Anti-Atherosclerotic Effects of a Phytoestrogen-Rich Herbal Preparation in Postmenopausal Women
Abstract
:1. Introduction
2. Results
2.1. Baseline Data
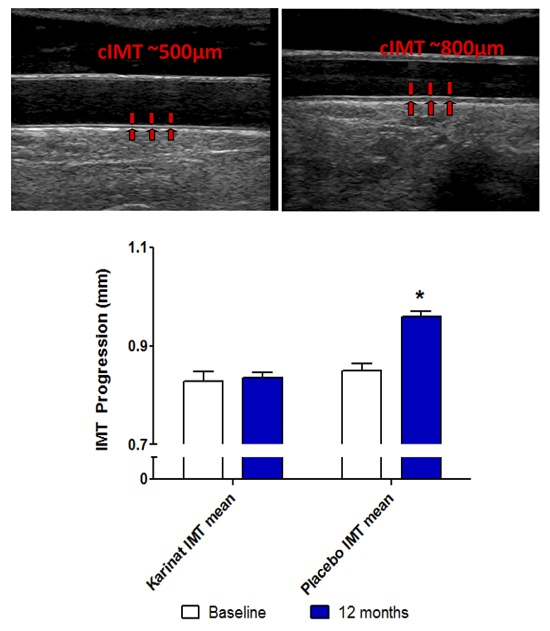
2.2. Follow-up
3. Discussion
4. Materials and Methods
4.1. Study Medication
4.2. Study Design
4.3. Baseline Examination
4.4. Follow-up Examination
4.5. Blood Sampling and Lipid Measurements
4.6. Calculation of Prognostic Cardiovascular Risk
4.7. Carotid Artery Ultrasound Examination
4.8. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rossi, R.; Grimaldi, T.; Origliani, G.; Fantini, G.; Coppi, F.; Modena, M.G. Menopause and cardiovascular risk. Pathophysiol. Haemost. Thromb. 2002, 32, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Rossi, R.; Cioni, E.; Nuzzo, A.; Origliani, G.; Modena, M.G. Endothelial-dependent vasodilation and incidence of type 2 diabetes in a population of healthy postmenopausal women. Diabetes Care 2005, 28, 702–707. [Google Scholar] [CrossRef] [PubMed]
- Hulley, S.; Grady, D.; Bush, T.; Furberg, C.; Herrington, D.; Riggs, B.; Vittinghoff, E. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. J. Am. Med. Assoc. 1998, 280, 605–613. [Google Scholar] [CrossRef]
- Grady, D.; Herrington, D.; Bittner, V.; Blumenthal, R.; Davidson, M.; Hlatky, M.; Hsia, J.; Hulley, S.; Herd, A.; Khan, S.; et al. HERS Research Group. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II). J. Am. Med. Assoc. 2002, 288, 49–57. [Google Scholar] [CrossRef]
- Gompel, A.; Santen, R.J. Hormone therapy and breast cancer risk 10 years after the WHI. Climacteric 2012, 15, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Justenhoven, C.; Obazee, O.; Brauch, H. The pharmacogenomics of sex hormone metabolism: Breast cancer risk in menopausal hormone therapy. Pharmacogenomics 2012, 13, 659–675. [Google Scholar] [CrossRef] [PubMed]
- Rossouw, J.E.; Anderson, G.L.; Prentice, R.L.; LaCroix, A.Z.; Kooperberg, C.; Stefanick, M.L.; Jackson, R.D.; Beresford, S.A.; Howard, B.V.; Johnson, K.C.; et al. Writing Group for the Women's Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women’s Health Initiative randomized controlled trial. J. Am. Med. Assoc. 2002, 288, 321–333. [Google Scholar]
- Rossouw, J.E.; Prentice, R.L.; Manson, J.E.; Wu, L.; Barad, D.; Barnabei, V.M.; Ko, M.; LaCroix, A.Z.; Margolis, K.L.; Stefanick, M.L. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. J. Am. Med. Assoc. 2007, 297, 1465–1477. [Google Scholar] [CrossRef] [PubMed]
- Manson, J.E.; Chlebowski, R.T.; Stefanick, M.L.; Aragaki, A.K.; Rossouw, J.E.; Prentice, R.L.; Anderson, G.; Howard, B.V.; Thomson, C.A.; LaCroix, A.Z.; et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women's Health Initiative randomized trials. J. Am. Med. Assoc. 2013, 310, 1353–1368. [Google Scholar] [CrossRef] [PubMed]
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG Committee Opinion No. 420, November 2008: Hormone therapy and heart disease. Obstet. Gynecol. 2008, 112, 1189–1192. [Google Scholar]
- Mosca, L.; Benjamin, E.J.; Berra, K.; Bezanson, J.L.; Dolor, R.J.; Lloyd-Jones, D.M.; Newby, L.K.; Piña, I.L.; Roger, V.L.; Shaw, L.J.; et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women -2011 Update: A Guideline from the American Heart Association. Circulation 2011, 123, 1243–1262. [Google Scholar] [CrossRef] [PubMed]
- Glazier, M.G.; Bowman, M.A. A review of the evidence for the use of phytoestrogens as a replacement for traditional estrogen replacement therapy. Arch. Intern. Med. 2001, 161, 1161–7112. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, M.J.; Cutini, P.H.; Rauschemberger, M.B.; Massheimer, V.L. The soyabean isoflavone genistein modulates endothelial cell behaviour. Br. J. Nutr. 2010, 104, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Finking, G.; Wohlfrom, M.; Lenz, C.; Wolkenhauer, M.; Eberle, C.; Hanke, H. The phytoestrogens genistein and daidzein, and 17 beta-estradiol inhibit development of neointima in aortas from male and female rabbits in vitro after injury. Coron. Artery Dis. 1999, 10, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; White, M.; Husband, A.J.; Hambly, B.D.; Bao, S. Phytoestrogen derivatives differentially inhibit arterial neointimal proliferation in a mouse model. Eur. J. Pharmacol. 2006, 548, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Safari, M.R.; Sheikh, N. Effects of flavonoids on the susceptibility of low-density lipoprotein to oxidative modification. Prostaglandins Leukot. Essent. Fatty Acids 2003, 69, 73–77. [Google Scholar] [CrossRef]
- Nikitina, N.A.; Sobenin, I.A.; Myasoedova, V.A.; Korennaya, V.V.; Mel’nichenko, A.A.; Khalilov, E.M.; Orekhov, A.N. Antiatherogenic effect of grape flavonoids in an ex vivo model. Bull. Exp. Biol. Med. 2006, 141, 712–715. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wu, G.; Su, X.; Yang, H.; Zhang, J. Antiobesity action of a daidzein derivative on male obese mice induced by a high-fat diet. Nutr. Res. 2009, 29, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Li, S.; Dai, X.; Chen, Y.; Feng, Z.; Zhao, Y.; Wu, J. Genistein inhibits proliferation and functions of hypertrophic scar fibroblasts. Burns 2009, 35, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Dharmappa, K.K.; Mohamed, R.; Shivaprasad, H.V.; Vishwanath, B.S. Genistein, a potent inhibitor of secretory phospholipase A2: A new insight in down regulation of inflammation. Inflammopharmacology 2010, 18, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Kwon, S.J.; Na, S.Y.; Lim, S.P.; Lee, J.H. Genistein supplementation inhibits atherosclerosis with stabilization of the lesions in hypercholesterolemic rabbits. J. Korean Med. Sci. 2004, 19, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zou, J.; Cao, K.; Hsieh, T.C.; Huang, Y.; Wu, J.M. Dealcoholized red wine containing known amounts of resveratrol suppresses atherosclerosis in hypercholesterolemic rabbits without affecting plasma lipid levels. Int. J. Mol. Med. 2005, 16, 533–540. [Google Scholar] [PubMed]
- Zou, J.; Huang, Y.; Cao, K.; Yang, G.; Yin, H.; Len, J.; Hsieh, T.C.; Wu, J.M. Effect of resveratrol on intimal hyperplasia after endothelial denudation in an experimental rabbit model. Life Sci. 2000, 68, 153–163. [Google Scholar] [CrossRef]
- Brito, P.; Almeida, L.M.; Dinis, T.C. The interaction of resveratrol with ferrylmyoglobin and peroxynitrite; protection against LDL oxidation. Free Radic. Res. 2002, 36, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Stein, J.H.; Korcarz, C.E.; Post, W.S. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: Summary and discussion of the American Society of Echocardiography consensus statement. Prev. Cardiol. 2009, 12, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Amato, M.; Montorsi, P.; Ravani, A.; Oldani, E.; Galli, S.; Ravagnani, P.M.; Tremoli, E.; Baldassarre, D. Carotid intima-media thickness by B-mode ultrasound as surrogate of coronary atherosclerosis: Correlation with quantitative coronary angiography and coronary intravascular ultrasound findings. Eur. Heart J. 2007, 28, 2094–2101. [Google Scholar] [CrossRef] [PubMed]
- Chambless, L.E.; Heiss, G.; Folsom, A.R.; Rosamond, W.; Szklo, M.; Sharrett, A.R.; Clegg, L.X. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: The Atherosclerosis Risk in Communities (ARIC) Study, 1987–1993. Am. J. Epidemiol. 1997, 146, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Hodis, H.N.; Mack, W.J.; Kono, N.; Azen, S.P.; Shoupe, D.; Hwang-Levine, J.; Petitti, D.; Whitfield-Maxwell, L.; Yan, M.; Franke, A.A.; et al. Women’s Isoflavone Soy Health Research Group. Isoflavone soy protein supplementation and atherosclerosis progression in healthy postmenopausal women: A randomized controlled trial. Stroke 2011, 42, 3168–3175. [Google Scholar] [CrossRef] [PubMed]
- Hodis, H.N.; Mack, W.J.; Henderson, V.W.; Shoupe, D.; Budoff, M.J.; Hwang-Levine, J.; Li, Y.; Feng, M.; Dustin, L.; Kono, N.; et al. ELITE Research Group. Vascular effects of early versus late postmenopausal treatment with estradiol. N. Engl. J. Med. 2016, 374, 1221–1231. [Google Scholar] [CrossRef] [PubMed]
- Harman, S.M. Effects of oral conjugated estrogen or transdermal estradiol plus oral progesterone treatment on common carotid artery intima media thickness (CIMT) and coronary artery calcium (CAC) in menopausal women: Initial results from the Kronos Early Estrogen Prevention Study (KEEPS). Menopause 2012, 19, 1365. [Google Scholar]
- Colacurci, N.; Fornaro, F.; Cobellis, L.; de Franciscis, P.; Torella, M.; Sepe, E.; Arciello, A.; Cacciapuoti, F.; Paolisso, G.; Manzella, D. Raloxifene slows down the progression of intima-media thickness in postmenopausal women. Menopause 2007, 14, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Bairey Merz, C.N.; Johnson, B.D.; Braunstein, G.D.; Pepine, C.J.; Reis, S.E.; Paul-Labrador, M.; Hale, G.; Sharaf, B.L.; Bittner, V.; Sopko, G.; et al. Phytoestrogens and lipoproteins in women. J. Clin. Endocrinol. Metab. 2006, 91, 2209–2213. [Google Scholar] [CrossRef] [PubMed]
- Crouse, J.R., 3rd; Morgan, T.; Terry, J.G.; Ellis, J.; Vitolins, M.; Burke, G.L. A randomized trial comparing the effect of casein with that of soy protein containing varying amounts of isoflavones on plasma concentrations of lipids and lipoproteins. Arch. Intern. Med. 1999, 159, 2070–2076. [Google Scholar] [CrossRef] [PubMed]
- Sobenin, I.A.; Chistiakov, D.A.; Bobryshev, Y.V.; Orekhov, A.N. Blood atherogenicity as a target for anti-atherosclerotic therapy. Curr. Pharm. Des. 2013, 19, 5954–5962. [Google Scholar] [CrossRef] [PubMed]
- Myasoedova, V.A.; Sobenin, I.A. Background, rationale and design of clinical study of the effect of isoflavonoid-rich botanicals on natural history of atherosclerosis in women. Atheroscler. Suppl. 2008, 9, 171. [Google Scholar] [CrossRef]
- Lorenz, M.W.; Markus, H.S.; Bots, M.L.; Rosvall, M.; Sitzer, M. Prediction of clinical cardiovascular events with carotid intima-media thickness: A systematic review and meta-analysis. Circulation 2007, 115, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Rossi, R.; Nuzzo, A.; Olaru, A.I.; Origliani, G.; Modena, M.G. Endothelial function affects early carotid atherosclerosis progression in hypertensive postmenopausal women. J. Hypertens. 2011, 29, 1136–1144. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, Y.; Zhu, L.; Wang, W.; Wan, Z.; Chen, F.; Wu, Y.; Zhou, J.; Yuan, Z. 17β-estradiol promotes cholesterol efflux from vascular smooth muscle cells through a liver X receptor α-dependent pathway. Int. J. Mol. Med. 2014, 33, 550–558. [Google Scholar] [PubMed]
- Seli, E.; Selam, B.; Mor, G.; Kayisli, U.A.; Pehlivan, T.; Arici, A. Estradiol regulates monocyte chemotactic protein-1 in human coronary artery smooth muscle cells: A mechanism for its antiatherogenic effect. Menopause 2001, 8, 296–301. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, M.G.; Goodrich, J.A.; Adams, M.R. Increased prostacyclin synthesis by atherosclerotic arteries from estrogen-treated monkeys. Life Sci. 2001, 69, 395–401. [Google Scholar] [CrossRef]
- Honisett, S.Y.; Stojanovska, L.; Sudhir, K.; Kingwell, B.A.; Dawood, T.; Komesaroff, P.A. Hormone therapy impairs endothelial function in postmenopausal women with type 2 diabetes mellitus treated with rosiglitazone. J. Clin. Endocrinol. Metab. 2004, 89, 4615–4619. [Google Scholar] [CrossRef] [PubMed]
- Gourdy, P.; Schambourg, A.; Filipe, C.; Douin-Echinard, V.; Garmy-Susini, B.; Calippe, B.; Tercé, F.; Bayard, F.; Arnal, J.F. Transforming growth factor activity is a key determinant for the effect of estradiol on fatty streak deposit in hypercholesterolemic mice. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2214–2221. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Ma, X.; Chen, Y.X.; Rayner, K.; Hibbert, B.; McNulty, M.; Dhaliwal, B.; Simard, T.; Ramirez, D.; O’Brien, E. Attenuation of atherogenesis via the anti-inflammatory effects of the selective estrogen receptor beta modulator 8β-VE2. J. Cardiovasc. Pharmacol. 2011, 58, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Chacko, B.K.; Chandler, R.T.; Mundhekar, A.; Khoo, N.; Pruitt, H.M.; Kucik, D.F.; Parks, D.A.; Kevil, C.G.; Barnes, S.; Patel, R.P. Revealing anti-inflammatory mechanisms of soy isoflavones by flow: Modulation of leukocyte-endothelial cell interactions. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H908–H915. [Google Scholar] [CrossRef] [PubMed]
- Simoncini, T.; Garibaldi, S.; Fu, X.D.; Pisaneschi, S.; Begliuomini, S.; Baldacci, C.; Lenzi, E.; Goglia, L.; Giretti, M.S.; Genazzani, A.R. Effects of phytoestrogens derived from red clover on atherogenic adhesion molecules in human endothelial cells. Menopause 2008, 15, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Andrade, C.M.; Sá, M.F.; Toloi, M.R. Effects of phytoestrogens derived from soy bean on expression of adhesion molecules on HUVEC. Climacteric 2012, 15, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Hou, D.D.; Zhao, Q.; Liu, W.; Zhen, P.P.; Xu, J.P.; Wang, K.; Huang, H.X.; Li, X.; Zhang, H.; et al. Phytoestrogen α-Zearalanol attenuates homocysteine-induced apoptosis in human umbilical vein endothelial cells. BioMed Res. Int. 2013, 2013, 813450. [Google Scholar] [PubMed]
- Orekhov, A.N.; Sobenin, I.A.; Korneev, N.V.; Kirichenko, T.V.; Myasoedova, V.A.; Melnichenko, A.A.; Balcells, M.; Edelman, E.R.; Bobryshev, Y.V. Anti-atherosclerotic therapy based on botanicals. Recent Pat. Cardiovasc. Drug Discov. 2013, 8, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Sobenin, I.A.; Myasoedova, V.A.; Orekhov, A.N. Phytoestrogen-rich dietary supplements in anti-atherosclerotic therapy in postmenopausal women. Curr. Pharm. Des. 2016, 22, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Alder, E. The Blatt-Kupperman menopausal index: A critique. Maturitas 1998, 29, 19–24. [Google Scholar] [CrossRef]
- Harman, S.M.; Brinton, E.A.; Cedars, M.; Lobo, R.; Manson, J.E.; Merriam, G.R.; Miller, V.M.; Naftolin, F.; Santoro, N. KEEPS: The Kronos Early Estrogen Prevention Study. Climacteric 2005, 8, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Artom, N.; Montecucco, F.; Dallegri, F.; Pende, A. Carotid atherosclerotic plaque stenosis: The stabilizing role of statins. Eur. J. Clin. Investig. 2014, 44, 1122–1134. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, W.; Dong, L.; Li, R.; Wu, Y. Effect of statin therapy on the progression of common carotid artery intima-media thickness: An updated systematic review and meta-analysis of randomized controlled trials. J. Atheroscler. Thromb. 2013, 20, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Papanas, N.; Maltezos, E. Oral antidiabetic agents: Anti-atherosclerotic properties beyond glucose lowering? Curr. Pharm. Des. 2009, 15, 3179–3192. [Google Scholar] [CrossRef] [PubMed]
- Katakami, N.; Kaneto, H.; Matsuhisa, M.; Shimomura, I.; Yamasaki, Y. Effects of glimepiride and glibenclamide on carotid atherosclerosis in type 2 diabetic patients. Diabetes Res. Clin. Pract. 2011, 92, e20–e22. [Google Scholar] [CrossRef] [PubMed]
- Mackinnon, A.D.; Jerrard-Dunne, P.; Sitzer, M.; Buehler, A.; von Kegler, S.; Markus, H.S. Rates and determinants of site-specific progression of carotid artery intima-media thickness: The carotid atherosclerosis progression study. Stroke 2004, 35, 2150–2154. [Google Scholar] [CrossRef] [PubMed]
- Koyasu, M.; Ishii, H.; Watarai, M.; Takemoto, K.; Inden, Y.; Takeshita, K.; Amano, T.; Yoshikawa, D.; Matsubara, T.; Murohara, T. Impact of acarbose on carotid intima-media thickness in patients with newly diagnosed impaired glucose tolerance or mild type 2 diabetes mellitus: A one-year, prospective, randomized, open-label, parallel-group study in Japanese adults with established coronary artery disease. Clin. Ther. 2010, 32, 1610–1617. [Google Scholar] [PubMed]
- Odell, P.M.; Anderson, K.M.; Kannel, W.B. New models for predicting cardiovascular events. J. Clin. Epidemiol. 1994, 47, 583–592. [Google Scholar] [CrossRef]
- Tunstall-Pedoe, H.; Kuulasmaa, K.; Mähönen, M.; Tolonen, H.; Ruokokoski, E.; Amouyel, P. Contribution of trends in survival and coronary-event rates to changes in coronary heart disease mortality: 10-year results from 37 WHO MONICA project populations. Monitoring trends and determinants in cardiovascular disease. Lancet 1999, 353, 1547–1557. [Google Scholar] [CrossRef]
- Salonen, R.; Nyyssönen, K.; Porkkala, E.; Rummukainen, J.; Belder, R.; Park, J.S.; Salonen, J.T. Kuopio Atherosclerosis Prevention Study (KAPS). A population-based primary preventive trial of the effect of LDL lowering on atherosclerotic progression in carotid and femoral arteries. Circulation 1995, 92, 1758–1764. [Google Scholar] [CrossRef] [PubMed]
- Baldassarre, D.; Nyyssönen, K.; Rauramaa, R.; de Faire, U.; Hamsten, A.; Smit, A.J.; Mannarino, E.; Humphries, S.E.; Giral, P.; Grossi, E.; et al. IMPROVE study group. Cross-sectional analysis of baseline data to identify the major determinants of carotid intima-media thickness in a European population: The IMPROVE study. Eur. Heart J. 2010, 31, 614–622. [Google Scholar] [CrossRef] [PubMed]

| Variable | Isoflavonoid-Rich Herbal Preparation Recipients, n = 77 | Placebo Recipients, n = 80 | p-Value |
|---|---|---|---|
| Age, years | 65 (7) | 65 (6) | 0.804 |
| Body mass index, kg/m2 | 27.1 (4.0) | 26.9 (3.8) | 0.782 |
| Systolic BP, mm·Hg | 127 (13) | 135 (18) | 0.006 |
| Diastolic BP, mm·Hg | 79 (8) | 83 (9) | 0.006 |
| Smoking, n (%) | 3 (5) | 7 (10) | 0.362 |
| Diabetes, n (%) | 6 (11) | 1 (1) | 0.022 |
| Hypertension, n (%) | 29 (51) | 41 (56) | 0.532 |
| Family history of CAD, n (%) | 16 (30) | 19 (26) | 0.634 |
| Family history of hypertension, n (%) | 30 (53) | 37 (51) | 0.827 |
| Family history of diabetes, n (%) | 5 (9) | 9 (14) | 0.387 |
| Total cholesterol, mg/dL | 271(55) | 252 (42) | 0.024 |
| Triglycerides, mg/dL | 134 (78) | 126 (51) | 0.456 |
| HDL-C, mg/dL | 74 (15) | 74 (18) | 0.745 |
| LDL-C, mg/dL | 170 (47) | 153 (42) | 0.034 |
| Risk of MI, PROCAM score, % | 1.64 (3.34) | 1.24 (1.40) | 0.363 |
| cIMT mean, mm | 0.829 (0.138) | 0.849 (0.133) | 0.415 |
| cIMT max, mm | 0.950 (0.172) | 0.981 (0.161) | 0.287 |
| Carotid plaque, relative size, score | 0.77 (0.78) | 0.76 (0.72) | 0.908 |
| Variable | Isoflavonoid-Rich Herbal Preparation Recipients, n = 56 | Placebo Recipients, n = 71 | ||
|---|---|---|---|---|
| Change | p-Value | Change | p-Value | |
| Body mass index, kg/m2 | −0.01 (0.8) | 0.978 | −0.07 (1.6) | 0.708 |
| Systolic BP, mm·Hg | 5 (19) | 0.051 | −1 (18) | 0.666 |
| Diastolic BP, mm·Hg | −1 (8) | 0.806 | −1 (9) | 0.150 |
| Total cholesterol, mg/dL | −17 (46) | 0.011 | −13 (41) | 0.020 |
| Triglycerides, mg/dL | −9 (53) | 0.232 | −9 (40) | 0.106 |
| HDL-C, mg/dL | −3 (11) | 0.114 | −3 (12) | 0.038 |
| LDL-C, mg/dL | −13 (45) | 0.040 | −8 (39) | 0.126 |
| Variable | Isoflavonoid-Rich Herbal Preparation Recipients, n = 56 | Placebo Recipients, n = 71 | ||
|---|---|---|---|---|
| Change | p-Value | Change | p-Value | |
| cIMT mean, μm | +6 (85) | 0.6 | +111 (91) | <0.001 |
| cIMT max, μm | +8 (101) | 0.6 | +4 (220) | 0.9 |
| Carotid plaque, score | +0.21 (0.59) | 0.009 | +0.31 (0.55) | <0.001 |
| Constituent | Mg Per Capsule | % |
|---|---|---|
| Humulus lupulus L. | 160 | 34.04 |
| Camellia sinensis L. | 115 | 24.46 |
| Allium sativum L. | 100 | 21.27 |
| Vitis vinifera L. (water ethanol extract, HPLC identification of the authenticity of the extract, UV measurement of proanthocyanidins (>95%) | 40 | 8.51 |
| Ascorbic acid (PubChem CID: 54670067) | 30 | 6.38 |
| Calcium stearate (PubChem CID: 15324) | 10 | 2.13 |
| Silicon dioxide (PubChem CID: 24261) | 8 | 1.70 |
| DL-alpha-tocopherol (PubChem CID: 2116) | 6.6 | 1.40 |
| Beta-carotene 99% (PubChem CID: 5280489) | 0.5 | 0.11 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Myasoedova, V.A.; Kirichenko, T.V.; Melnichenko, A.A.; Orekhova, V.A.; Ravani, A.; Poggio, P.; Sobenin, I.A.; Bobryshev, Y.V.; Orekhov, A.N. Anti-Atherosclerotic Effects of a Phytoestrogen-Rich Herbal Preparation in Postmenopausal Women. Int. J. Mol. Sci. 2016, 17, 1318. https://doi.org/10.3390/ijms17081318
Myasoedova VA, Kirichenko TV, Melnichenko AA, Orekhova VA, Ravani A, Poggio P, Sobenin IA, Bobryshev YV, Orekhov AN. Anti-Atherosclerotic Effects of a Phytoestrogen-Rich Herbal Preparation in Postmenopausal Women. International Journal of Molecular Sciences. 2016; 17(8):1318. https://doi.org/10.3390/ijms17081318
Chicago/Turabian StyleMyasoedova, Veronika A., Tatyana V. Kirichenko, Alexandra A. Melnichenko, Varvara A. Orekhova, Alessio Ravani, Paolo Poggio, Igor A. Sobenin, Yuri V. Bobryshev, and Alexander N. Orekhov. 2016. "Anti-Atherosclerotic Effects of a Phytoestrogen-Rich Herbal Preparation in Postmenopausal Women" International Journal of Molecular Sciences 17, no. 8: 1318. https://doi.org/10.3390/ijms17081318
APA StyleMyasoedova, V. A., Kirichenko, T. V., Melnichenko, A. A., Orekhova, V. A., Ravani, A., Poggio, P., Sobenin, I. A., Bobryshev, Y. V., & Orekhov, A. N. (2016). Anti-Atherosclerotic Effects of a Phytoestrogen-Rich Herbal Preparation in Postmenopausal Women. International Journal of Molecular Sciences, 17(8), 1318. https://doi.org/10.3390/ijms17081318