62.5 C | 15.6/62.5 C | >1000/>1000 N | >1000/>1000 N | >1000/>1000 N | >1000/>1000 | >1000/>1000 N | 62.5/62.5 C | 125/500 C | 125/1000 S | 125/500 C |
| P.M × P.TRI.W/E | 31.3/>1000 N | 62.5/125 C | 62.5/125 C | 15.6/15.6 C | 62.5/62.5 C | 62.5/62.5 C | >1000/Nd N | >1000/Nd N | >1000/Nd N | >1000/Nd | >1000/Nd N | 62.5/125 C | 125/125 C | 125/125 C | 125/250 C |
| P.N.1.ETOH | 62.5/250 C | 125/125 C | 125/250 C | 31.3/31.3 C | 125/250 C | 125/125 C | >1000/>1000 N | >1000/>1000 N | >1000/>1000 N | >1000/>1000 | >1000/>1000 N | 62.5/62.5 C | 125/125 C | 125/250 C | 125/500 C |
| P.N.1.W/E | >1000/Nd N | 250/500 C | 500/>1000 N | 125/500 C | 250/500 C | 500/1000 C | >1000/Nd N | >1000/Nd N | >1000/Nd N | >1000/Nd | >1000/Nd N | 125/250 C | 250/250 C | 500/500 C | 250/500 C |
| P.N.2.ETOH | 125/>1000 N | 125/125 C | 250/500 C | 62.5/125 C | 125/250 C | 250/500 C | >1000/>1000 N | >1000/>1000 N | >1000/>1000 N | >1000/>1000 | >1000/>1000 N | 62.5/125 C | 125/125 C | 125/250 C | 62.5/500 S |
| P.N.2.W/E | >1000/Nd N | 125/125 C | 125/250 C | 62.5/62.5 C | 125/125 C | 250/250 C | >1000/Nd N | >1000/Nd N | >1000/Nd N | >1000/Nd | >1000/Nd N | 125/250 C | 125/125 C | 125/250 C | 125/250 C |
| P.N.3.EtOH | 31.3/62.5 C | 31.3/62.5 C | 125/250 C | 31.3/62.5 C | 62.5/62.5 C | 62.5/125 C | >1000/>1000 N | >1000/>1000 N | >1000/>1000 N | >1000/>1000 | >1000/>1000 N | 31.3/31.3 C | 62.5/125 C | 125/125 C | 125/250 C |
| P.N.3.W/E | >1000/Nd N | 62.5/62.5 C | 250/250 C | 62.5/62.5 C | 62.5/62.5 C | 62.5/125 C | >1000/Nd N | >1000/Nd N | >1000/Nd N | >1000/Nd | >1000/Nd N | 250/250 C | 62.5/125 C | 125/125 C | 125/125 C |
| P. × PE1.EtOH | 62.5/>1000 N | 62.5/250 C | 125/250 C | 31.3/62.5 C | 62.5/125 C | 125/125 C | >1000/>1000 N | >1000/>1000 N | >1000/>1000 N | >1000/>1000 | >1000/>1000 N | 62.5/62.5 C | 125/125 C | 125/125 C | 62.5/250 C |
| P. × PE1.W/E | >1000/Nd N | 125/125 C | 250/250 C | 31.3/62.5 C | 62.5/125 C | 125/250 C | >1000/Nd N | >1000/Nd N | >1000/Nd N | >1000/Nd | >1000/Nd N | 250/250 C | 125/125 C | 125/125 C | 125/250 C |
| P. × PE2.EtOH | 125/>1000 N | 125/125 C | 500/1000 C | 125/125 C | 125/250 C | 250/1000 C | >1000/>1000 N | >1000/>1000 N | >1000/>1000 N | >1000/>1000 | >1000/>1000 N | 62.5/62.5 C | 125/250 C | 250/500 C | 250/500 C |
| P. × PE2.W/E | >1000/Nd N | 500/>1000 N | 1000/>1000 N | 125/500 C | 250/1000 C | 250/500 C | >1000/Nd N | >1000/Nd N | >1000/Nd N | >1000/Nd | >1000/Nd N | 1000/>1000 N | 500/500 C | 500/1000 C | 500/1000 C |
| P. × RA.EtOH | 62.5/>1000 N | 125/125 C | 500/500 C | 31.3/62.5 C | 125/250 C | 125/250 C | >1000/>1000 N | >1000/>1000 N | >1000/>1000 N | >1000/>1000 | >1000/>1000 N | 62.5/62.5 C | 125/500 C | 250/500 C | 125/125 C |
| P. × RA.W/E | 125/>1000 N | 125/125 C | 500/500 C | 62.5/62.5 C | 125/125 C | 250/250 C | >1000/Nd N | >1000/Nd N | >1000/Nd N | >1000/Nd | >1000/Nd N | 500/500 C | 250/250 C | 250/250 C | 125/250 C |
| P.RO.EtOH | 62.5/>1000 N | 31.3/62.5 C | 62.5/250 C | 15.6/31.3 C | 62.5/62.5 C | 15.6/31.3 C | >1000/>1000 N | >1000/>1000 N | >1000/>1000 N | >1000/>1000 | >1000/>1000 N | 31.3/31.3 C | 62.5/62.5 C | 125/125 C | 62.5/250 C |
| P.RO.W/E | >1000/Nd N | 31.3/62.5 C | 62.5/125 C | 15.6/31.3 C | 31.3/62.5 C | 31.3/31.3 C | >1000/Nd N | >1000/Nd N | >1000/Nd N | >1000/Nd | >1000/Nd N | 62.5/125 C | 62.5/250 C | 125/125 C | 62.5/125 C |
| P.SI.EtOH | 62.5/>1000 N | 125/250 C | 250/500 C | 62.5/125 C | 125/250 C | 125/125 C | >1000/>1000 N | >1000/>1000 N | 1000/>1000 N | >1000/>1000 | >1000/>1000 N | 62.5/62.5 C | 125/125 C | 125/250 C | 125/250 C |
| P.SI.W/E | 125/>1000 N | 125/125 C | 250/500 C | 62.5/250 C | 125/250 C | 125/500 C | >1000/Nd N | >1000/Nd N | >1000/Nd N | >1000/Nd | >1000/Nd N | 250/500 C | 125/250 C | 250/250 C | 250/250 C |
| P.SU.EtOH | 62.5/>1000 N | 62.5/125 C | 250/250 C | 31.3/62.5 C | 62.5/125 C | 125/125 C | >1000/>1000 N | >1000/>1000 N | >1000/>1000 N | >1000/>1000 | >1000/>1000 N | 31.3/62.5 C | 125/125 C | 125/250 C | 125/250 C |
| P.SU.W/E | 125/>1000 N | 125/125 C | 250/500 C | 62.5/62.5 C | 125/125 C | 125/250 C | >1000/Nd N | >1000/Nd N | >1000/Nd N | >1000/Nd | >1000/Nd N | 62.5/62.5 C | 125/125 C | 125/125 C | 125/250 C |
| P.TA.1.EtOH | 62.5/500 S | 125/125 C | 500/500 C | 31.3/62.5 C | 125/125 C | 125/250 C | >1000/>1000 N | >1000/>1000 N | >1000/>1000 N | >1000/>1000 | >1000/>1000 N | 62.5/62.5 C | 125/125 C | 125/250 C | 125/500 C |
| P.TA.1.W/E | >1000/Nd N | 125/125 C | 250/>1000 N | 62.5/62.5 C | 125/250 C | 125/500 C | >1000/Nd N | >1000/Nd N | >1000/Nd N | >1000/Nd | >1000/Nd N | 250/>1000 N | 125/250 C | 125/250 C | 125/250 C |
| P.TA.2.EtOH | 250/>1000 N | 125/125 C | 62.5/500 S | 31.3/62.5 C | 125/250 C | 125/250 C | >1000/>1000 N | >1000/>1000 N | >1000/>1000 N | >1000/>1000 | >1000/>1000 N | 62.5/62.5 C | 125/125 C | 125/250 C | 125/250 C |
| P.TA.2.W/E | >1000/Nd N | 250/250 C | 500/>1000 N | 125/500 C | 250/500 C | 250/500 C | >1000/Nd N | >1000/Nd N | >1000/Nd N | >1000/Nd | >1000/Nd N | 500/>1000 N | 250/250 C | 250/500 C | 500/500 C |
| P.TA1 × PTRI.EtOH | 15.6/1000 S | 62.5/62.5 C | 125/250 C | 15.6/15.6 C | 62.5/125 C | 31.3/62.5 C | >1000/>1000 N | >1000/>1000 N | >1000/>1000 N | >1000/>1000 | >1000/>1000 N | 62.5/62.5 C | 125/125 C | 125/500 C | 125/1000 S |
| P.TA1 × PTRI.W/E | >1000/Nd N | 62.5/125 C | 125/250 C | 15.6/31.3 C | 125/125 C | 62.5/62.5 C | >1000/Nd N | >1000/Nd N | >1000/Nd N | >1000/Nd | >1000/Nd N | 125/250 C | 125/250 C | 250/250 C | 250/250 C |
| P.TA.2 × P.TRI.EtOH | 31.3/500 S | 31.3/31.3 C | 15.6/500 S | 7.8/7.8 C | 31.3/31.3 C | 7.8/15.6 | >1000/>1000 N | >1000/>1000 N | >1000/>1000 N | >1000/>1000 | >1000/>1000 N | 31.3/31.3 C | 125/125 C | 125/500 C | 1000/1000 C |
| P.TA.2 × P.TRI.W/E | 31.3/500 S | 62.5/62.5 C | 62.5/125 C | 31.3/31.3 C | 62.5/62.5 C | 31.3/125 | >1000/Nd N | >1000/Nd N | >1000/Nd N | >1000/Nd | >1000/Nd N | 62.5/125 C | 125/500 C | 125/500 C | 125/500 C |
| P.TRI.EtOH | 31.3/>1000 N | 62.5/62.5 C | 62.5/125 C | 15.6/15.6 C | 62.5/62.5 C | 31.3/31.3 C | >1000/>1000 N | >1000/>1000 N | >1000/>1000 N | >1000/>1000 | >1000/>1000 N | 31.3/31.3 C | 62.5/125 C | 125/250 C | 125/500 C |
| P.TRI.W/E | >1000/Nd N | 62.5/62.5 C | 62.5/125 C | 15.6/31.3 C | 62.5/62.5 C | 31.3/500 | >1000/Nd N | >1000/Nd N | >1000/Nd N | >1000/Nd N | >1000/Nd N | 62.5/125 C | 62.5/62.5 C | 125/250 C | 62.5/250 C |
| P. × WCA.EtOH | >1000/>1000 N | >1000/>1000 N | >1000/>1000 N | 1000/1000 C | 1000/1000 C | 1000/>1000 N | >1000/>1000 N | >1000/>1000 N | >1000/>1000 N | >1000/>1000 N | >1000/>1000 N | 250/250 C | >1000/>1000 N | >1000/>1000 N | >1000/>1000 N |
| P. × WCA.W/E | >1000/Nd N | >1000/Nd N | >1000/Nd N | 1000/Nd N | >1000/Nd N | >1000/Nd N | >1000/Nd N | >1000/Nd N | >1000/Nd N | >1000/Nd N | >1000/Nd N | 500/1000 C | >1000/Nd N | >1000/Nd N | >1000/Nd N |
| P.WIL.EtOH | >1000/>1000 N | 1000/>1000 N | >1000/>1000 N | 500/1000 C | 1000/1000 C | 250/1000 C | >1000/>1000 N | >1000/>1000 N | >1000/>1000 N | >1000/>1000 N | >1000/>1000 N | 250/250 C | >1000/>1000 N | >1000/>1000 N | >1000/>1000 N |
| P.WIL.W/E | >1000/>1000 N | 1000/>1000 N | 1000/>1000 N | 500/>1000 N | 1000/>1000 N | 1000/>1000 N | >1000/Nd N | >1000/Nd N | >1000/Nd N | >1000/Nd N | >1000/Nd N | 1000/>1000 N | >1000/Nd N | 1000/Nd N | >1000/Nd N |
| Reference drugs | 0.98 VAN | 0.24 VAN | 1.95 VAN | 0.12 VAN | 0.98 VAN | 0.98 VAN | 0.015 CIP | 0.12 CIP | 0.03 CIP | 0.49 CIP | 0.06 CIP | 31.3 MET | 0.24 NYS | 0.48 NYS | 0.24 NYS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okińczyc, P.; Widelski, J.; Nowak, K.; Radwan, S.; Włodarczyk, M.; Kuś, P.M.; Susniak, K.; Korona-Głowniak, I. Phytochemical Profiles and Antimicrobial Activity of Selected Populus spp. Bud Extracts. Molecules 2024, 29, 437. https://doi.org/10.3390/molecules29020437
Okińczyc P, Widelski J, Nowak K, Radwan S, Włodarczyk M, Kuś PM, Susniak K, Korona-Głowniak I. Phytochemical Profiles and Antimicrobial Activity of Selected Populus spp. Bud Extracts. Molecules. 2024; 29(2):437. https://doi.org/10.3390/molecules29020437
Chicago/Turabian StyleOkińczyc, Piotr, Jarosław Widelski, Kinga Nowak, Sylwia Radwan, Maciej Włodarczyk, Piotr Marek Kuś, Katarzyna Susniak, and Izabela Korona-Głowniak. 2024. "Phytochemical Profiles and Antimicrobial Activity of Selected Populus spp. Bud Extracts" Molecules 29, no. 2: 437. https://doi.org/10.3390/molecules29020437
APA StyleOkińczyc, P., Widelski, J., Nowak, K., Radwan, S., Włodarczyk, M., Kuś, P. M., Susniak, K., & Korona-Głowniak, I. (2024). Phytochemical Profiles and Antimicrobial Activity of Selected Populus spp. Bud Extracts. Molecules, 29(2), 437. https://doi.org/10.3390/molecules29020437