Starch from Unripe Apples (Malus domestica Borkh) as an Alternative for Application in the Food Industry
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Composition
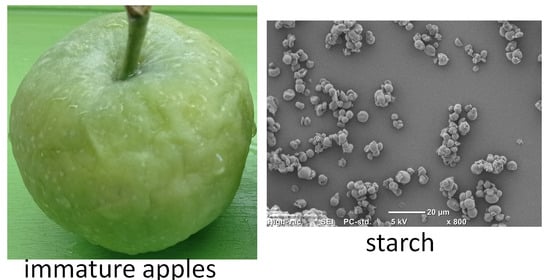
2.2. SEM and Granularity
2.3. Swelling Characteristics
2.4. Thermal Characteristics Performed by Differential Scanning Calorimetry (DSC)
2.5. Retrogradation
2.6. Assessment of Viscous Properties
2.7. Assessment of Viscoelastic Properties
- -
- The total elasticity of the network Ge of gelatinized apple starches SP and SO is comparable, with only a slight advantage—about 4 Pa—for the sample of SO starch paste.
- -
- Equilibrium compliance Je, and thus, the possibility of storing energy, is slightly higher for SP starch paste.
- -
- Quite high values of the viscoelastic modulus of the plateau GN0 show that the tested samples of apple starch are media with a structure that shows the behavior typical of viscoelastic quasi-solids with a strong structure. The values of the GN0 modulus indicate that the cross-linking of the structure is stronger for SO starch. What is more, for this starch, aging processes take place slower over time.
- -
- Plateau compliance JN0 is, however, 30% lower for SO starch than for SP starch, which means that SP starch has such network entanglements that suppress all kinds of long-range configuration rearrangements.
- -
- Newtonian viscosity under steady flow conditions η0 has a much higher value for apple starch SO, which means that the flow capacity of the set of elements closed with the minimum number of network nodes for this starch is lower than for starch SP, and thus starch paste SO is characterized by greater gel stiffness.
- -
- Cross-linking densities ω0 of both apple starch pastes are at a comparable level.
- -
- The width of the viscoelastic plateau L has a higher value, equal to 5.141, for SO apple starch; therefore, this starch has a higher polydispersity than SP starch.
- -
- The values of the average molecular weights for Me entanglement and Mc cross-linking have the same order of magnitude and similar values, but with a slight advantage in favor of SP apple starch.
- -
- Apple starch SP also creates a network, with the mesh size ξ being 3.5 nm larger than in the case of SO starch.
| Rheological Parameters | Starch Pastes Sample | Unit | |
|---|---|---|---|
| SO | SP | ||
| Ge | 42.185 ± 0.107 | 38.927 ± 0.083 | (Pa) |
| Je | 0.0237 ± 0.0003 | 0.0257 ± 0.0005 | (1/Pa) |
| GN0 | 216.89 ± 1.631 | 150.54 ± 0.890 | (Pa) |
| JN0 | 0.0046 ± 0.0001 | 0.0066 ± 0.0003 | (1/Pa) |
| η0 | 1082.13 ± 3.213 | 637.65 ± 1.846 | (Pa·s) |
| τm | 25.652 ± 0.026 | 16.381 ± 0.017 | (s) |
| τ0 | 4.989 ± 0.090 | 4.236 ± 0.062 | (s) |
| ω0 | 0.200 ± 0.004 | 0.236 ± 0.006 | (1/s) |
| L | 5.141 ± 0.085 | 3.867 ± 0.032 | (-) |
| Me | 1.238 × 104 ± 0.129 | 1.784 × 104 ± 0.114 | (kg/mol) |
| Mchem | 6.366 × 104 ± 0.328 | 6.899 × 104 ± 0.386 | (kg/mol) |
| ξ | 27.391 ± 0.019 | 30.937 ± 0.028 | (nm) |
3. Materials and Methods
3.1. Materials
3.2. Starch Extraction
3.3. Scanning Electron Microscopy
3.4. Granule Diameter and Particle Size Distribution
3.5. Amylose Content
3.6. Determination of Weight-Average Molecular Weight
3.7. Content of Non-Polysaccharide Components
3.8. Water-Binding Capacity and Solubility
3.9. Gelatinization Measurements
3.10. Retrogradation Measurement
3.11. Degree of Retrogradation
3.12. Rheological Measurements
3.13. Measurement of Viscous Properties
- -
- Increasing shear rate from 0.1 s−1 to 100 s−1 for 300 s.
- -
- Constant shear rate 100 s−1 for 60 s.
- -
- Decreasing shear rate from 100 s−1 to 0.1 s−1 for 300 s.
- -
- Ostwald de Waele model.
- -
- Herschel–Bulkley model.
- -
- Casson model.
3.14. Measurement of Viscoelastic Properties
- -
- The equilibrium modulus, i.e., the modulus of elasticity in a steady state, Ge, is responsible for the total elasticity of the medium:
- -
- Viscoelastic plateau modulus GN0 is responsible for cross-linking of the structure, and its high values also indicate the possibility of slowing down the aging effects of the medium over time. For polydisperse media—such as gelatinized apple starches—it is determined by the following relationship:
- -
- Newtonian viscosity in conditions of steady flow η0 characterizes the flow capacity of a set of elements closed with a minimum number of nodes of the biopolymer network that are capable of individual movement:
- -
- The equilibrium compliance Je, which is a measure of the energy stored during a steady state of the biopolymer under low stress conditions:
- -
- Plateau compliance JN0, representing the strength with which the entanglements of the biopolymer network suppress all kinds of long-range configuration rearrangements:
- -
- Weighted average relaxation time τm equated with the longest relaxation time:
- -
- Numerically average relaxation time τ0 equated with the shortest relaxation time:
- -
- Cross-linking density of the structure ω0, defining at the same time the end of the viscoelastic area of the plateau:
- -
- Dimensionless width of the viscoelastic plateau L, combining fast and slow dissipation processes, determining the degree of biopolymer polydispersion:
- -
- Average molecular weight by entanglement Me, which is the average molecular weight between the topological bonds of the biopolymer network, resulting from the physical (mechanical) entanglement of long biopolymer chains:
- -
- Average molecular weight at Mc cross-linking, which is the average molecular weight of biopolymer chains between successive nodes of the network, which can be chemical cross-links, crystalline regions, and even polymer complexes:
- -
- Mesh size of the resulting network ξ, which is one of the characteristic linear dimensions of the created network of viscoelastic material:
3.15. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, F.; Xie, Q. Structure and Physicochemical Properties of Starch. In Physical Modifications of Starch; Sui, Z., Kong, X., Eds.; Springer: Singapore, 2018; pp. 1–14. ISBN 978-981-13-0724-9. [Google Scholar]
- Zhu, F. Encapsulation and delivery of food ingredients using starch based systems. Food Chem. 2017, 229, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Guo, Y.; Cheng, Z.; Qiao, D. Physicochemical Properties of Starch. In Physical Modifications of Starch; Sui, Z., Kong, X., Eds.; Springer Nature: Singapore, 2023; pp. 27–47. ISBN 978-981-99-5389-9. [Google Scholar]
- Apriyanto, A.; Compart, J.; Fettke, J. A review of starch, a unique biopolymer—Structure, metabolism and in planta modifications. Plant Sci. 2022, 318, 111223. [Google Scholar] [CrossRef] [PubMed]
- Melani, L.; Kim, H.-J. Physicochemical Properties of Starches from Several Agricultural Sources: Application to a Starch-based Adhesive. J. Korea TAPPI 2019, 51, 100–107. [Google Scholar] [CrossRef]
- Sathaporn, S.; Jay-Lin, J. Physicochemical properties of starch affected by molecular composition and structure: A review. Food Sci. Biotechnol. 2007, 16, 663–674. [Google Scholar]
- Fan, Y.; Picchioni, F. Modification of starch: A review on the application of “green” solvents and controlled functionalization. Carbohydr. Polym. 2020, 241, 116350. [Google Scholar] [CrossRef] [PubMed]
- Kringel, D.H.; Dias, A.R.G.; Da Zavareze, E.R.; Gandra, E.A. Fruit wastes as promising sources of starch: Extraction, properties, and applications. Starch-Stärke 2020, 72, 1900200. [Google Scholar] [CrossRef]
- USDA. Fresh Apples, Grapes, and Pears: World Markets and Trade. Available online: https://downloads.usda.library.cornell.edu/usda-esmis/files/1z40ks800/zc77vb19h/sj13bn99q/fruit.pdf (accessed on 13 February 2024).
- Fresh Plaza. Asia Not Yet a Solution to Apple Overproduction in Europe. Available online: https://www.internationalproducegroup.com/asia-not-yet-a-solution-to-apple-overproduction-in-europe (accessed on 18 March 2024).
- Fresh Plaza. Kashmir Apple Farmers Panic Because of Overproduction, Lack of Cold Storage and Tanking Prices. Available online: https://www.freshplaza.com/asia/article/9480924/kashmir-apple-farmers-panic-because-of-overproduction-lack-of-cold-storage-and-tanking-prices (accessed on 18 March 2024).
- Sad24.pl. Poland’s Apple Production Should Be Reduced by 1 Million Tons? Available online: https://www.sad24.pl/sady/produkcja-jablek-w-polsce-powinna-zostac-zmniejszona-o-1-milion-ton/ (accessed on 18 March 2024).
- Rabetafika, H.N.; Bchir, B.; Blecker, C.; Richel, A. Fractionation of apple by-products as source of new ingredients: Current situation and perspectives. Trends Food Sci. Technol. 2014, 40, 99–114. [Google Scholar] [CrossRef]
- European Commission. The Apple Market in the EU: Vol. 1: Production, Areas and Yields. Available online: https://agriculture.ec.europa.eu/document/download/bd71aca7-dd45-4ab0-8dc1-f27321084916_en?filename=apples-production_en.pdf&prefLang=pl (accessed on 13 February 2024).
- Peirs, A.; Scheerlinck, N.; Perez, A.B.; Jancsók, P.; Nicolaï, B.M. Uncertainty analysis and modelling of the starch index during apple fruit maturation. Postharvest Biol. Technol. 2002, 26, 199–207. [Google Scholar] [CrossRef]
- Gambuś, H.; Gumul, D.; Juszczak, L. Rheological properties of pastes obtained from starches derived from immature cereal kernels. Starch-Stärke 2004, 56, 225–231. [Google Scholar] [CrossRef]
- Liu, Q.; Weber, E.; Currie, V.; Yada, R. Physicochemical properties of starches during potato growth. Carbohydr. Polym. 2003, 51, 213–221. [Google Scholar] [CrossRef]
- Stevenson, D.; Domoto, P.; Jane, J. Structures and functional properties of apple (Malus domestica Borkh) fruit starch. Carbohydr. Polym. 2006, 63, 432–441. [Google Scholar] [CrossRef]
- Kaur, A.; Singh, N.; Ezekiel, R.; Guraya, H.S. Physicochemical, thermal and pasting properties of starches separated from different potato cultivars grown at different locations. Food Chem. 2007, 101, 643–651. [Google Scholar] [CrossRef]
- Kaur, A.; Singh, N.; Ezekiel, R.; Sodhi, N.S. Properties of starches separated from potatoes stored under different conditions. Food Chem. 2009, 114, 1396–1404. [Google Scholar] [CrossRef]
- Tong, C.; Ahmed, S.; Pang, Y.; Zhou, X.; Bao, J. Fine structure and gelatinization and pasting properties relationships among starches from pigmented potatoes. Food Hydrocoll. 2018, 83, 45–52. [Google Scholar] [CrossRef]
- de Barros Mesquita, C.; Leonel, M.; Franco, C.M.L.; Leonel, S.; Garcia, E.L.; Dos Santos, T.P.R. Characterization of banana starches obtained from cultivars grown in Brazil. Int. J. Biol. Macromol. 2016, 89, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Bertoft, E. Understanding starch structure: Recent progress. Agronomy 2017, 7, 56. [Google Scholar] [CrossRef]
- Doerflinger, F.C.; Miller, W.B.; Nock, J.F.; Watkins, C.B. Relationships between starch pattern indices and starch concentrations in four apple cultivars. Postharvest Biol. Technol. 2015, 110, 86–95. [Google Scholar] [CrossRef]
- Manca, M.; Woortman, A.J.J.; Loos, K.; Loi, M.A. Imaging inclusion complex formation in starch granules using confocal laser scanning microscopy. Starch-Stärke 2015, 67, 132–138. [Google Scholar] [CrossRef]
- Dhital, S.; Shrestha, A.K.; Hasjim, J.; Gidley, M.J. Physicochemical and structural properties of maize and potato starches as a function of granule size. J. Agric. Food Chem. 2011, 59, 10151–10161. [Google Scholar] [CrossRef]
- Lizarazo, S.P.; Hurtado, G.G.; Rodríguez, L.F. Physicochemical and morphological characterization of potato starch (Solanum tuberosum L.) as raw material for the purpose of obtaining bioethanol. Agron. Colomb. 2015, 33, 244–252. [Google Scholar] [CrossRef]
- Gunaratne, A.; Hoover, R. Effect of heat–moisture treatment on the structure and physicochemical properties of tuber and root starches. Carbohydr. Polym. 2002, 49, 425–437. [Google Scholar] [CrossRef]
- Heo, H.; Lee, Y.-K.; Chang, Y.H. Effect of cross-linking on physicochemical and in vitro digestibility properties of potato starch. Emir. J. Food Agric. 2017, 29, 463–469. [Google Scholar] [CrossRef]
- Ashogbon, A.O.; Akintayo, E.T.; Oladebeye, A.O.; Oluwafemi, A.D.; Akinsola, A.F.; Imanah, O.E. Developments in the isolation, composition, and physicochemical properties of legume starches. Crit. Rev. Food Sci. Nutr. 2021, 61, 2938–2959. [Google Scholar] [CrossRef] [PubMed]
- Yousif, E.I.; Gadallah, M.G.; Sorour, A.M. Physico-chemical and rheological properties of modified corn starches and its effect on noodle quality. Ann. Agric. Sci. 2012, 57, 19–27. [Google Scholar] [CrossRef]
- Lorenz, K.; Collins, F.; Kulp, K. Steeping of barley starch. Effects on physicochemical properties and functional characteristics. Starch-Stärke 1984, 36, 122–126. [Google Scholar] [CrossRef]
- Park, H.-S.; Chung, H.-S. Evaluation of the physicochemical properties of starch isolated from thinned young ‘Fuji’ apples compared to corn and potato starches. Korean J. Food Preserv. 2021, 28, 501–509. [Google Scholar] [CrossRef]
- Alcázar-Alay, S.C.; Meireles, M.A.A. Physicochemical properties, modifications and applications of starches from different botanical sources. Food Sci. Technol. 2015, 35, 215–236. [Google Scholar] [CrossRef]
- Carrín, M. Characterization of starch in apple juice and its degradation by amylases. Food Chem. 2004, 87, 173–178. [Google Scholar] [CrossRef]
- Stevenson, D.; Yoo, S.; Hurst, P.; Jane, J. Structural and physicochemical characteristics of winter squash (Cucurbita maxima D.) fruit starches at harvest. Carbohydr. Polym. 2005, 59, 153–163. [Google Scholar] [CrossRef]
- Sugimoto, Y.; Yamamoto, M.; Abe, K.; Fuwa, H. Developmental changes in the properties of kiwi fruit starches (Actinidia chinensis Planch.). J. Japn. Soc. Starch Sci. 1988, 35, 1–10. [Google Scholar] [CrossRef]
- Singh, N.; Singh, J.; Kaur, L.; Singh Sodhi, N.; Singh Gill, B. Morphological, thermal and rheological properties of starches from different botanical sources. Food Chem. 2003, 81, 219–231. [Google Scholar] [CrossRef]
- Kaur, L.; Singh, J.; McCarthy, O.J.; Singh, H. Physico-chemical, rheological and structural properties of fractionated potato starches. J. Food Eng. 2007, 82, 383–394. [Google Scholar] [CrossRef]
- Swinkels, J.J.M. Composition and Properties of Commercial Native Starches. Starch-Stärke 1985, 37, 1–5. [Google Scholar] [CrossRef]
- Kaur, P.; Kaur, K.; Basha, S.J.; Kennedy, J.F. Current trends in the preparation, characterization and applications of oat starch—A review. Int. J. Biol. Macromol. 2022, 212, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Tester, R.F.; Karkalas, J.; Qi, X. Starch—Composition, fine structure and architecture. J. Cereal Sci. 2004, 39, 151–165. [Google Scholar] [CrossRef]
- Pietrzyk, S.; Fortuna, T.; Wojtanowska, O. Effect of oxidation level of potato starch on its acetylation and physicochemical properties. Food Sci. Technol. Qual. 2012, 19, 54–65. [Google Scholar] [CrossRef]
- Khan, H.K.; Ali, M.T.; Hasnain, A. Effect of chemical modifications on the functional and rheological properties of potato (Solanum tuberosum) starches. J. Anim. Plant Sci. 2014, 24, 550–555. [Google Scholar]
- Ratnayake, W.S.; Otani, C.; Jackson, D.S. DSC enthalpic transitions during starch gelatinisation in excess water, dilute sodium chloride and dilute sucrose solutions. J. Sci. Food Agric. 2009, 89, 2156–2164. [Google Scholar] [CrossRef]
- Liu, Q.; Lu, X.; Yada, R. The effect of various potato cultivars at different times during growth on starch content determined by DSC. J. Therm. Anal. Calorim. 2005, 79, 13–18. [Google Scholar] [CrossRef]
- Majzoobi, M.; Farahnaky, A.; Amiri, S. Physicochemical characteristics of starch component of wheat flours obtained from fourteen Iranian wheat cultivars. Int. J. Food Prop. 2011, 14, 685–696. [Google Scholar] [CrossRef]
- Inouchi, N.; Glover, D.V.; Sugimoto, Y.; Fuwa, H. DSC characteristics of gelatinization of starches of single-, double-, and triple-mutants and their normal counterpart in the inbred Oh43 maize (Zea mays L.) background. Starch-Stärke 1991, 43, 468–472. [Google Scholar] [CrossRef]
- Sikora, M.; Kowalski, S.; Tomasik, P. Binary hydrocolloids from starches and xanthan gum. Food Hydrocoll. 2008, 22, 943–952. [Google Scholar] [CrossRef]
- Chen, Y.Y.; McPherson, A.E.; Radoslavjevic, M.; Lee, V.; Wong, K.S.; Jane, L. Effects of starch chemical structures on gelatinization and pasting properties. Food Sci. Technol. Qual. 1998, 17, 63–71. [Google Scholar]
- Husemann, E. Methods in Carbohydrate Chemistry. Herausgeg. v. R. L.; Whistler, R.J.; Smith, J.N. BeMiller und M. L. Wolfrom. Vol. IV: Starch. Academic Press, New York-London 1964. 1. Aufl., XVI, 335 S., zahlr. Abb. u. Tab., geb. $ 13.50. Angew. Chem. 1965, 77, 226–227. [Google Scholar] [CrossRef]
- BeMiller, J.; Whistler, R. (Eds.) Starch: Chemistry and Technology, 3rd ed.; Academic Press: Amsterdam, The Netherlands, 2009; ISBN 9780127462752. [Google Scholar]
- BeMiller, J.N. Pasting, paste, and gel properties of starch–hydrocolloid combinations. Carbohydr. Polym. 2011, 86, 386–423. [Google Scholar] [CrossRef]
- Funami, T.; Kataoka, Y.; Omoto, T.; Goto, Y.; Asai, I.; Nishinari, K. Food hydrocolloids control the gelatinization and retrogradation behavior of starch. 2b. Functions of guar gums with different molecular weights on the retrogradation behavior of corn starch. Food Hydrocoll. 2005, 19, 25–36. [Google Scholar] [CrossRef]
- Funami, T.; Kataoka, Y.; Omoto, T.; Goto, Y.; Asai, I.; Nishinari, K. Effects of non-ionic polysaccharides on the gelatinization and retrogradation behavior of wheat starch☆. Food Hydrocoll. 2005, 19, 1–13. [Google Scholar] [CrossRef]
- Kim, C.; Lee, S.-P.; Yoo, B. Dynamic Rheology of Rice Starch-Galactomannan Mixtures in the Aging Process. Starch-Stärke 2006, 58, 35–43. [Google Scholar] [CrossRef]
- Kaur, L.; Singh, N.; Sodhi, N.S. Some properties of potatoes and their starches II. Morphological, thermal and rheological properties of starches. Food Chem. 2002, 79, 183–192. [Google Scholar] [CrossRef]
- Espinosa-Solis, V.; Jane, J.; Bello-Perez, L.A. Physicochemical Characteristics of Starches from Unripe Fruits of Mango and Banana. Starch-Stärke 2009, 61, 291–299. [Google Scholar] [CrossRef]
- Singh, J.; Dartois, A.; Kaur, L. Starch digestibility in food matrix: A review. Trends Food Sci. Technol. 2010, 21, 168–180. [Google Scholar] [CrossRef]
- Wei, M.; Wang, H.; Ma, T.; Ge, Q.; Fang, Y.; Sun, X. Comprehensive Utilization of Thinned Unripe Fruits from Horticultural Crops. Foods 2021, 10, 2043. [Google Scholar] [CrossRef] [PubMed]
- Rosicka-Kaczmarek, J.; Komisarczyk, A.; Nebesny, E. Heteropolysaccharide preparations from rye and wheat bran as sources of antioxidants. J. Cereal Sci. 2018, 81, 37–43. [Google Scholar] [CrossRef]
- Bai, Y.; Cai, L.; Doutch, J.; Gilbert, E.P.; Shi, Y.-C. Structural changes from native waxy maize starch granules to cold-water-soluble pyrodextrin during thermal treatment. J. Agric. Food Chem. 2014, 62, 4186–4194. [Google Scholar] [CrossRef] [PubMed]
- Latimer, G.W. (Ed.) Official Methods of Analysis of AOAC International, 21st ed.; AOAC International: Rockville, MD, USA, 2019; ISBN 978-0935584899. [Google Scholar]
- PN-EN ISO 3946:2000; Skrobia i Produkty Pochodne-Oznaczanie Całkowitej Zawartości Fosforu-Metoda Spektrofotometryczna. Polish Committee for Standardization: Warszawa, Poland, 2000.
- Ulfa, G.M.; Putri, W.D.R.; Fibrianto, K.; Prihatiningtyas, R.; Widjanarko, S.B. The influence of temperature in swelling power, solubility, and water binding capacity of pregelatinised sweet potato starch. IOP Conf. Ser. Earth Environ. Sci. 2020, 475, 12036. [Google Scholar] [CrossRef]
- Kapusniak, K.; Lubas, K.; Wojcik, M.; Rosicka-Kaczmarek, J.; Pavlyuk, V.; Kluziak, K.; Gonçalves, I.; Lopes, J.; Coimbra, M.A.; Kapusniak, J. Effect of Continuous and Discontinuous Microwave-Assisted Heating on Starch-Derived Dietary Fiber Production. Molecules 2021, 26, 5619. [Google Scholar] [CrossRef]
- Ferguson, J.; Kembłowski, Z. Applied Fluid Rheology; Elsevier Applied Science: London, UK, 1991; ISBN 978-1-85166-588-4. [Google Scholar]
- Ferry, J.D. Viscoelastic Properties of Polymers, 3rd ed.; Wiley: New York, NY, USA, 1980; ISBN 0-471-04894-1. [Google Scholar]


| Starch | Chemical Composition and the Weight-Average Molecular Weight (Mw) | |||||
| Amylose (%) | Protein (%) × 6.25 | Lipids (%) | Ash (%) | Phosphorus (%) | Mw (g/mol) | |
| SO | 15.5 a ± 0.5 | 0.567 b ± 0.06 | 1.20 a ± 0.02 | 0.76 b ± 0.01 | 0.108 b ± 0.001 | 1.68 × 108 |
| SP | 16.3 a ± 0.1 | 0.514 a ± 0.04 | 1.21 a ± 0.01 | 0.51 a ± 0.01 | 0.092 a ± 0.001 | 1.64 × 108 |
| Granularity | ||||||
| Average diameter (µm) | Min (1%) (µm) | Max (1%) (µm) | <30 µm (%) | 30–70 µm (%) | >70 µm (%) | |
| SO | 6.4 | 0.4 | 132.7 | 67.3 | 20.4 | 12.3 |
| SP | 5.2 | 0.4 | 100.3 | 75.2 | 16.2 | 8.6 |
| Starch | Thermal Characteristics | ||||
| ΔH (J/g d.m.) | To (°C) | Tp (°C) | Tc (°C) | Tc − To (°C) | |
| SO | 7.59 b ± 0.12 | 60.50 a ± 0.01 | 64.19 a ± 0.02 | 71.31 b ± 0.01 | 10.81 b ± 0.01 |
| SP | 5.44 a ± 0.19 | 60.88 b ± 0.01 | 64.94 b ± 0.00 | 71.13 a ± 0.03 | 10.25 a ± 0.04 |
| Temperature | |||||
| 25 °C | 40 °C | 60 °C | 75 °C | 95 °C | |
| Solubility S (% d.m.) | |||||
| SO | 1.65 b ± 0.04 | 2.03 b ± 0.11 | 2.33 b ± 0.10 b | 3.94 b ± 0.46 | 6.98 a ± 0.85 |
| SP | 0.68 a ± 0.01 | 0.94 a ± 0.04 | 0.95 a ± 0.06 a | 2.20 a ± 0.08 | 6.86 a ± 0.04 |
| Water-binding capacity WBC (g water/g starch d.m.) | |||||
| SO | 1.22 a ± 0.01 | 1.39 a ± 0.03 | 1.49 a ± 0.00 | 5.48 b ± 0.00 | 8.00 b ± 0.69 |
| SP | 1.25 a ± 0.04 | 1.33 a ± 0.05 | 1.56 a ± 0.02 | 4.51 a ± 0.26 | 6.64 a ± 0.18 |
| Retrogradation by DSC | |||||
| Starch | Time of Storage (h) | To (°C) | Tp (°C) | Tc (°C) | ΔHr (J/g d.m.) |
| SO | 24 | 43.25 a ± 0.01 | 49.00 a ± 0.02 | 52.18 a ± 0.03 | 0.71 b ± 0.03 |
| 48 | 51.00 b ± 0.03 | 47.36 a ± 0.01 | 57.30 a ± 0.00 | 0.83 b ± 0.01 | |
| 72 | 45.03 a ± 0.02 | 51.73 b ± 0.03 | 59.61 a ± 0.01 | 0.91 b ± 0.02 | |
| SP | 24 | 46.28 b ± 0.01 | 52.10 b ± 0.00 | 61.09 b ± 0.02 | 0.59 a ± 0.05 |
| 48 | 41.90 a ± 0.00 | 50.04 b ± 0.02 | 58.70 b ± 0.01 | 0.68 a ± 0.04 | |
| 72 | 49.11 b ± 0.01 | 51.67 a ± 0.00 | 63.43 b ± 0.01 | 0.79 a ± 0.02 | |
| Degree of retrogradation (%) | |||||
| Temperature of storage | |||||
| 20 °C | 8 °C | −20 °C | |||
| SO | 24 | 3.07 b ± 0.07 | 4.44 b ± 0.08 | 49.51 b ± 0.54 | |
| 48 | 4.94 b ± 0.00 | 6.82 b ± 0.06 | - | ||
| 72 | 5.83 b ± 0.07 | 8.63 b ± 0.06 | - | ||
| SP | 24 | 2.59 a ± 0.05 | 3.91 a ± 0.02 | 42.58 a ± 0.27 | |
| 48 | 3.81 a ± 0.10 | 4.53 a ± 0.05 | - | ||
| 72 | 4.59 a ± 0.03 | 7.59 a ± 0.00 | - | ||
| Ostwald de Waele Model | ||||||||||
| Starch | Upper Curve | Lower Curve | ||||||||
| τy | n | k | R2 | RMSE | τy | n | k | R2 | RMSE | |
| SO | - | 0.176 ± 0.001 | 11.177 ± 0.160 | 0.998 | 0.912 | - | 0.361 ± 0.002 | 4.155 ± 0.106 | 0.991 | 1.071 |
| SP | - | 0.308 ± 0.003 | 4.269 ± 0.081 | 0.993 | 1.060 | - | 0.303 ± 0.001 | 3.915 ± 0.074 | 0.983 | 1.282 |
| Herschel–Bulkley Model | ||||||||||
| Upper Curve | Lower Curve | |||||||||
| τy | n | k | R2 | RMSE | τy | n | k | R2 | RMSE | |
| SO | 1.947 ± 0.016 | 0.499 ± 0.004 | 2.046 ± 0.007 | 0.998 | 0.470 | 1.832 ± 0.011 | 0.497 ± 0.002 | 1.731 ± 0.018 | 0.998 | 0.432 |
| SP | 5.230 ± 0.033 | 0.292 ± 0.001 | 5.622 ± 0.026 | 0.998 | 0.600 | 1.240 ± 0.008 | 0.463 ± 0.002 | 2.689 ± 0.021 | 0.998 | 0.564 |
| Casson Model | ||||||||||
| Upper Curve | Lower Curve | |||||||||
| τy | n | k | R2 | RMSE | τy | n | k | R2 | RMSE | |
| SO | 8.748 ± 0.114 | - | 0.271 ± 0.002 | 0.990 | 1.432 | 2.251 ± 0.010 | - | 0.416 ± 0.005 | 0.988 | 1.557 |
| SP | 2.575 ± 0.025 | - | 0.346 ± 0.003 | 0.989 | 1.056 | 2.373 ± 0.013 | - | 0.327 ± 0.002 | 0.997 | 0.679 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gumul, D.; Korus, J.; Orczykowska, M.; Rosicka-Kaczmarek, J.; Oracz, J.; Areczuk, A. Starch from Unripe Apples (Malus domestica Borkh) as an Alternative for Application in the Food Industry. Molecules 2024, 29, 1707. https://doi.org/10.3390/molecules29081707
Gumul D, Korus J, Orczykowska M, Rosicka-Kaczmarek J, Oracz J, Areczuk A. Starch from Unripe Apples (Malus domestica Borkh) as an Alternative for Application in the Food Industry. Molecules. 2024; 29(8):1707. https://doi.org/10.3390/molecules29081707
Chicago/Turabian StyleGumul, Dorota, Jarosław Korus, Magdalena Orczykowska, Justyna Rosicka-Kaczmarek, Joanna Oracz, and Anna Areczuk. 2024. "Starch from Unripe Apples (Malus domestica Borkh) as an Alternative for Application in the Food Industry" Molecules 29, no. 8: 1707. https://doi.org/10.3390/molecules29081707
APA StyleGumul, D., Korus, J., Orczykowska, M., Rosicka-Kaczmarek, J., Oracz, J., & Areczuk, A. (2024). Starch from Unripe Apples (Malus domestica Borkh) as an Alternative for Application in the Food Industry. Molecules, 29(8), 1707. https://doi.org/10.3390/molecules29081707