1. Introduction
Nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH) are increasingly recognized as common clinicopathological entities that occur in individuals without significant alcohol use [
1]. The former is believed to have a benign clinical course, whereas the latter represents a form of liver injury that carries a risk for progressive fibrosis, liver cirrhosis (LC), and hepatocellular carcinoma (HCC) [
2,
3]. Due to the obesity epidemic and the increasing prevalence of metabolic syndrome, NAFLD and its progressive form, NASH, are seen more commonly in different parts of the world [
4,
5]. NAFLD has become a serious public health issue not only in Western countries, but also in many Asian countries, including Japan [
6,
7,
8]. NASH is characterized by parenchymal injuries, including macrovesicular steatosis, ballooning degeneration, Mallory-Denk bodies, and inflammation in hepatic lobes [
9]. On the other hand, chronic hepatitis C (CHC) is characterized by portal tract infiltration of dense aggregates of lymphocytes with follicle formation, and mild macrovesicular steatosis can be seen in lobules, particular in periportal hepatocytes [
10,
11]. Thus, the manner of fibrosis progression in NASH is different from that in CHC. Although there is currently no validated test involving serum biomarkers available to diagnose NASH, and histologic evaluation with a liver biopsy remains the gold standard, and screening for fibrosis is recommended in patients with suspected NASH. However, liver biopsy has some clinical problems related to its invasiveness and complications. On the other hand, there are validated tests with serum biomarkers available to diagnose the stage of hepatic fibrosis (e.g., Wisteria floribunda agglutinin positive Mac-2-binding protein (WFA
+-M2BP), hyaluronic acid (HA), 7S domain of type IV collagen, tissue inhibitor of metalloproteinase-1 (TIMP-1), type III procollagen N peptide (PIIIP), FIB4-index, etc.). Recently, WFA
+-M2BP has been reported to be a useful marker for staging in patients with NAFLD [
12] and CHC [
13,
14]. Especially in CHC patients, WFA
+-M2BP can be a useful surrogate marker not only as a fibrotic marker, but also for the risk of HCC development [
13,
14]. However, there are few reports about WFA
+-M2BP on the basis of the etiology of chronic liver disease (CLD).
Since the liver receives blood flow from both the portal vein and hepatic artery, which account for 70% and 30%, respectively, this double blood supply mechanism is a specific characteristic of the liver. The portal vein receives the blood supply from the intestine, which engages in metabolism as a functional vessel. Xenon-CT has been established as a non-invasive technique to visualize tissue blood flow (TBF) in the neurosurgical field [
15,
16]. Xe-CT can also be applied to obtain separate measurements of hepatic arterial and venous blood flow to detect changes in hepatic blood flow (HBF). We previously reported that PVTBF and total hepatic TBF (THTBF) decrease with the progression of liver fibrosis in patients with CHC [
17,
18] and NAFLD [
19,
20]. However, few reports have addressed the association between HBF and liver function; no report has examined the progression of liver function according to the etiology of CLD. Moreover we previously reported that hepatic TBF in patients with liver cirrhosis varied according to the etiology of the disease and there is a close correlation between liver function and hepatic blood flow in patients with alcoholic liver cirrhosis [
21,
22]. In the present study, we investigated the difference in the fibrosis markers between patients with initial chronic hepatitis and those with advanced chronic hepatitis in NASH and CH-C. Furthermore, we attempted to clarify the relationship between hepatic TBF and liver function. It is extremely important to understand the characteristics of CLD progression for the management and treatment of CLD. The aim of this study was to elucidate the difference in disease progression between CHC and NAFLD in CLD by comparing the cutoff values for fibrosis markers and the associations of liver function and HBF.
3. Discussion
A definite diagnosis of NASH requires liver biopsy, though various non-invasive measures are under development [
6]. NASH is characterized by parenchymal injury, including macrovesicular steatosis, ballooning degeneration, Mallory-Denk bodies, and inflammation in hepatic lobes [
9]. Fibrosis begins in zone 3 or the centrilobular area of the hepatic lobule. Periportal and bridging fibrosis develop with progression of the disease, and once cirrhosis is established, features of steatohepatitis and perisinusoidal fibrosis may be obscured. It is well known that exercise, itself, is an important factor to treat NASH and, therefore, the role of exercise should be emphasized. Exercise, in fact, improves NASH-related fibrosis markers (collagen 1α1 mRNA,
p < 0.05 and fibrosis score,
p < 0.01) and the inflammation score; exercise increases the hepatic stellate cell senescence marker CCN1 [
25,
26].
On the other hand, fibrosis begins in zone 1 or the periportal area of the hepatic lobule in patients with CHC. CHC is characterized by a portal tract that is infiltrated by dense aggregates of lymphocytes with follicle formation, and mild macrovesicular steatosis can be seen in lobules, particularly in periportal hepatocytes [
10,
11]. Moreover, it has been reported that daily use of recreational drugs, in particular cannabis, has a deleterious effect on the speed of progression of fibrosis and steatosis in patients suffering from chronic hepatitis C [
27]. There are other differences between NAFLD and CHC. Previous reports indicated that at the early stages of CLD the numbers of liver monocytes/macrophages were elevated without the evidence of local proliferation, supporting a role for infiltrating monocyte-derived macrophages in disease progression in patients with both CHC and NAFLD. However, CHC and NAFLD differentially affected the circulating monocyte phenotype, suggesting that unique injury-induced signals may contribute to the intrahepatic monocyte recruitment and the systemic activation state. Moreover, it was also shown that monocyte function was similarly impaired in patients with both CHC and NAFLD, particularly in advanced disease [
28]. Thus, the manner of fibrosis progression resulting from inflammation could be different between NASH and CHC.
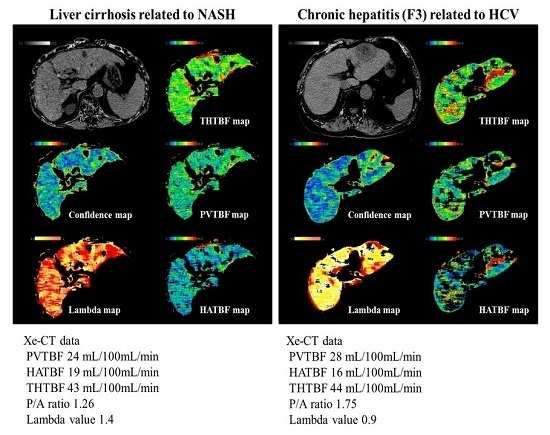
The results of present study showed the relationship between liver function and PVTBF (
Table 5 and
Table 6). PVTBF was well correlated with hepatic synthesis capacity, which included Alb, ChE, TC, and PT. The reason why liver function tests in NASH was better than that in CHC is suggested the excess energy intake and lipid hypermetabolism [
29]. ICG-R
15 is the indicator which reflects liver function [
30] and the presence of portal hypertension. Furthermore ICG-R
15 is well correlated to the hepatic tissue blood flow [
31]. Lisotti et al. reported that the ICG-R
15 test is an effective tool for assessment of portal hypertension in patients with compensated cirrhosis [
30]. We confirmed that the hemodynamic changes occurred earlier in NAFLD relative to CHC. For example, 15% of ICG-R
15 correspond to the stage 3 in NASH and LC in CHC (
Table 3 and
Table 4). Yamazaki reported that the average of ICG-R
15 was 15.4% in which the presence of esophageal varices cases [
32], and their data, supported our results.
Alteration in hepatic microcirculation in human donor livers with steatosis was first reported during organ retrieval before mobilization by Seifalian et al. [
33] using laser Doppler flowmetry. A significant decrease in hepatic microcirculation in liver donors with steatosis was observed in comparison with that in normal liver donors [
34]. Experimental studies in animal models with fatty liver showed that steatosis led to reduce hepatic blood flow and microcirculation, and that there was an inverse correlation between the degree of steatosis and both total hepatic blood flow and flow in the microcirculation [
30]. The severity of fatty infiltration has a greater effect on the microcirculation than on total hepatic blood flow [
35,
36]. In spite of steatosis alone, hepatic blood flow reduced. Moreover, hepatic blood flow reduced with fibrosis development, in addition to steatosis [
37]. Fat-laden hepatocytes are swollen, and in steatohepatitis, further swelling occurs due to the ballooning of hepatocytes, causing sinusoidal distortion, as visualized by in vivo microscopy, reducing intrasinusoidal volume and microcirculation [
38].
In addition to steatosis, a mechanism of decreasing portal blood flow other than steatosis has been reported in NAFLD. In livers with perfusion from cafeteria diet-fed rats, there was increased portal pressure and decreased endothelium-dependent vasodilation. This was associated with decreased Akt-dependent endothelial nitric-oxide synthase (eNOS) phosphorylation and NOS activity. They demonstrated in a rat model of the metabolic syndrome that hepatic endothelial dysfunction occurs before the development of fibrosis and inflammation [
39]. Ying-Ying Yang et al. reported that hyperleptinemia increases hepatic endocannabinoid production, promotes hepatic fibrogenesis, enhances the hepatic vasoconstrictive response to endothelin-1, and aggravates hepatic microcirculatory dysfunction. These events subsequently increase intrahepatic resistance and portal hypertension in NASH cirrhotic rats [
40].
The present data show that the liver function was better in initial NASH-LC than in C-LC. However, because PVTBF was lower in NASH than in C-LC, portal hypertension might occur at an earlier stage in NASH than in CHC. In fact, portal hypertension occurs without LC in NASH [
41,
42,
43]. Mendes et al. investigated the prevalence of portal hypertension in NAFLD patients, and found that clinical signs of portal hypertension, including esophageal varices, splenomegaly, portosystemic encephalopathy, and ascites, were present in 25% of patients at the time of diagnosis. Furthermore, portal hypertension can occur in a small proportion of patients with mild or no fibrosis and is associated with the extent of steatosis [
44]. Brunt et al. reported that hepatic fibrosis in NAFLD patients was found in the pericellular space around the central vein and in the presinusoidal region in zone 3 in the early stage [
23]. The pericellular fibrosis in the early stage of NAFLD patients may lead to an elevated portal vascular resistance and result in a change of hepatic blood flow [
45]. Therefore, we considered that the hemodynamic changes occurred earlier in NAFLD relative to CHC.
In this study, there are two limitations, such as sampling error during liver biopsy and by permeation of Xe gas. Xe-CT cannot monitor the exact result in the patients with chronic lung disease (e.g., chronic obstructive pulmonary disease, lung cancer) and heart failure, because Xe gas is taken up by the lung via the respiratory tract. On the contrary, we believe that there are also many strong points of Xe-CT which objectively and repeatedly measure hepatic blood flow with reproducibility. Moreover, we have safely performed a Xe-CT for patients with acute or chronic renal failure because there are no complications associated with the contrast agent, such as allergic reactions and radiocontrast nephropathy.
Thus, in the present study, the difference between NAFLD and CHC was investigated based on TBF, fibrosis markers, and liver function. In conclusion, compared to C-LC, PVTBF decreased significantly in the Child-Pugh A stage of NASH-LC, indicating that portal hemodynamic changes could occur earlier in NASH-LC without impaired liver function. Therefore, patients with NASH should be monitored carefully for portal hypertensive complications in the early fibrosis stage.