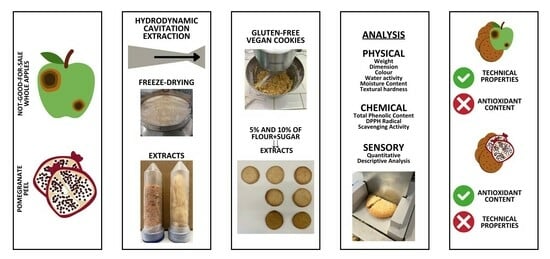
Can a Fraction of Flour and Sugar Be Replaced with Fruit By-Product Extracts in a Gluten-Free and Vegan Cookie Recipe?
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Characterization of Extracts
2.2. Physical Properties of Cookies Immediately after Baking
2.3. Chemical Properties of Cookies Immediately after Baking
2.4. Sensory Evaluation of Cookies Immediately after Baking
2.5. Physical and Chemical Properties after One Month of Conservation
3. Materials and Methods
3.1. Samples
3.1.1. HC-Based Extraction of Whole Apple and Pomegranate Peel
3.1.2. Physical and Chemical Characterization of the Extracts
3.2. Vegan and Gluten-Free Cookie Preparation
3.3. Physical Properties
3.3.1. Weight, Dimension and Color Analysis
3.3.2. Water Activity, Moisture Content, and Texture (Textural Hardness)
3.4. Total Phenolic Content and DPPH Radical Scavenging Activity
3.5. Sensory Analyses
3.6. Data Processing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Girotto, F.; Alibardi, L.; Cossu, R. Food Waste Generation and Industrial Uses: A Review. Waste Manag. 2015, 45, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Mirabella, N.; Castellani, V.; Sala, S. Current Options for the Valorization of Food Manufacturing Waste: A Review. J. Clean. Prod. 2014, 65, 28–41. [Google Scholar] [CrossRef]
- Roy, P.; Mohanty, A.K.; Dick, P.; Misra, M. A Review on the Challenges and Choices for Food Waste Valorization: Environmental and Economic Impacts. ACS Environ. Au 2023, 3, 58–75. [Google Scholar] [CrossRef] [PubMed]
- Vilas-Boas, A.A.; Pintado, M.; Oliveira, A.L.S. Natural Bioactive Compounds from Food Waste: Toxicity and Safety Concerns. Foods 2021, 10, 1564. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Yadav, A.N.; Kumar, V.; Vyas, P.; Dhaliwal, H.S. Food Waste: A Potential Bioresource for Extraction of Nutraceuticals and Bioactive Compounds. Bioresour. Bioprocess. 2017, 4, 18. [Google Scholar] [CrossRef]
- Perussello, C.A.; Zhang, Z.; Marzocchella, A.; Tiwari, B.K. Valorization of Apple Pomace by Extraction of Valuable Compounds. Comp. Rev. Food Sci. Food Safe 2017, 16, 776–796. [Google Scholar] [CrossRef] [PubMed]
- Putra, N.R.; Rizkiyah, D.N.; Abdul Aziz, A.H.; Che Yunus, M.A.; Veza, I.; Harny, I.; Tirta, A. Waste to Wealth of Apple Pomace Valorization by Past and Current Extraction Processes: A Review. Sustainability 2023, 15, 830. [Google Scholar] [CrossRef]
- Liu, Z.; De Souza, T.S.P.; Holland, B.; Dunshea, F.; Barrow, C.; Suleria, H.A.R. Valorization of Food Waste to Produce Value-Added Products Based on Its Bioactive Compounds. Processes 2023, 11, 840. [Google Scholar] [CrossRef]
- Asma, U.; Morozova, K.; Ferrentino, G.; Scampicchio, M. Apples and Apple By-Products: Antioxidant Properties and Food Applications. Antioxidants 2023, 12, 1456. [Google Scholar] [CrossRef]
- Szabo, K.; Mitrea, L.; Călinoiu, L.F.; Teleky, B.-E.; Martău, G.A.; Plamada, D.; Pascuta, M.S.; Nemeş, S.-A.; Varvara, R.-A.; Vodnar, D.C. Natural Polyphenol Recovery from Apple-, Cereal-, and Tomato-Processing By-Products and Related Health-Promoting Properties. Molecules 2022, 27, 7977. [Google Scholar] [CrossRef]
- Da Silva, L.C.; Viganó, J.; De Souza Mesquita, L.M.; Dias, A.L.B.; De Souza, M.C.; Sanches, V.L.; Chaves, J.O.; Pizani, R.S.; Contieri, L.S.; Rostagno, M.A. Recent Advances and Trends in Extraction Techniques to Recover Polyphenols Compounds from Apple By-Products. Food Chem. X 2021, 12, 100133. [Google Scholar] [CrossRef]
- Alberti, A.; Zielinski, A.A.F.; Zardo, D.M.; Demiate, I.M.; Nogueira, A.; Mafra, L.I. Optimisation of the Extraction of Phenolic Compounds from Apples Using Response Surface Methodology. Food Chem. 2014, 149, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Xu, Y.; Fan, Y. Upgrading Pectin Production from Apple Pomace by Acetic Acid Extraction. Appl. Biochem. Biotechnol. 2019, 187, 1300–1311. [Google Scholar] [CrossRef] [PubMed]
- Morales-Contreras, B.E.; Wicker, L.; Rosas-Flores, W.; Contreras-Esquivel, J.C.; Gallegos-Infante, J.A.; Reyes-Jaquez, D.; Morales-Castro, J. Apple Pomace from Variety “Blanca de Asturias” as Sustainable Source of Pectin: Composition, Rheological, and Thermal Properties. LWT 2020, 117, 108641. [Google Scholar] [CrossRef]
- Sood, A.; Gupta, M. Extraction Process Optimization for Bioactive Compounds in Pomegranate Peel. Food Biosci. 2015, 12, 100–106. [Google Scholar] [CrossRef]
- Cecchi, L.; Khatib, M.; Bellumori, M.; Civa, V.; Domizio, P.; Innocenti, M.; Balli, D.; Mulinacci, N. Industrial Drying for Agrifood By-Products Re-Use: Cases Studies on Pomegranate Peel (Punica granatum L.) and Stoned Olive Pomace (Pâtè, Olea europaea L.). Food Chem. 2023, 403, 134338. [Google Scholar] [CrossRef]
- Balli, D.; Khatib, M.; Cecchi, L.; Adessi, A.; Melgarejo, P.; Nunes, C.; Coimbra, M.A.; Mulinacci, N. Pomegranate Peel as a Promising Source of Pectic Polysaccharides: A Multi-Methodological Analytical Investigation. Food Chem. 2022, 397, 133550. [Google Scholar] [CrossRef]
- Canuti, V.; Cecchi, L.; Khatib, M.; Guerrini, L.; Mulinacci, N.; Zanoni, B. A New Extract from Pomegranate (Punica granatum L.) By-Products as a Potential Oenological Tannin: Preliminary Characterization and Comparison with Existing Commercial Products. Molecules 2020, 25, 4460. [Google Scholar] [CrossRef]
- Akhtar, S.; Ismail, T.; Fraternale, D.; Sestili, P. Pomegranate Peel and Peel Extracts: Chemistry and Food Features. Food Chem. 2015, 174, 417–425. [Google Scholar] [CrossRef]
- Cano-Lamadrid, M.; Martínez-Zamora, L.; Castillejo, N.; Artés-Hernández, F. From Pomegranate Byproducts Waste to Worth: A Review of Extraction Techniques and Potential Applications for Their Revalorization. Foods 2022, 11, 2596. [Google Scholar] [CrossRef]
- El-Shamy, S.; Farag, M.A. Novel Trends in Extraction and Optimization Methods of Bioactives Recovery from Pomegranate Fruit Biowastes: Valorization Purposes for Industrial Applications. Food Chem. 2021, 365, 130465. [Google Scholar] [CrossRef]
- Talekar, S.; Patti, A.F.; Vijayraghavan, R.; Arora, A. Rapid, Enhanced and Eco-Friendly Recovery of Punicalagin from Fresh Waste Pomegranate Peels via Aqueous Ball Milling. J. Clean. Prod. 2019, 228, 1238–1247. [Google Scholar] [CrossRef]
- Magangana, T.P.; Makunga, N.P.; Fawole, O.A.; Opara, U.L. Processing Factors Affecting the Phytochemical and Nutritional Properties of Pomegranate (Punica granatum L.) Peel Waste: A Review. Molecules 2020, 25, 4690. [Google Scholar] [CrossRef] [PubMed]
- Lampakis, D.; Skenderidis, P.; Leontopoulos, S. Technologies and Extraction Methods of Polyphenolic Compounds Derived from Pomegranate (Punica granatum) Peels. A Mini Review. Processes 2021, 9, 236. [Google Scholar] [CrossRef]
- Minutolo, A.; Gismondi, A.; Chirico, R.; Di Marco, G.; Petrone, V.; Fanelli, M.; D’Agostino, A.; Canini, A.; Grelli, S.; Albanese, L.; et al. Antioxidant Phytocomplexes Isolated from Pomegranate (Punica granatum L.) Using Hydrodynamic Cavitation Reveal Potential Application as Adjuvants in Cancer Therapies. Biol. Life Sci. 2023. preprints. [Google Scholar]
- Arya, S.S.; More, P.R.; Ladole, M.R.; Pegu, K.; Pandit, A.B. Non-Thermal, Energy Efficient Hydrodynamic Cavitation for Food Processing, Process Intensification and Extraction of Natural Bioactives: A Review. Ultrason. Sonochem. 2023, 98, 106504. [Google Scholar] [CrossRef]
- Askarniya, Z.; Sun, X.; Wang, Z.; Boczkaj, G. Cavitation-Based Technologies for Pretreatment and Processing of Food Wastes: Major Applications and Mechanisms—A Review. Chem. Eng. J. 2023, 454, 140388. [Google Scholar] [CrossRef]
- Ciriminna, R.; Scurria, A.; Pagliaro, M. Natural Product Extraction via Hydrodynamic Cavitation. Sustain. Chem. Pharm. 2023, 33, 101083. [Google Scholar] [CrossRef]
- Panda, D.; Saharan, V.K.; Manickam, S. Controlled Hydrodynamic Cavitation: A Review of Recent Advances and Perspectives for Greener Processing. Processes 2020, 8, 220. [Google Scholar] [CrossRef]
- Meneguzzo, F.; Brunetti, C.; Fidalgo, A.; Ciriminna, R.; Delisi, R.; Albanese, L.; Zabini, F.; Gori, A.; Dos Santos Nascimento, L.; De Carlo, A.; et al. Real-Scale Integral Valorization of Waste Orange Peel via Hydrodynamic Cavitation. Processes 2019, 7, 581. [Google Scholar] [CrossRef]
- Nuzzo, D.; Cristaldi, L.; Sciortino, M.; Albanese, L.; Scurria, A.; Zabini, F.; Lino, C.; Pagliaro, M.; Meneguzzo, F.; Di Carlo, M.; et al. Exceptional Antioxidant, Non-Cytotoxic Activity of Integral Lemon Pectin from Hydrodynamic Cavitation. ChemistrySelect 2020, 5, 5066–5071. [Google Scholar] [CrossRef]
- Asaithambi, N.; Singha, P.; Dwivedi, M.; Singh, S.K. Hydrodynamic Cavitation and Its Application in Food and Beverage Industry: A Review. J. Food Process Eng. 2019, 42, e13144. [Google Scholar] [CrossRef]
- Nuzzo, D.; Scurria, A.; Picone, P.; Guiducci, A.; Pagliaro, M.; Pantaleo, G.; Albanese, L.; Meneguzzo, F.; Ciriminna, R. A Gluten-Free Biscuit Fortified with Lemon IntegroPectin. ChemistrySelect 2022, 7, e202104247. [Google Scholar] [CrossRef]
- Parenti, O.; Albanese, L.; Guerrini, L.; Zanoni, B.; Zabini, F.; Meneguzzo, F. Whole Wheat Bread Enriched with Silver Fir (Abies Alba Mill.) Needles Extract: Technological and Antioxidant Properties. J. Sci. Food Agric. 2022, 102, 3581–3589. [Google Scholar] [CrossRef] [PubMed]
- Taylor, T.P.; Fasina, O.; Bell, L.N. Physical Properties and Consumer Liking of Cookies Prepared by Replacing Sucrose with Tagatose. J. Food Sci. 2008, 73, S145–S151. [Google Scholar] [CrossRef] [PubMed]
- Handa, C.; Goomer, S.; Siddhu, A. Physicochemical Properties and Sensory Evaluation of Fructoligosaccharide Enriched Cookies. J. Food Sci. Technol. 2012, 49, 192–199. [Google Scholar] [CrossRef]
- Mancebo, C.M.; Picón, J.; Gómez, M. Effect of Flour Properties on the Quality Characteristics of Gluten Free Sugar-Snap Cookies. LWT-Food Sci. Technol. 2015, 64, 264–269. [Google Scholar] [CrossRef]
- Masih, L.P.; Sonkar, C.; Singh, S.; Chauhan, R. Physico-Chemical Properties of Biscuits Influenced by Different Ratios of Hydrogenated Fat (Vanaspati) and Peanut Butter. Int. J. Curr. Microbiol. App. Sci. 2017, 6, 1804–1811. [Google Scholar] [CrossRef]
- Miller, R.A.; Hoseney, R.C.; Morris, C.F. Effect of Formula Water Content on the Spread of Sugar-Snap Cookies. Cereal Chem. 1997, 74, 669–671. [Google Scholar] [CrossRef]
- Gujral, H.S.; Mehta, S.; Samra, I.S.; Goyal, P. Effect of Wheat Bran, Coarse Wheat Flour, and Rice Flour on the Instrumental Texture of Cookies. Int. J. Food Prop. 2003, 6, 329–340. [Google Scholar] [CrossRef]
- Hamdani, A.M.; Wani, I.A.; Bhat, N.A. Gluten Free Cookies from Rice-Chickpea Composite Flour Using Exudate Gums from Acacia, Apricot and Karaya. Food Biosci. 2020, 35, 100541. [Google Scholar] [CrossRef]
- Kulp, K.; Olewnik, M.; Lorenz, K.; Collins, F. Starch Functionality in Cookie Systems. Starch-Stärke 1991, 43, 53–57. [Google Scholar] [CrossRef]
- Ashwath Kumar, K.; Sudha, M.L. Effect of Fat and Sugar Replacement on Rheological, Textural and Nutritional Characteristics of Multigrain Cookies. J. Food Sci. Technol. 2021, 58, 2630–2640. [Google Scholar] [CrossRef]
- Wüstenberg, T. Cellulose and Cellulose Derivatives in the Food Industry: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Kissel, L.T.; Pomeranz, Y.; Amazaki, W.T. Effects of Flour Lipids on Cookie Quality. Cereal Chem. 1971, 48, 655. [Google Scholar]
- Yamamoto, H.; Worthington, S.T.; Hou, G.; Ng, P.K.W. Rheological Properties and Baking Qualities of Selected Soft Wheats Grown in the United States. Cereal Chem. 1996, 73, 215–221. [Google Scholar]
- Bose, D.; Shams-Ud-Din, M. The Effect of Chickpea (Cicer arietinim) Husk on the Properties of Cracker Biscuits. J. Bangladesh Agric. Univ. 1970, 8, 147–152. [Google Scholar] [CrossRef]
- Pareyt, B.; Talhaoui, F.; Kerckhofs, G.; Brijs, K.; Goesaert, H.; Wevers, M.; Delcour, J.A. The Role of Sugar and Fat in Sugar-Snap Cookies: Structural and Textural Properties. J. Food Eng. 2009, 90, 400–408. [Google Scholar] [CrossRef]
- Barak, S.; Mudgil, D.; Singh Khatkar, B. Effect of Composition of Gluten Proteins and Dough Rheological Properties on the Cookie-making Quality. Br. Food J. 2013, 115, 564–574. [Google Scholar] [CrossRef]
- Kruczek, M.; Gumul, D.; Korus, A.; Buksa, K.; Ziobro, R. Phenolic Compounds and Antioxidant Status of Cookies Supplemented with Apple Pomace. Antioxidants 2023, 12, 324. [Google Scholar] [CrossRef]
- Najjar, Z.; Alkaabi, M.; Alketbi, K.; Stathopoulos, C.; Ranasinghe, M. Physical Chemical and Textural Characteristics and Sensory Evaluation of Cookies Formulated with Date Seed Powder. Foods 2022, 11, 305. [Google Scholar] [CrossRef]
- Nuzzo, D.; Picone, P.; Lozano Sanchez, J.; Borras-Linares, I.; Guiducci, A.; Muscolino, E.; Giacomazza, D.; Sanfilippo, T.; Guggino, R.; Bulone, D.; et al. Recovery from Food Waste—Biscuit Doughs Enriched with Pomegranate Peel Powder as a Model of Fortified Aliment. Biology 2022, 11, 416. [Google Scholar] [CrossRef] [PubMed]
- Özer, E.A. Optimization of Gluten Free Cookies Produced with Nutritious Ingredients: Evaluating a New Food Product. Food Process. Preserv. 2022, 46, e16302. [Google Scholar] [CrossRef]
- Mudgil, D.; Barak, S.; Khatkar, B.S. Cookie Texture, Spread Ratio and Sensory Acceptability of Cookies as a Function of Soluble Dietary Fiber, Baking Time and Different Water Levels. LWT 2017, 80, 537–542. [Google Scholar] [CrossRef]
- Piga, A.; Catzeddu, P.; Farris, S.; Roggio, T.; Sanguinetti, A.; Scano, E. Texture Evolution of “Amaretti” Cookies during Storage. Eur. Food Res. Technol. 2005, 221, 387–391. [Google Scholar] [CrossRef]
- Lisiecka, K.; Wójtowicz, A.; Gancarz, M. Characteristics of Newly Developed Extruded Products Supplemented with Plants in a Form of Microwave-Expanded Snacks. Materials 2021, 14, 2791. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Bonina, F.; Puglia, C.; Tomaino, A.; Saija, A.; Mulinacci, N.; Romani, A.; Vincieri, F.F. In-Vitro Antioxidant and In-Vivo Photoprotective Effect of Three Lyophilized Extracts of Sedum telephium L. Leaves. J. Pharm. Pharmacol. 2010, 52, 1279–1285. [Google Scholar] [CrossRef]
- Cereals & Grains Association. AACC Approved Methods of Analysis, 11th ed.; Method 10-54.01 Baking Quality of Cookie Flour—Micro Wire-Cut Formulation, Approved November 3; Cereals & Grains Association: St. Paul, MN, USA, 1999. [Google Scholar] [CrossRef]
- Cereals & Grains Association. AACC Approved Methods of Analysis, 11th ed.; Method 44-15.02 Moisture—Air-Oven Methods, Approved November 3; Cereals & Grains Association: St. Paul, MN, USA, 1999. [Google Scholar] [CrossRef]
- Gao, L.; Oomah, B.D.; Mazza, G. Wheat quality: Antioxidant activity of wheat millstreams. Wheat Qual. Elucidation 2002, 1, 219–233. [Google Scholar]
- Cecchi, L.; Schuster, N.; Flynn, D.; Bechtel, R.; Bellumori, M.; Innocenti, M.; Mulinacci, N.; Guinard, J. Sensory Profiling and Consumer Acceptance of Pasta, Bread, and Granola Bar Fortified with Dried Olive Pomace (Pâté): A Byproduct from Virgin Olive Oil Production. J. Food Sci. 2019, 84, 2995–3008. [Google Scholar] [CrossRef]


| AE | PPE | |
|---|---|---|
| Calories (kcal/100 g) | 246 | 79 |
| Calories (kJ/100 g) | 1043 | 335 |
| Total fats (g/100 g) | 0.9 | <0.1 |
| Saturated fats (g/100 g) | 0.4 | <0.1 |
| Total carbohydrates (g/100 g) | 58.1 | 16.0 |
| Total sugars (g/100 g) | 58.1 | 8.7 |
| Dietary Fiber (g/100 g) | 9.6 | 0.2 |
| Total proteins (g/100 g) | 1.3 | 3.7 |
| Diameter (cm) | Thickness (cm) | Spread Ratio | L* | a* | b* | Hardness (N) | |
|---|---|---|---|---|---|---|---|
| CON | 5.8 ± 0.2 b | 1.11 ± 0.03 a | 5.2 ± 0. 2 d | 73.8 ± 3.7 a | 4.1 ± 2.2 b | 27.9 ± 3.6 | 23.4 ± 4.0 b |
| W05 | 5.6 ± 0.1 bc | 1.05 ± 0.03 ab | 5.3 ± 0.1 d | 71.8 ± 3.2 a | 4.2 ± 1.1 b | 25.1 ± 2.4 | 23.0 ± 4.2 b |
| W10 | 5.8 ± 0.1 b | 1.05 ± 0.02 ab | 5.6 ± 0.2 cd | 74.6 ± 1.9 a | 3.1 ± 1.4 b | 25.7 ± 1.5 | 25.5 ± 6.2 ab |
| A05 | 5.9 ± 0.2 ab | 0.99 ± 0.08 bc | 6.0 ± 0.3 bc | 73.9 ± 0.7 a | 2.8 ± 0.1 b | 26.9 ± 0.6 | 29.4 ± 1.9 a |
| A10 | 6.1 ± 0.1 a | 0.91 ± 0.03 c | 6.7 ± 0.3 a | 69.5 ± 2.9 a | 3.9 ± 0.6 b | 25.7 ± 3.6 | 33.5 ± 5.7 a |
| P05 | 5.5 ± 0.1 c | 0.99 ± 0.08 bc | 5.6 ± 0.4 cd | 57.8 ± 0.5 b | 7.8 ± 0.2 a | 32.1 ± 7.7 | 41.0 ± 10.7 a |
| P10 | 5.7 ± 0.1 bc | 0.93 ± 0.04 c | 6.2 ± 0.3 b | 56.7 ± 2.2 b | 7.7 ± 1.0 a | 32.3 ± 7.7 | 35.6 ± 7.0 a |
| p-value | ** | ** | *** | *** | *** | ns | * |
| TPC (mgGAE/g) | DPPH (IC50 µg/mL) | |
|---|---|---|
| CON | 0.75 ± 0.35 c | 25.6 ± 4.3 bc |
| W05 | 0.61 ± 0.02 c | 50.4 ± 6.5 a |
| W10 | 0.59 ± 0.10 c | 46.9 ± 2.6 a |
| A05 | 0.92 ± 0.18 c | 28.9 ± 3.9 b |
| A10 | 1.30 ± 0.12 c | 20.7 ± 0.9 c |
| P05 | 6.15 ± 0.37 b | 3.1 ± 1.9 d |
| P10 | 9.77 ± 0.95 a | 1.2 ± 0.3 d |
| p-value | *** | *** |
| Extract Name | Water (L) | Lemon Juice (L) | Fresh Raw Material (kg) | Relative Humidity (%) | Time (min) | Temperature Range (°C) | Specific Energy (kWh/kg DW) |
|---|---|---|---|---|---|---|---|
| AE | 44 | 5 | 96.9 | 84.3 | 117 | 24.0–78.0 | 0.852 |
| PPE | 108 | 2 | 47.3 | 75.0 | 20 | 31.0–39.0 | 0.216 |
| Without Substitution | With AE | With PPE | |||||
|---|---|---|---|---|---|---|---|
| CON | W05 | W10 | A05 | A10 | P05 | P10 | |
| All-purpose flour (g) | 120 | 114 | 108 | 114 | 108 | 114 | 108 |
| White Sugar (g) | 60 | 57 | 54 | 57 | 54 | 57 | 54 |
| Extract (g) | 0 | 0 | 0 | 9 | 18 | 9 | 18 |
| Baking Powder (g) | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Margarine (g) | 48 | 48 | 48 | 48 | 48 | 48 | 48 |
| Water (g) | 30 | 30 | 30 | 30 | 30 | 30 | 30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Breschi, C.; D’Agostino, S.; Meneguzzo, F.; Zabini, F.; Chini, J.; Lovatti, L.; Tagliavento, L.; Guerrini, L.; Bellumori, M.; Cecchi, L.; et al. Can a Fraction of Flour and Sugar Be Replaced with Fruit By-Product Extracts in a Gluten-Free and Vegan Cookie Recipe? Molecules 2024, 29, 1102. https://doi.org/10.3390/molecules29051102
Breschi C, D’Agostino S, Meneguzzo F, Zabini F, Chini J, Lovatti L, Tagliavento L, Guerrini L, Bellumori M, Cecchi L, et al. Can a Fraction of Flour and Sugar Be Replaced with Fruit By-Product Extracts in a Gluten-Free and Vegan Cookie Recipe? Molecules. 2024; 29(5):1102. https://doi.org/10.3390/molecules29051102
Chicago/Turabian StyleBreschi, Carlotta, Silvia D’Agostino, Francesco Meneguzzo, Federica Zabini, Jasmine Chini, Luca Lovatti, Luca Tagliavento, Lorenzo Guerrini, Maria Bellumori, Lorenzo Cecchi, and et al. 2024. "Can a Fraction of Flour and Sugar Be Replaced with Fruit By-Product Extracts in a Gluten-Free and Vegan Cookie Recipe?" Molecules 29, no. 5: 1102. https://doi.org/10.3390/molecules29051102
APA StyleBreschi, C., D’Agostino, S., Meneguzzo, F., Zabini, F., Chini, J., Lovatti, L., Tagliavento, L., Guerrini, L., Bellumori, M., Cecchi, L., & Zanoni, B. (2024). Can a Fraction of Flour and Sugar Be Replaced with Fruit By-Product Extracts in a Gluten-Free and Vegan Cookie Recipe? Molecules, 29(5), 1102. https://doi.org/10.3390/molecules29051102