Visible Light-Promoted Oxidative Cross-Coupling of Alcohols to Esters
Abstract
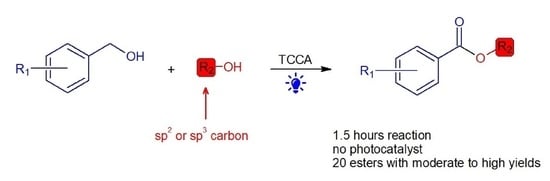
:1. Introduction
2. Results and Discussion
2.1. Study of the Methodology and Optimization Conditions
2.2. Investigation of Reaction Applicability
2.3. Proposed Mechanism
3. Materials and Methods
3.1. Materials
3.2. General Procedure for the Preparation of Methyl Benzoate 5a via Solar Simulator Irradiation
3.3. General Procedure for the Preparation of Methyl Benzoate 5a via Sunlight Irradiation
3.4. General Procedure for the Synthesis of Esters 5a–g, 6a–g and 7a–f via Blue LED Irradiation
3.5. Detection and Isolation of Benzoyl Chloride 3
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ishihara, K. Dehydrative condensation catalyses. Tetrahedron 2009, 65, 1085–1109. [Google Scholar] [CrossRef]
- Moser, B.R. Biodiesel Production, Properties, and Feedstocks. In Biofuels; Springer: New York, NY, USA, 2011; pp. 285–347. [Google Scholar]
- Ambat, I.; Srivastava, V.; Sillanpaa, M. Recent advancement in biodiesel production methodologies using various feedstock: A review. Renew. Sustain. Energy Rev. 2018, 90, 356–369. [Google Scholar] [CrossRef]
- Petibon, R.; Aiken, C.P.; Ma, L.; Xiong, D.; Dahn, J.R. The use of ethyl acetate as a sole solvent in highly concentrated electrolyte for Li-ion batteries. Electrochim. Acta 2015, 154, 287–293. [Google Scholar] [CrossRef]
- De Nazare de Oliveira, A.; de Oliveira, D.T.; Angèlica, R.S.; de Aguiar Andrade, E.H.; do Rosàrio da Silva, J.K.; da Rocha Filo, G.N.; Coral, N.; de Oliveira Pires, L.H.; Luque, R.; Santos do Nascimento, L.A. Efficient esterification of eugenol using a microwave-activated waste kaolin. React. Kinet. Mech. Catal. 2020, 130, 633–653. [Google Scholar] [CrossRef]
- Pereira, C.S.; Silva, V.M.; Rodrigues, A.E. Ethyl lactate as a solvent: Properties, applications and production processes—A review. Green Chem. 2011, 13, 2658–2671. [Google Scholar] [CrossRef]
- Tang, X.; Chen, E.Y.-X. Toward Infinitely Recyclable Plastics Derived from Renewable Cyclic Esters. Chem 2019, 5, 284–312. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, W. Antioxidant and antibacterial chitosan film with tea polyphenols-mediated green synthesis silver nanoparticle via a novel one-pot method. Int. J. Biol. Macromol. 2020, 155, 1252–1261. [Google Scholar] [CrossRef]
- Zare, M.; Golmakani, M.-T.; Sardarian, A. Green synthesis of banana flavor using different catalysts: A comparative study of different methods. Green Chem. Lett. Rev. 2020, 13, 83–92. [Google Scholar] [CrossRef]
- De Barros, D.P.C.; Azevedo, A.M.; Cabral, J.M.S.; Fonseca, L.P. Optimization of flavor esters synthesis by Fusarium solani pisi cutinase. J. Food Biochem. 2012, 36, 275–284. [Google Scholar] [CrossRef]
- Almeida, S.A.A.G.; de Meneses, A.C.; de Araùjo, P.H.H.; de Oliveira, D. A review on enzymatic synthesis of aromatic esters used as flavor ingredients for food, cosmetics and pharmaceuticals industries. Trends Food Sci. Technol. 2017, 69, 95–105. [Google Scholar]
- Steele, J.H.; Bozor, M.X.; Boyce, G.R. Transumtation of scent: An evaluation of the synthesis of methyl cinnamate, a commercial fragrance, via a Fischer esterification for the second-year organic laboratory. J. Chem. Educ. 2020, 97, 4127–4132. [Google Scholar] [CrossRef]
- Otera, J.; Dan-oh, N.; Nozaki, H. Novel template effects of distannoxane catalysts in highly efficient transesterification and esterification. J. Org. Chem. 1991, 56, 5307–5311. [Google Scholar] [CrossRef]
- Ishihara, K.; Ohara, S.; Yamamoto, H. 3,4,5-Trifluorobenzeneboronic Acid as an Extremely Active Amidation Catalyst. J. Org. Chem. 1996, 61, 4196–4197. [Google Scholar] [CrossRef] [PubMed]
- Storck, S.; Maier, W.F.; Salvado, I.M.M.; Ferreira, J.M.F.; Guhl, D.; Souverijins, W.; Martens, J.A. Amorphous Sn/Si Mixed Oxides, Mild Solid Lewis Acid Catalysts for Esterification and Etherification Reactions. J. Catal. 1997, 172, 414–426. [Google Scholar] [CrossRef]
- Mustafa, A.; Faisal, S.; Ahmed, I.A.; Munir, M.; Pereira Cipolatti, E.; Manoel, E.A.; Pastore, C.; di Bitonto, L.; Hanelt, D.; Nitbani, F.O.; et al. Has the time finally come for green oleochemicals and biodiesel production using large-scale enzyme technologies? Current status and new developments. Biotechnol. Adv. 2023, 69, 108275–108293. [Google Scholar] [CrossRef]
- Otera, J.; Nishikido, J. Esterification: Methods, Reactions, and Applications, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2010. [Google Scholar]
- Wipf, P. Handbook of Reagents for Organic Synthesis; Wiley: New York, NY, USA, 2005. [Google Scholar]
- Valeur, E.; Bradley, M. Amide bond formation: Beyond the myth of coupling reagents. Chem. Soc. Rev. 2009, 38, 606–631. [Google Scholar] [CrossRef] [PubMed]
- Enache, D.I.; Edwards, J.K.; Landon, P.; Solsona-Espriu, B.; Carley, A.F.; Herzing, A.A.; Watanabe, M.; Kiely, C.J.; Knight, D.W.; Hutchings, G.J. Solvent-free oxidation of primary alcohols to aldehydes using Au-Pd/TiO2 catalysts. Science 2006, 311, 362–365. [Google Scholar] [CrossRef]
- Mallat, T.; Baiker, A. Oxidation of alcohols with molecular oxygen on solid catalysts. Chem. Rev. 2004, 104, 3037–3058. [Google Scholar] [CrossRef]
- Liu, C.; Tang, S.; Zheng, L.; Liu, D.; Zhang, H.; Lei, A. Covalently Bound Benzyl Ligand Promotes Selective Palladium-Catalyzed Oxidative Esterification of Aldehydes with Alcohols. Angew. Chem. Int. Ed. 2012, 51, 5662–5666. [Google Scholar] [CrossRef]
- Tang, S.; Yuan, J.; Liu, C.; Lei, A. Direct oxidative esterification of alcohols. Dalton Trans. 2014, 43, 13460–13470. [Google Scholar] [CrossRef]
- Ekoue-Kovi, K.; Wolf, C. One-pot oxidative esterification and amidation of aldehydes. Chem. Eur. J. 2008, 14, 6302–6315. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Liang, S.; Chen, S.-Y.; Zhang, J.; Fu, S.-S.; Yu, X.-Q. A Metal-Free Oxidative Esterification of the Benzyl C–H Bond. Adv. Synth. Catal. 2012, 354, 1287–1292. [Google Scholar] [CrossRef]
- Liu, C.; Tang, S.; Lei, A. Oxidant controlled Pd-catalysed selective oxidation of primary alcohols. Chem. Commun. 2013, 49, 1324–1326. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, C.; Yuan, J.; Lei, A. Transition-metal-free aerobic oxidation of primary alcohols to carboxylic acids. New J. Chem. 2013, 37, 1700–1703. [Google Scholar] [CrossRef]
- Xia, J.; Shao, A.; Tang, S.; Gao, X.; Gao, M.; Lei, A. Palladium-catalysed oxidative cross-esterification between two alcohols. Org. Biomol. Chem. 2015, 13, 6154–6157. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, J.; Meng, L.; Deng, Y.; Li, Y.; Lei, A. Palladium-Catalyzed Aerobic Oxidative Direct Esterification of Alcohols. Angew. Chem. Int. Ed. 2011, 50, 5144–5148. [Google Scholar] [CrossRef]
- Nielsen, M.; Junge, H.; Kammer, A.; Beller, M. Towards a Green Process for Bulk-Scale Synthesis of Ethyl Acetate: Efficient Acceptorless Dehydrogenation of Ethanol. Angew. Chem. Int. Ed. 2012, 51, 5711–5713. [Google Scholar] [CrossRef]
- Fogler, E.; Garg, J.A.; Hu, P.; Leitus, G.; Shimon, L.J.W.; Milstein, D. System with Potential Dual Modes of Metal–Ligand Cooperation: Highly Catalytically Active Pyridine-Based PNNH–Ru Pincer Complexes. Chem. Eur. J. 2014, 20, 15727–15731. [Google Scholar] [CrossRef]
- Paudel, K.; Pandey, B.; Xu, S.; Taylor, D.K.; Tyer, D.L.; Lopez Torres, C.; Gallagher, S.; Kong, L.; Ding, K. Cobalt-Catalyzed Acceptorless Dehydrogenative Coupling of Primary Alcohols to Esters. Org. Lett. 2018, 20, 4478–4481. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, F.; Tao, C.; Yu, H.; Ruhlmann, L.; Wei, Y. Oxidative esterification of alcohols by a single-side organically decorated Anderson-type chrome-based catalyst. Green Chem. 2021, 23, 2652–2657. [Google Scholar] [CrossRef]
- Samanta, S.; Pappula, V.; Dinda, M.; Adimurthy, A. Transition metal-free oxidative esterification of benzylic alcohols in aqueous medium. Org. Biomol. Chem. 2014, 12, 9453–9456. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, Z.; Liu, H.; Xie, Z.; Mei, Q.; Han, B. Transformation of alcohols to esters promoted by hydrogen bonds using oxygen as the oxidant under metal-free conditions. Sci. Adv. 2018, 4, eaas9319. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Chen, J.; Zhang, A. Commercially available natural benzyl esters and their synthetic analogs exhibit different toxicities against insect pests. Sci. Rep. 2018, 8, 7902. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, P.; Dollimore, D.; Alexander, K. The use of thermogravimetry to follow the rate of evaporation of an ingredient used in perfumes. J. Ther. Anal. Calorim. 1997, 49, 595–599. [Google Scholar] [CrossRef]
- Wardzińska, E.; Penczek, P. Influence of the glycol component in dibenzoate plasticizers on the properties of plasticized PVC films. J. Appl. Polym. Sci. 2005, 97, 822–824. [Google Scholar] [CrossRef]
- Tekin, E.; de Gans, B.J.; Schubert, U.S. Ink-jet printing of polymers–from single dots to thin film libraries. J. Mater. Chem. 2004, 14, 2627–2632. [Google Scholar] [CrossRef]
- Patel, J.P.; Deshmukh, S.; Zhao, C.; Wamuo, O.; Hsu, S.L.; Schoch, A.B.; Carleen, S.A.; Matsumoto, D. An analysis of the role of nonreactive plasticizers in the crosslinking reactions of a rigid resin. J. Polym. Sci. B Polym. Phys. 2017, 55, 206–213. [Google Scholar] [CrossRef]
- Stephenson, C.R.J.; Yoon, T.P.; MacMillan, D.W.C. Visible Light Photocatalysis in Organic Chemistry; Wiley-VCH: Weinheim, Germany, 2017. [Google Scholar]
- Konig, B. Photocatalysis in Organic Synthesis—Past, Present, and Future. Eur. J. Org. Chem. 2017, 15, 1979–1981. [Google Scholar] [CrossRef]
- Prier, C.K.; Rankic, D.A.; MacMillan, D.W. Visible Light Photoredox Catalysis with Transition Metal Complexes: Applications in Organic Synthesis. Chem. Rev. 2013, 113, 5322–5363. [Google Scholar] [CrossRef]
- Dettori, G.; Gaspa, S.; Porcheddu, A.; De Luca, L. A two-step tandem reaction to prepare hydroxamic acids directly from alcohols. Org. Biomol. Chem. 2014, 12, 4582–4585. [Google Scholar] [CrossRef]
- Gaspa, S.; Carraro, M.; Pisano, L.; Porcheddu, A.; De Luca, L. Trichloroisocianuric acid: A versatile and efficient chlorinationg and oxidizing reagent. Eur. J. Org. Chem. 2019, 2019, 3544–3552. [Google Scholar] [CrossRef]
- Tilstam, U.; Weinmann, H. Trichloroisocyanuric acid: A safe and efficient oxidant. Org. Process Res. Dev. 2002, 6, 384–393. [Google Scholar] [CrossRef]
- Protti, S.; Ravelli, M.; Fagnoni, M.; Albini, A. Solar light-driven photocatalyzed alkylations. Chemistry on the window ledge. Chem. Commun. 2009, 47, 7351–7353. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Muniz, K. Selective piperidine synthesis exploiting iodine-catalyzed Csp3–H amination under visible light. ACS Catal. 2017, 7, 4122–4125. [Google Scholar] [CrossRef]
- Saika, A.J.; Boran, P.; Phukan, P. Use of bromine and bromo-organic compounds in organic synthesis. Chem. Rev. 2016, 116, 6837–7042. [Google Scholar] [CrossRef] [PubMed]
- Beckwith, A.L.J.; Goodrich, J.E. Free-radical rearrangement of N-chlor-anides: A synthesis of lactones. Aust. J. Chem. 1965, 18, 747–757. [Google Scholar] [CrossRef]
- Veisi, H. Direct oxidative conversion of alcohols, amines, aldehydes and benzyl halides into the corresponding nitriles with tricholroisocianuric acid in aqueous ammonia. Synthesis 2010, 2010, 2631–2635. [Google Scholar] [CrossRef]
- Srilakshmi Krishanaveni, N.; Suredra, K.; Rama Rao, K. A Simple and Highly Selective Biomimetic Oxidation of Alcohols and Epoxides with N-Bromosuccinimide in the Presence of β-Cyclodextrin in Water. Adv. Synth. Catal. 2004, 346, 346–350. [Google Scholar] [CrossRef]
- Filler, R. Oxidations and Dehydrogenations with N-Bromosuccinimide and Related N-Haloimides. Chem. Rev. 1963, 63, 21–43. [Google Scholar] [CrossRef]
- Wilson, S.R.; Tofigh, S.; Misra, R.N. A novel, nonoxidative method for the conversion of aldehydes to esters. J. Org. Chem. 1982, 47, 1360–1361. [Google Scholar] [CrossRef]
- Zhao, J.; Sun, H.; Lu, Y.; Li, J.; Yu, Z.; Zhu, H.; Ma, C.; Meng, Q.; Peg, X. A divergent photocatalysis strategy for selective aerobic oxidation of C(sp3)–H bonds promoted by disulfides. Green Chem. 2022, 24, 8503–8511. [Google Scholar] [CrossRef]
- Chen, P.-S.; Chou, C.-H. Pyrolytic Chemistry of Thenyl Benzoates. Tetrahedron 1996, 52, 13615–13622. [Google Scholar] [CrossRef]
- Ruso, J.S.; Rajendiran, N.; Kumaran, R.S. Metal-free synthesis of aryl esters by coupling aryl carboxylic acids and aryl boroni cacids. Tetrahedron Lett. 2014, 55, 2345–2347. [Google Scholar] [CrossRef]
- Gondo, K.; Oyamada, J.; Kitamura, T. Palladium-Catalyzed Desilylative Acyloxylation of Silicon–CarbonBonds on (Trimethylsilyl)arenes: Synthesis of Phenol Derivativesfrom Trimethylsilylarenes. Org. Lett. 2015, 17, 4778–4781. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Yuan, L.; Wang, T.; Wang, C.; Ke, J.; Zhao, J. Palladium-catalyzed oxidative carbonylation of aryl hydrazines with CO and O2 at atmospheric pressure. J. Org. Chem. 2017, 82, 4970–4976. [Google Scholar] [CrossRef] [PubMed]
- Ramanjaneyulu, B.T.; Reddy, V.; Arde, P.; Mahesh, S.; Anand, R.V. Combining Oxidative N-Heterocyclic Carbene Catalysis with Click Chemistry: A Facile One-Pot Approach to 1, 2, 3-Triazole Derivatives. Chem. Asian J. 2013, 8, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Ullah, E.; McNulty, J.; Sliwinski, M.; Robertson, A. One-step synthesis of reusable, polymer-supported tri-alkyl phosphine ligands. Application in Suzuki–Miyaura and alkoxycarbonylation reactions. Tetrahedron Lett. 2012, 53, 3990–3993. [Google Scholar] [CrossRef]
- Dai, M.-S.; Zheng, Z.-M.; Zhang, S.L. High-valent Cu (III)–CF 3 compound-mediated esterification reaction. Org. Biomol. Chem. 2023, 21, 935–939. [Google Scholar] [CrossRef]
- Rout, S.K.; Guin, S.; Ghara, K.K.; Banerjee, A.; Patel, B.K. Copper catalyzed oxidative esterification of aldehydes with alkylbenzenes via cross dehydrogenative coupling. Org. Lett. 2012, 14, 3982–3985. [Google Scholar] [CrossRef]
- Liu, H.; Dong, C.; Zhang, Z.; Wu, P.; Jiang, X. Transition-Metal-Free Aerobic Oxidative Cleavage of C–C Bonds in α-Hydroxy Ketones and Mechanistic Insight to the Reaction Pathway. Angew. Chem. Int. Ed. 2012, 51, 12570–12574. [Google Scholar] [CrossRef]
- Liu, H.; Shi, G.; Pan, S.; Jiang, Y.; Zhang, Y. Palladium-catalyzed benzylation of carboxylic acids with toluene via benzylic C–H activation. Org. Lett. 2013, 15, 4098–4101. [Google Scholar] [CrossRef] [PubMed]
- Finney, E.E.; Ogawa, K.A.; Boydston, A.J. Organocatalyzed anodic oxidation of aldehydes. J. Am. Chem. Soc. 2012, 134, 12374–12377. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, T.; Hirose, D.; Ishibashi, H. Esterification via iron-catalyzed activation of triphenylphosphine with air. ACS Catal. 2011, 1, 1469–1474. [Google Scholar] [CrossRef]
- But, T.Y.S.; Lu, J.; Toy, P.H. Organocatalytic Mitsunobu reactions with 3, 5-dinitrobenzoic acid. Synlett 2010, 2010, 1115–1117. [Google Scholar]
- Ackermann, L.; Gschrei, C.J.; Althammer, A.; Riederer, M. Cross-coupling reactions of aryl and vinyl chlorides catalyzed by a palladium complex derived from an air-stable H-phosphonate. Chem. Comm. 2006, 2006, 1419–1421. [Google Scholar] [CrossRef] [PubMed]
- Inamoto, K.; Kuroda, J.I.; Hiroya, K.; Noda, Y.; Watanabe, M.; Sakamoto, T. Synthesis and catalytic activity of a pincer-type bis (imidazolin-2-ylidene) nickel (II) complex. Organometallics 2006, 25, 3095–3098. [Google Scholar] [CrossRef]
- Tian, Y.; Ling, A.; Fang, R.; Tan, R.X.; Liu, Z.Q. Free-radical anti-Markovnikov hydroalkylation of unactivated alkenes with simple alkanes. Green Chem. 2018, 20, 3432–3435. [Google Scholar] [CrossRef]
- Chen, X.; Hu, S.; Chen, R.; Wang, J.; Wu, M.; Guo, H.; Sun, S. Fe-catalyzed esterification of amides via C–N bond activation. RSC Adv. 2018, 8, 4571–4576. [Google Scholar] [CrossRef]
- Hoffmann, H.M.R.; Haase, K. The synthesis of acyl iodides. Synthesis 1981, 1981, 715–719. [Google Scholar] [CrossRef]











| Entry | Irradiation Source | Irradiation Time | TCCA (mmol) | Solvent | Yield 1 [%] |
|---|---|---|---|---|---|
| 1 | Solar simulator | 1 | 1.1 | DCM (1 mL) | 67% |
| 2 | Solar simulator | 1 | 1.2 | DCM (1 mL) | 77% |
| 3 | Solar simulator | 1 | 1.3 | DCM (1 mL) | 80% |
| 4 | Solar simulator | 1 | 1.3 | DCM (2 mL) | 87% |
| 5 | Solar simulator | 1.5 | 1.3 | DCM (2 mL) | 99% |
| 6 | Sun | 1.5 | 1.3 | DCM (2 mL) | 98% |
| 7 | Blue LED | 1.5 | 1.3 | DCM (2 mL) | 99% |
| 8 | Blue LED | 1.5 | 1.1 | DCM (2 mL) | 99% |
| 9 | Blue LED | 1.5 | 1.1 | CPME (2 mL) | - |
| 10 | Blue LEDd | 1.5 | 1.1 | THF (2 mL) | - |
| 11 | Blue LED | 1.5 | 1.1 | CH3CN (2 mL) | - |
| 12 | Blue LED | 1.5 | 1.1 | AcOEt (2 mL) | - |
| 13 | Blue LED | 1.5 | 1.1 | DCE (2 mL) | Trace |
| 14 2 | None | 1.5 | 1.1 | DCM (2 mL) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dellisanti, A.; Chessa, E.; Porcheddu, A.; Carraro, M.; Pisano, L.; De Luca, L.; Gaspa, S. Visible Light-Promoted Oxidative Cross-Coupling of Alcohols to Esters. Molecules 2024, 29, 570. https://doi.org/10.3390/molecules29030570
Dellisanti A, Chessa E, Porcheddu A, Carraro M, Pisano L, De Luca L, Gaspa S. Visible Light-Promoted Oxidative Cross-Coupling of Alcohols to Esters. Molecules. 2024; 29(3):570. https://doi.org/10.3390/molecules29030570
Chicago/Turabian StyleDellisanti, Andrea, Elisa Chessa, Andrea Porcheddu, Massimo Carraro, Luisa Pisano, Lidia De Luca, and Silvia Gaspa. 2024. "Visible Light-Promoted Oxidative Cross-Coupling of Alcohols to Esters" Molecules 29, no. 3: 570. https://doi.org/10.3390/molecules29030570
APA StyleDellisanti, A., Chessa, E., Porcheddu, A., Carraro, M., Pisano, L., De Luca, L., & Gaspa, S. (2024). Visible Light-Promoted Oxidative Cross-Coupling of Alcohols to Esters. Molecules, 29(3), 570. https://doi.org/10.3390/molecules29030570