The Effect of Temozolomide/Poly(lactide-co-glycolide) (PLGA)/Nano-Hydroxyapatite Microspheres on Glioma U87 Cells Behavior
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of TMZ/nHA Synthesis
2.2. The Characteristics of TMZ/PLGA/nHA Microspheres
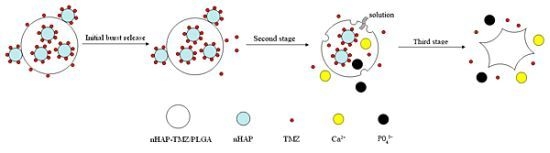
2.3. In Vitro Drug Release
2.4. Effect of TMZ/PLGA/nHA on Proliferation of Glioma Cells
2.5. Effect of TMZ/PLGA/nHA on Apoptosis
2.6. Invasion of U87 Glioma Cells Treated with TMZ/PLGA/nHA
2.7. The Expression of αVβ3 Integrin Was Reduced by TMZ/PLGA/nHA
3. Experimental Section
3.1. Materials
3.2. Preparation of nHA Powder and Absorption Experiment of the TMZ/nHA
3.3. Fabrication of TMZ/PLGA/nHA Microspheres by S/O/W Method
3.4. Characterization of Microspheres
3.5. Determination of TMZ Encapsulation Efficiency in Microspheres
3.6. In Vitro Release of TMZ from Microspheres
3.7. Cell Culture
3.8. Cell Viability
3.9. Flow Cytometry (FCM) Analysis
3.10. Invasion Assay
3.11. Semiquantitative Detection of αVβ3 Integrin mRNA with Reverse Transcription Polymerase Chain Reaction (RT-PCR)
3.12. Western Blot Analysis
3.13. Statistical Analysis
4. Conclusions
Acknowledgments
References
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med 2005, 352, 987–996. [Google Scholar]
- Mason, W.P.; Cairncross, J.G. Drug insight: Temozolomide as a treatment for malignant glioma—Impact of a recent trial. Nat. Clin. Pract. Neurol 2005, 1, 88–95. [Google Scholar]
- Baker, S.D.; Wirth, M.; Statkevich, P.; Reidenberg, P.; Alton, K.; Sartorius, S.E.; Dugan, M.; Cutler, D.; Batra, V.; Grochow, L.B.; et al. Absorption, metabolism, and excretion of 14c-temozolomide following oral administration to patients with advanced cancer. Clin. Cancer Res 1999, 5, 309–317. [Google Scholar]
- Chakravarti, A.; Erkkinen, M.G.; Nestler, U.; Stupp, R.; Mehta, M.; Aldape, K.; Gilbert, M.R.; Black, P.M.; Loeffler, J.S. Temozolomide-mediated radiation enhancement in glioblastoma: A report on underlying mechanisms. Clin. Cancer Res 2006, 12, 4738–4746. [Google Scholar]
- Zhang, H.; Gao, S. Temozolomide/plga microparticles and antitumor activity against glioma c6 cancer cells in vitro. Int. J. Pharm 2007, 329, 122–128. [Google Scholar]
- Zhang, Y.H.; Yue, Z.J.; Zhang, H.; Tang, G.S.; Wang, Y.; Liu, J.M. Temozolomide/plga microparticles plus vatalanib inhibits tumor growth and angiogenesis in an orthotopic glioma model. Eur. J. Pharm. Biopharm 2010, 76, 371–375. [Google Scholar]
- Yang, Z.J.; Best, S.M.; Cameron, R.E. The influence of α-tricalcium phosphate nanoparticles and microparticles on the degradation of poly(d,l-lactide-co-glycolide). Adv. Mater 2009, 21, 3900–3904. [Google Scholar]
- Wei, G.; Ma, P.X. Partially nanofibrous architecture of 3d tissue engineering scaffolds. Biomaterials 2009, 30, 6426–6434. [Google Scholar]
- Xue, J.M.; Shi, M. Plga/mesoporous silica hybrid structure for controlled drug release. J. Control. Release 2004, 98, 209–217. [Google Scholar]
- Ara, M.; Watanabe, M.; Imai, Y. Effect of blending calcium compounds on hydrolytic degradation of poly(dl-lactic acid-co-glycolic acid). Biomaterials 2002, 23, 2479–2483. [Google Scholar]
- Ho, M.L.; Fu, Y.C.; Wang, G.J.; Chen, H.T.; Chang, J.K.; Tsai, T.H.; Wang, C.K. Controlled release carrier of bsa made by w/o/w emulsion method containing plga and hydroxyapatite. J. Control. Release 2008, 128, 142–148. [Google Scholar]
- Xu, Q.; Tanaka, Y.; Czernuszka, J.T. Encapsulation and release of a hydrophobic drug from hydroxyapatite coated liposomes. Biomaterials 2007, 28, 2687–2694. [Google Scholar]
- Yang, P.; Quan, Z.; Li, C.; Kang, X.; Lian, H.; Lin, J. Bioactive, luminescent and mesoporous europium-doped hydroxyapatite as a drug carrier. Biomaterials 2008, 29, 4341–4347. [Google Scholar]
- Liu, T.Y.; Chen, S.Y.; Liu, D.M.; Liou, S.C. On the study of bsa-loaded calcium-deficient hydroxyapatite nano-carriers for controlled drug delivery. J. Control. Release 2005, 107, 112–121. [Google Scholar]
- Nie, H.; Wang, C.H. Fabrication and characterization of plga/hap composite scaffolds for delivery of bmp-2 plasmid DNA. J. Control. Release 2007, 120, 111–121. [Google Scholar]
- Shi, X.; Wang, Y.; Ren, L.; Gong, Y.; Wang, D.A. Enhancing alendronate release from a novel plga/hydroxyapatite microspheric system for bone repairing applications. Pharm. Res 2009, 26, 422–430. [Google Scholar]
- Shen, H.; Hu, X.; Yang, F.; Bei, J.; Wang, S. An injectable scaffold: Rhbmp-2-loaded poly (lactide-co-glycolide)/hydroxyapatite composite microspheres. Acta Biomater 2010, 6, 455–465. [Google Scholar]
- Okada, M.; Furukawa, K.; Serizawa, T.; Yanagisawa, Y.; Tanaka, H.; Kawai, T.; Furuzono, T. Interfacial interactions between calcined hydroxyapatite nanocrystals and substrates. Langmuir 2009, 25, 6300–6306. [Google Scholar]
- Tian, A.; Wang, C.; Xue, X.; Wu, A.; Guan, G.; Wang, L.; Qiu, B. Inhibitory effect of nano-ha particles on human u87 glioblastoma cells viability. J. Inorg. Mater 2010, 25, 101–106. [Google Scholar]
- Bodmeier, R.; Chen, H. Preparation and characterization of microspheres containing the anti-inflammatory agents, indomethacin, ibuprofen, and ketoprofen. J. Control. Release 1989, 10, 167–175. [Google Scholar]
- Liu, J.J.; Lin, M.; Yu, J.Y.; Liu, B.; Bao, J.K. Targeting apoptotic and autophagic pathways for cancer therapeutics. Cancer Lett 2011, 300, 105–114. [Google Scholar]
- Bello, L.; Lucini, V.; Giussani, C.; Carrabba, G.; Pluderi, M.; Scaglione, F.; Tomei, G.; Villani, R.; Black, P.M.; Bikfalvi, A.; et al. Is20i, a specific alphavbeta3 integrin inhibitor, reduces glioma growth in vivo. Neurosurgery 2003, 52, 177–186. [Google Scholar]
- Hood, J.D.; Cheresh, D.A. Role of integrins in cell invasion and migration. Nat. Rev. Cancer 2002, 2, 91–100. [Google Scholar]
- Bello, L.; Francolini, M.; Marthyn, P.; Zhang, J.; Carroll, R.S.; Nikas, D.C.; Strasser, J.F.; Villani, R.; Cheresh, D.A.; Black, P.M. Alpha(v)beta3 and alpha(v)beta5 integrin expression in glioma periphery. Neurosurgery 2001, 49, 380–390. [Google Scholar]
- D’Abaco, G.M.; Kaye, A.H. Integrins: Molecular determinants of glioma invasion. J. Clin. Neurosci 2007, 14, 1041–1048. [Google Scholar]
- Deryugina, E.I.; Bourdon, M.A.; Luo, G.X.; Reisfeld, R.A.; Strongin, A. Matrix metalloproteinase-2 activation modulates glioma cell migration. J. Cell Sci 1997, 110, 2473–2482. [Google Scholar]
- Cai, Y.; Liu, Y.; Yan, W.; Hu, Q.; Tao, J.; Zhang, M.; Shi, Z.; Tang, R. Role of hydroxyapatite nanoparticle size in bone cell proliferation. J. Mater. Chem 2007, 17, 3780–3787. [Google Scholar]
- Guo, X.; Gough, J.E.; Xiao, P.; Liu, J.; Shen, Z. Fabrication of nanostructured hydroxyapatite and analysis of human osteoblastic cellular response. J. Biomed. Mater. Res. A 2007, 82, 1022–1032. [Google Scholar]









| Samples | TMZ content (wt %) | Amount of TMZ/nHA used in fabricating (mg) |
|---|---|---|
| TMZ/R-nHA | 46.4 ± 2.03 | 21.6 |
| TMZ/S-nHA | 54.5 ± 3.28 | 18.3 |
| Samples | EE (%) | Microspheres Size (μm) | Concentration of the nHA (wt %) |
|---|---|---|---|
| TMZ/PLGA/R-nHA | 84.19 ± 1.48 | 62.7 | 4.49 |
| TMZ/PLGA/S-nHA | 82.47 ± 1.9 | 63.9 | 4.39 |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhang, D.; Tian, A.; Xue, X.; Wang, M.; Qiu, B.; Wu, A. The Effect of Temozolomide/Poly(lactide-co-glycolide) (PLGA)/Nano-Hydroxyapatite Microspheres on Glioma U87 Cells Behavior. Int. J. Mol. Sci. 2012, 13, 1109-1125. https://doi.org/10.3390/ijms13011109
Zhang D, Tian A, Xue X, Wang M, Qiu B, Wu A. The Effect of Temozolomide/Poly(lactide-co-glycolide) (PLGA)/Nano-Hydroxyapatite Microspheres on Glioma U87 Cells Behavior. International Journal of Molecular Sciences. 2012; 13(1):1109-1125. https://doi.org/10.3390/ijms13011109
Chicago/Turabian StyleZhang, Dongyong, Ang Tian, Xiangxin Xue, Mei Wang, Bo Qiu, and Anhua Wu. 2012. "The Effect of Temozolomide/Poly(lactide-co-glycolide) (PLGA)/Nano-Hydroxyapatite Microspheres on Glioma U87 Cells Behavior" International Journal of Molecular Sciences 13, no. 1: 1109-1125. https://doi.org/10.3390/ijms13011109
APA StyleZhang, D., Tian, A., Xue, X., Wang, M., Qiu, B., & Wu, A. (2012). The Effect of Temozolomide/Poly(lactide-co-glycolide) (PLGA)/Nano-Hydroxyapatite Microspheres on Glioma U87 Cells Behavior. International Journal of Molecular Sciences, 13(1), 1109-1125. https://doi.org/10.3390/ijms13011109