Dermal Contributions to Human Interfollicular Epidermal Architecture and Self-Renewal
Abstract
:1. Introduction

2. Human Interfollicular Epidermal Architecture and Stem Cells
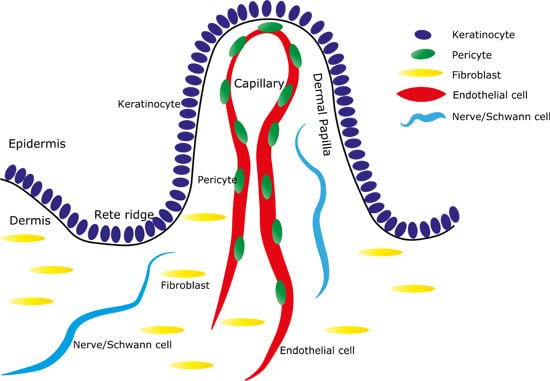
3. Rete Ridges and Capillaries
4. Endothelial Cells and Pericytes
5. Nerves
6. Fibroblasts
7. Adipocytes
8. Dermal Heterogeneity and the Basement Membrane
9. Dermal Heterogeneity for Regenerative Medicine
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kaur, P. Interfollicular epidermal stem cells: Identification, challenges, potential. J. Investig. Dermatol. 2006, 126, 1450–1458. [Google Scholar] [CrossRef] [PubMed]
- Mascré, G.; Dekoninck, S.; Drogat, B.; Youssef, K.K.; Broheé, S.; Sotiropoulou, P.A.; Simons, B.D.; Blanpain, C. Distinct contribution of stem and progenitor cells to epidermal maintenance. Nature 2012, 489, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Clayton, E.; Doupé, D.P.; Klein, A.M.; Winton, D.J.; Simons, B.D.; Jones, P.H. A single type of progenitor cell maintains normal epidermis. Nature 2007, 446, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Doupé, D.P.; Klein, A.M.; Simons, B.D.; Jones, P.H. The ordered architecture of murine ear epidermis is maintained by progenitor cells with random fate. Dev. Cell 2010, 18, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Lim, X.; Tan, S.H.; Koh, W.L.C.; Chau, R.M.W.; Yan, K.S.; Kuo, C.J.; van Amerongen, R.; Klein, A.M.; Nusse, R. interfollicular epidermal stem cells self-renew via autocrine wnt signaling. Science 2013, 342, 1226–1230. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.W.; Sorkin, M.; Glotzbach, J.P.; Longaker, M.T.; Gurtner, G.C. Surgical approaches to create murine models of human wound healing. J. Biomed. Biotechnol. 2011, 969618, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.H.; Limthongkul, B.; Humphreys, T.R. The biomechanical properties of the skin. Dermatol. Surg. 2013, 39, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Kippenberger, S.; Bernd, A.; Loitsch, S.; Guschel, M.; Müller, J.; Bereiter-Hahn, J.; Kaufmann, R. Signaling of mechanical stretch in human keratinocytes via MAP kinases. J. Investig. Dermatol. 2000, 114, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Discher, D.E.; Janmey, P.; Wang, Y.-L. Tissue cells feel and respond to the stiffness of their substrate. Science 2005, 310, 1139–1143. [Google Scholar] [CrossRef] [PubMed]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Orr, A.W.; Helmke, B.P.; Blackman, B.R.; Schwartz, M.A. Mechanisms of mechanotransduction. Dev. Cell 2006, 10, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Reichelt, J. Mechanotransduction of keratinocytes in culture and in the epidermis. Eur. J. Cell Biol. 2007, 86, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Trappmann, B.; Gautrot, J.E.; Connelly, J.T.; Strange, D.G.T.; Li, Y.; Oyen, M.L.; Cohen Stuart, M.A.; Boehm, H.; Li, B.; Vogel, V.; et al. Extracellular-matrix tethering regulates stem-cell fate. Nat. Mater. 2012, 11, 742–742. [Google Scholar] [CrossRef]
- Evans, N.D.; Oreffo, R.O.C.; Healy, E.; Thurner, P.J.; Man, Y.H. Epithelial mechanobiology, skin wound healing, and the stem cell niche. J. Mech. Behav. Biomed. Mater. 2013, 28, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Kupper, T.S.; Fuhlbrigge, R.C. Immune surveillance in the skin: Mechanisms and clinical consequences. Nat. Rev. Immunol. 2004, 4, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Merad, M.; Ginhoux, F.; Collin, M. Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. Nat. Rev. Immunol. 2008, 8, 935–947. [Google Scholar] [CrossRef] [PubMed]
- Nestle, F.O.; di Meglio, P.; Qin, J.-Z.; Nickoloff, B.J. Skin immune sentinels in health and disease. Nat. Rev. Immunol. 2009, 9, 679–691. [Google Scholar] [CrossRef] [PubMed]
- Pasparakis, M.; Haase, I.; Nestle, F.O. Mechanisms regulating skin immunity and inflammation. Nat. Rev. Immunol. 2014, 14, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-N.; McGovern, N.; Gunawan, M.; Richardson, C.; Windebank, M.; Siah, T.-W.; Lim, H.-Y.; Fink, K.; Li, J.L.Y.; Ng, L.G.; et al. A three-dimensional atlas of human dermal leukocytes, lymphatics, and blood vessels. J. Investig. Dermatol. 2014, 134, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Lavker, R.M.; Sun, T.T. Heterogeneity in epidermal basal keratinocytes: Morphological and functional correlations. Science 1982, 215, 1239–1241. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.H.; Harper, S.; Watt, F.M. Stem cell patterning and fate in human epidermis. Cell 1995, 80, 83–93. [Google Scholar] [CrossRef]
- Jensen, U.B.; Lowell, S.; Watt, F.M. The spatial relationship between stem cells and their progeny in the basal layer of human epidermis: A new view based on whole-mount labelling and lineage analysis. Development 1999, 126, 2409–2418. [Google Scholar] [PubMed]
- Jones, P.H.; Watt, F.M. Separation of human epidermal stem cells from transit amplifying cells on the basis of differences in integrin function and expression. Cell 1993, 73, 713–724. [Google Scholar] [CrossRef]
- Li, A.; Simmons, P.J.; Kaur, P. Identification and isolation of candidate human keratinocyte stem cells based on cell surface phenotype. Proc. Natl. Acad. Sci. USA 1998, 95, 3902–3907. [Google Scholar]
- Li, A.; Pouliot, N.; Redvers, R.; Kaur, P. Extensive tissue-regenerative capacity of neonatal human keratinocyte stem cells and their progeny. J. Clin. Investig. 2004, 113, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Schlüter, H.; Paquet-Fifield, S.; Gangatirkar, P.; Li, J.; Kaur, P. Functional characterization of quiescent keratinocyte stem cells and their progeny reveals a hierarchical organization in human skin epidermis. Stem Cells 2011, 29, 1256–1268. [Google Scholar] [CrossRef] [PubMed]
- Webb, A.; Li, A.; Kaur, P. Location and phenotype of human adult keratinocyte stem cells of the skin. Differentiation 2004, 72, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Briggaman, R.A.; Wheeler, C.E. The epidermal-dermal junction. J. Investig. Dermatol. 1975, 65, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Lavker, R.M. Structural Alterations in Exposed and Unexposed Aged Skin. J. Investig. Dermatol. 1979, 73, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Braverman, I.M. The cutaneous microcirculation. J. Investig. Dermatol. Symp. Proc. 2000, 5, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Stücker, M.; Struk, A.; Altmeyer, P.; Herde, M.; Baumgärtl, H.; Lübbers, D.W. The cutaneous uptake of atmospheric oxygen contributes significantly to the oxygen supply of human dermis and epidermis. J. Physiol. 2002, 538, 985–994. [Google Scholar] [CrossRef] [PubMed]
- Boutin, A.T.; Weidemann, A.; Fu, Z.; Mesropian, L.; Gradin, K.; Jamora, C.; Wiesener, M.; Eckardt, K.-U.; Koch, C.J.; Ellies, L.G.; et al. Epidermal Sensing of Oxygen Is Essential for Systemic Hypoxic Response. Cell 2008, 133, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Romanovsky, A.A. Skin temperature: Its role in thermoregulation. Acta Physiol. 2014, 210, 498–507. [Google Scholar] [CrossRef]
- Flavahan, N.A. A vascular mechanistic approach to understanding Raynaud phenomenon. Nat. Rev. Rheumatol. 2014, 11, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Breathnach, A.S. Development and differentiation of dermal cells in man. J. Investig. Dermatol. 1978, 71, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.T.; Holbrook, K.A. Embryogenesis of the dermis in human skin. Pediatr. Dermatol. 1986, 3, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.L.; Holbrook, K.A. Development of human embryonic and fetal dermal vasculature. J. Investig. Dermatol. 1989, 93, 10S–17S. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, S.K.; Kusumbe, A.P.; Adams, R.H. Regulation of tissue morphogenesis by endothelial cell-derived signals. Trends Cell Biol. 2015, 25, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Smola, H.; Thiekötter, G.; Fusenig, N.E. Mutual induction of growth factor gene expression by epidermal-dermal cell interaction. J. Cell Biol. 1993, 122, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Supp, D.M.; Wilson-Landy, K.; Boyce, S.T. Human dermal microvascular endothelial cells form vascular analogs in cultured skin substitutes after grafting to athymic mice. FASEB J. 2002, 16, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Braverman, I.M.; Sibley, J. Ultrastructural and three-dimensional analysis of the contractile cells of the cutaneous microvasculature. J. Investig. Dermatol. 1990, 95, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Armulik, A.; Genové, G.; Betsholtz, C. Pericytes: Developmental, physiological, and pathological perspectives, problems, and promises. Dev. Cell 2011, 21, 193–215. [Google Scholar] [CrossRef] [PubMed]
- Dulauroy, S.; di Carlo, S.E.; Langa, F.; Eberl, G.; Peduto, L. Lineage tracing and genetic ablation of ADAM12+ perivascular cells identify a major source of profibrotic cells during acute tissue injury. Nat. Med. 2012, 18, 1262–1270. [Google Scholar] [CrossRef] [PubMed]
- Helmbold, P.; Lautenschläger, C.; Marsch, W.C.; Nayak, R.C. Detection of a physiological juvenile phase and the central role of pericytes in human dermal microvascular aging. J. Investig. Dermatol. 2006, 126, 1419–1421. [Google Scholar] [CrossRef] [PubMed]
- Paquet-Fifield, S.; Schlüter, H.; Li, A.; Aitken, T.; Gangatirkar, P.; Blashki, D.; Koelmeyer, R.; Pouliot, N.; Palatsides, M.; Ellis, S.; et al. A role for pericytes as microenvironmental regulators of human skin tissue regeneration. J. Clin. Investig. 2009, 119, 2795–2806. [Google Scholar] [CrossRef] [PubMed]
- Crisan, M.; Yap, S.; Casteilla, L.; Chen, C.-W.; Corselli, M.; Park, T.S.; Andriolo, G.; Sun, B.; Zheng, B.; Zhang, L.; et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 2008, 3, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Woo, W.-M.; Nagao, K.; Li, W.; Terunuma, A.; Mukouyama, Y.; Oro, A.E.; Vogel, J.C.; Brownell, I. Perivascular hair follicle stem cells associate with a venule annulus. J. Investig. Dermatol. 2013, 133, 2324–2331. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Saunders, T.L.; Enikolopov, G.; Morrison, S.J. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 2012, 481, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Kunisaki, Y.; Bruns, I.; Scheiermann, C.; Ahmed, J.; Pinho, S.; Zhang, D.; Mizoguchi, T.; Wei, Q.; Lucas, D.; Ito, K.; et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature 2013, 502, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Ottone, C.; Krusche, B.; Whitby, A.; Clements, M.; Quadrato, G.; Pitulescu, M.E.; Adams, R.H.; Parrinello, S. Direct cell-cell contact with the vascular niche maintains quiescent neural stem cells. Nat. Cell Biol. 2014, 16, 1045–1056. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Zeve, D.; Suh, J.M.; Bosnakovski, D.; Kyba, M.; Hammer, R.E.; Tallquist, M.D.; Graff, J.M. White fat progenitor cells reside in the adipose vasculature. Science 2008, 322, 583–586. [Google Scholar] [CrossRef] [PubMed]
- Kostallari, E.; Baba-Amer, Y.; Alonso-Martin, S.; Ngoh, P.; Relaix, F.; Lafuste, P.; Gherardi, R.K. Pericytes in the myovascular niche promote post-natal myofiber growth and satellite cell quiescence. Development 2015, 142, 1242–1253. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C.M.; Bautista, D.M.; Lumpkin, E.A. Mammalian touch catches up. Curr. Opin. Neurobiol. 2015, 34, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Tschachler, E.; Reinisch, C.M.; Mayer, C.; Paiha, K.; Lassmann, H.; Weninger, W. Sheet preparations expose the dermal nerve plexus of human skin and render the dermal nerve end organ accessible to extensive analysis. J. Investig. Dermatol. 2004, 122, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Reinisch, C.M.; Tschachler, E. The dimensions and characteristics of the subepidermal nerve plexus in human skin—Terminal Schwann cells constitute a substantial cell population within the superficial dermis. J. Dermatol. Sci. 2012, 65, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, S.T.; Lin, W.M. Modulation of keratinocyte proliferation by skin innervation. J. Investig. Dermatol. 1999, 113, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Huang, I.T.; Lin, W.M.; Shun, C.T.; Hsieh, S.T. Influence of cutaneous nerves on keratinocyte proliferation and epidermal thickness in mice. Neuroscience 1999, 94, 965–973. [Google Scholar] [CrossRef]
- Roggenkamp, D.; Köpnick, S.; Stäb, F.; Wenck, H.; Schmelz, M.; Neufang, G. Epidermal nerve fibers modulate keratinocyte growth via neuropeptide signaling in an innervated skin model. J. Investig. Dermatol. 2013, 133, 1620–1628. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.-H.; Nguyen, H. Epidermal expression of Lgr6 is dependent on nerve endings and Schwann cells. Exp. Dermatol. 2014, 23, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Scheibert, J.; Leurent, S.; Prevost, A.; Debrégeas, G. The role of fingerprints in the coding of tactile information probed with a biomimetic sensor. Science 2009, 323, 1503–1506. [Google Scholar] [CrossRef] [PubMed]
- Doucet, Y.S.; Woo, S.-H.; Ruiz, M.E.; Owens, D.M. The touch dome defines an epidermal niche specialized for mechanosensory signaling. Cell Rep. 2013, 3, 1759–1765. [Google Scholar] [CrossRef] [PubMed]
- Leary, T.; Jones, P.L.; Appleby, M.; Blight, A.; Parkinson, K.; Stanley, M. Epidermal keratinocyte self-renewal is dependent upon dermal integrity. J. Investig. Dermatol. 1992, 99, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Tuan, T.L.; Keller, L.C.; Sun, D.; Nimni, M.E.; Cheung, D. Dermal fibroblasts activate keratinocyte outgrowth on collagen gels. J. Cell Sci. 1994, 107, 2285–2289. [Google Scholar] [PubMed]
- Sorrell, J.M. Fibroblast heterogeneity: More than skin deep. J. Cell Sci. 2004, 117, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Driskell, R.R.; Watt, F.M. Understanding fibroblast heterogeneity in the skin. Trends Cell Biol. 2015, 25, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Harper, R.; Grove, G. Human skin fibroblasts derived from papillary and reticular dermis: Differences in growth potential in vitro. Science 1979, 204, 526–527. [Google Scholar] [CrossRef] [PubMed]
- Sorrell, J.M.; Baber, M.A.; Caplan, A.I. Site-matched papillary and reticular human dermal fibroblasts differ in their release of specific growth factors/cytokines and in their interaction with keratinocytes. J. Cell. Physiol. 2004, 200, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Sorrell, J.M.; Baber, M.A.; Caplan, A.I. Clonal characterization of fibroblasts in the superficial layer of the adult human dermis. Cell Tissue Res. 2007, 327, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Mine, S.; Fortunel, N.O.; Pageon, H.; Asselineau, D. Aging alters functionally human dermal papillary fibroblasts but not reticular fibroblasts: a new view of skin morphogenesis and aging. PLoS ONE 2008, 3, e4066. [Google Scholar] [CrossRef] [PubMed]
- Janson, D.; Saintigny, G.; Mahé, C.; Ghalbzouri, A. El Papillary fibroblasts differentiate into reticular fibroblasts after prolonged in vitro culture. Exp. Dermatol. 2013, 22, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.Y.; Chi, J.-T.; Dudoit, S.; Bondre, C.; van de Rijn, M.; Botstein, D.; Brown, P.O. Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc. Natl. Acad. Sci. USA 2002, 99, 12877–12882. [Google Scholar] [CrossRef] [PubMed]
- Rinn, J.L.; Bondre, C.; Gladstone, H.B.; Brown, P.O.; Chang, H.Y. Anatomic demarcation by positional variation in fibroblast gene expression programs. PLoS Genet. 2006, 2, e119. [Google Scholar] [CrossRef] [PubMed]
- Driskell, R.R.; Lichtenberger, B.M.; Hoste, E.; Kretzschmar, K.; Simons, B.D.; Charalambous, M.; Ferron, S.R.; Herault, Y.; Pavlovic, G.; Ferguson-Smith, A.C.; et al. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature 2013, 504, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Rinkevich, Y.; Walmsley, G.G.; Hu, M.S.; Maan, Z.N.; Newman, A.M.; Drukker, M.; Januszyk, M.; Krampitz, G.W.; Gurtner, G.C.; Lorenz, H.P.; et al. Identification and isolation of a dermal lineage with intrinsic fibrogenic potential. Science 2015, 348. [Google Scholar] [CrossRef] [PubMed]
- Sorrell, J.M.; Baber, M.A.; Caplan, A.I. Human dermal fibroblast subpopulations; differential interactions with vascular endothelial cells in coculture: Nonsoluble factors in the extracellular matrix influence interactions. Wound Repair Regen. 2008, 16, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Festa, E.; Fretz, J.; Berry, R.; Schmidt, B.; Rodeheffer, M.; Horowitz, M.; Horsley, V. Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell 2011, 146, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.A.; Horsley, V. Intradermal adipocytes mediate fibroblast recruitment during skin wound healing. Development 2013, 140, 1517–1527. [Google Scholar] [CrossRef] [PubMed]
- Donati, G.; Proserpio, V.; Lichtenberger, B.M.; Natsuga, K.; Sinclair, R.; Fujiwara, H.; Watt, F.M. Epidermal Wnt/-catenin signaling regulates adipocyte differentiation via secretion of adipogenic factors. Proc. Natl. Acad. Sci. USA 2014, 111, E1501–E1509. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Gonzalez, G.; Shook, B.; Horsley, V. Adipocytes in skin health and disease. Cold Spring Harb. Perspect. Med. 2014, 4, a015271–a015271. [Google Scholar] [CrossRef] [PubMed]
- Driskell, R.; Colin, J.; Chuong, C.-M.; Watt, F.; Horsley, V. Defining dermal adipose tissue. Exp. Dermatol. 2015, 23, 629–631. [Google Scholar] [CrossRef] [PubMed]
- Wojciechowicz, K.; Gledhill, K.; Ambler, C.A.; Manning, C.B.; Jahoda, C.A.B. Development of the mouse dermal adipose layer occurs independently of subcutaneous adipose tissue and is marked by restricted early expression of FABP4. PLoS ONE 2013, 8, e59811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geerligs, M.; Peters, G.W.M.; Ackermans, P.A.J.; Oomens, C.W.J.; Baaijens, F.P.T. Linear viscoelastic behavior of subcutaneous adipose tissue. Biorheology 2008, 45, 677–688. [Google Scholar] [PubMed]
- Crandall, D.L.; Hausman, G.J.; Kral, J.G. A review of the microcirculation of adipose tissue: Anatomic, metabolic, and angiogenic perspectives. Microcirculation 1997, 4, 211–232. [Google Scholar] [CrossRef] [PubMed]
- Watt, F.M.; Fujiwara, H. Cell-extracellular matrix interactions in normal and diseased skin. Cold Spring Harb. Perspect. Biol. 2011, 3, a005124–a005124. [Google Scholar] [CrossRef] [PubMed]
- Yurchenco, P.D. Basement membranes: Cell scaffoldings and signaling platforms. Cold Spring Harb. Perspect. Biol. 2011, 3, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Burgeson, R.E.; Christiano, A.M. The dermal—Epidermal junction. Curr. Opin. Cell Biol. 1997, 9, 651–658. [Google Scholar] [CrossRef]
- Bruckner-Tuderman, L.; Has, C. Disorders of the cutaneous basement membrane zone—The paradigm of epidermolysis bullosa. Matrix Biol. 2014, 33, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Marinkovich, M.P.; Keene, D.R.; Rimberg, C.S.; Burgeson, R.E. Cellular origin of the dermal-epidermal basement membrane. Dev. Dyn. 1993, 197, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Andriani, F.; Margulis, A.; Lin, N.; Griffey, S.; Garlick, J.A. Analysis of microenvironmental factors contributing to basement membrane assembly and normalized epidermal phenotype. J. Investig. Dermatol. 2003, 120, 923–931. [Google Scholar] [CrossRef] [PubMed]
- El Ghalbzouri, A.; Jonkman, M.F.; Dijkman, R.; Ponec, M. Basement membrane reconstruction in human skin equivalents is regulated by fibroblasts and/or exogenously activated keratinocytes. J. Investig. Dermatol. 2005, 124, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Pouliot, N.; Saunders, N.A.; Kaur, P. Laminin 10/11: An alternative adhesive ligand for epidermal keratinocytes with a functional role in promoting proliferation and migration. Exp. Dermatol. 2002, 11, 387–397. [Google Scholar] [CrossRef] [PubMed]
- MacNeil, S. Progress and opportunities for tissue-engineered skin. Nature 2007, 445, 874–880. [Google Scholar] [CrossRef] [PubMed]
- Shevchenko, R.V.; James, S.L.; James, S.E. A review of tissue-engineered skin bioconstructs available for skin reconstruction. J. R. Soc. Interface 2010, 7, 229–258. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lawlor, K.T.; Kaur, P. Dermal Contributions to Human Interfollicular Epidermal Architecture and Self-Renewal. Int. J. Mol. Sci. 2015, 16, 28098-28107. https://doi.org/10.3390/ijms161226078
Lawlor KT, Kaur P. Dermal Contributions to Human Interfollicular Epidermal Architecture and Self-Renewal. International Journal of Molecular Sciences. 2015; 16(12):28098-28107. https://doi.org/10.3390/ijms161226078
Chicago/Turabian StyleLawlor, Kynan T., and Pritinder Kaur. 2015. "Dermal Contributions to Human Interfollicular Epidermal Architecture and Self-Renewal" International Journal of Molecular Sciences 16, no. 12: 28098-28107. https://doi.org/10.3390/ijms161226078
APA StyleLawlor, K. T., & Kaur, P. (2015). Dermal Contributions to Human Interfollicular Epidermal Architecture and Self-Renewal. International Journal of Molecular Sciences, 16(12), 28098-28107. https://doi.org/10.3390/ijms161226078