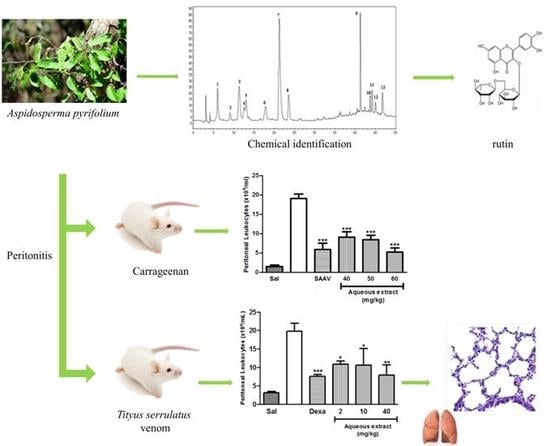
Aspidosperma pyrifolium Has Anti-Inflammatory Properties: An Experimental Study in Mice with Peritonitis Induced by Tityus serrulatus Venom or Carrageenan
Abstract
:1. Introduction
2. Results
2.1. High Performance Liquid Chromatography Coupled with Diode Array Detector (HPLC-DAD)
2.2. Liquid Chromatography Coupled with Mass Spectrometry with Diode Array Detection Analysis (LC-DAD-MS)
2.3. Cell Viability
2.4. Evaluation of Rutin and Aqueous Extract of the Leaves of Aspidosperma pyrifolium in Carrageenan-Induced Peritonitis Model
2.5. Effect of Dose and Time-Kinetics of Tityus serrulatus Venom-Induced Inflammation
2.6. Evaluation of Rutin and Aqueous Extract of the Leaves of Aspidosperma pyrifolium in Tityus serrulatus Venom-Induced Peritonitis Model
2.7. Histopathology Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Extraction
4.3. Qualitative High-Performance Liquid Chromatography Coupled with Diode Array (HPLC-DAD) Analysis
4.4. Liquid Chromatography Coupled with Mass Spectrometry with Diode Array Detection Analysis (LC-DAD-MS) Analyses
4.5. Animals
4.6. Venom and Antivenom
4.7. Cell Proliferation Assay
4.8. Carrageenan-Induced Peritonitis Model
4.9. Dose and Time-Kinetics of Tityus Serrulatus Venom-Induced Inflammation
4.10. Venom-Induced Peritonitis Model
4.11. Histological Analysis
4.12. Statistical Analyses
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mazzei de Dàvila, C.A.; Dàvila, D.F.; Donis, J.H.; de Bellabarba, G.A.; Villarreal, V.; Barboza, J.S. Sympathetic nervous system activation, antivenin administration and cardiovascular manifestations of scorpion envenomation. Toxicon 2002, 40, 1339–1346. [Google Scholar] [CrossRef]
- Fukuhara, Y.D.; Reis, M.L.; Dellalibera-Joviliano, R.; Cunha, F.Q.; Donadi, E.A. Increased plasma levels of IL-1 β, IL-6, IL-8, IL-10 and TNF-α in patients moderately or severely envenomed by Tityus serrulatus scorpion sting. Toxicon 2003, 41, 49–55. [Google Scholar] [CrossRef]
- Guerra, C.M.N.; Carvalho, L.F.A.; Colosimo, E.A.; Freire, H.B.M. Análise de variáveis relacionadas à evolução letal do escorpionismo em crianças e adolescentes no estado de Minas Gerais no período de 2001 a 2005. J. Pediatr. (Rio. J.) 2008, 84, 509–515. [Google Scholar] [CrossRef]
- Zuliani, J.P.; Freitas, T.A.; Conceição, I.M.; Kwasniewski, F.H. Tityus serrulatus venom increases vascular permeability in selected airway tissues in a mast cell-independent way. Exp. Toxicol. Pathol. 2013, 65, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Matos, I.M.; Rocha, O.A.; Leite, R.; Freire-Maia, L. Lung oedema induced by Tityus serrulatus scorpion venom in the rat. Comp. Biochem. Physiol. Part C Pharmacol. Toxicol. Endocrinol. 1997, 118, 143–148. [Google Scholar] [CrossRef]
- Oliveira, F.N.; Mortari, M.R.; Carneiro, F.P.; Guerrero-Vargas, J.A.; Santos, D.M.; Pimenta, A.M.C.; Schwartz, E.F. Another record of significant regional variation in toxicity of Tityus serrulatus venom in Brazil: A step towards understanding the possible role of sodium channel modulators. Toxicon 2013, 73, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Murthy, K.R.K. On scorpion envenoming syndrome: Problems of medical ethics and accountability in medical research in India. J. Venom. Anim. Toxins 2002, 8, 3–17. [Google Scholar] [CrossRef]
- Petricevich, V.L. Scorpion venom and the inflammatory response. Mediators Inflamm. 2010, 2010, 903295. [Google Scholar] [CrossRef] [PubMed]
- Venancio, E.J.; Portaro, F.C.V.; Kuniyoshi, A.K.; Carvalho, D.C.; Pidde-Queiroz, G.; Tambourgi, D.V. Enzymatic properties of venoms from Brazilian scorpions of Tityus genus and the neutralisation potential of therapeutical antivenoms. Toxicon 2013, 69, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, F.; Sampaio, S.V.; Garófalo, M.A.R.; Guimarães, L.F.L.; Giglio, J.R.; Arantes, E.C. Insulin-like effects of Bauhinia forficata aqueous extract upon Tityus serrulatus scorpion envenoming. J. Ethnopharmacol. 2004, 95, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, P.A.; Nipate, S.S.; Sonpetkar, J.M.; Salvi, N.C.; Waghmare, A.B.; Chaudhari, P.D. Anti-snake venom activities of ethanolic extract of fruits of Piper longum L. (Piperaceae) against Russell’s viper venom: Characterization of piperine as active principle. J. Ethnopharmacol. 2013, 147, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Warrell, D.A.; Williams, D.J.; Jensen, S.; Brown, N.; Calvete, J.J.; Harrison, R.A. The need for full integration of snakebite envenoming within a global strategy to combat the neglected tropical diseases: The way forward. PLoS Negl. Trop. Dis. 2013, 7, e2162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Félix-Silva, J.; Souza, T.; Menezes, Y.A.S.; Cabral, B.; Câmara, R.B.G.; Silva-Junior, A.A.; Rocha, H.A.O.; Rebecchi, I.M.M.; Zucolotto, S.M.; Fernandes-Pedrosa, M.F. Aqueous leaf extract of Jatropha gossypiifolia L. (Euphorbiaceae) inhibits enzymatic and biological actions of Bothrops jararaca snake venom. PLoS ONE 2014, 9, e104952. [Google Scholar] [CrossRef] [PubMed]
- Hutt, M.J.; Houghton, P.J. A survey from the literature of plants used to treat scorpion stings. J. Ethnopharmacol. 1998, 60, 97–110. [Google Scholar] [CrossRef]
- Lima, M.C.; Bitencourt, M.A.; Furtado, A.A.; Rocha, H.A.; Oliveira, R.M.; Silva-Júnior, A.A.; Tabosa Do Egito, E.S.; Tambourgi, D.V.; Zucolotto, S.M.; Fernandes-Pedrosa Mde, F. Ipomoea asarifolia neutralizes inflammation induced by Tityus serrulatus scorpion venom. J. Ethnopharmacol. 2014, 153, 890–895. [Google Scholar] [CrossRef] [PubMed]
- Bitencourt, M.A.O.; Lima, M.C.J.S.; Torres-Rêgo, M.; Fernandes, J.M.; Silva-Júnior, A.A.; Tambourgi, D.V.; Zucolotto, S.M.; Fernandes-Pedrosa, M.F. Neutralizing effects of Mimosa tenuiflora extracts against inflammation caused by Tityus serrulatus scorpion venom. BioMed Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Araújo, J.X.; Antheaume, C.; Trindade, R.C.P.; Schmitt, M.; Bourguignon, J.-J.; Sant’Ana, A.E.G. Isolation and characterisation of the monoterpenoid indole alkaloids of Aspidosperma pyrifolium. Phytochem. Rev. 2007, 6, 183–188. [Google Scholar] [CrossRef]
- De Souza Lima, M.C.; Soto-Blanco, B. Poisoning in goats by Aspidosperma pyrifolium Mart.: Biological and cytotoxic effects. Toxicon 2010, 55, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Koch, I.; Rapini, A.; Simões, A.O.; Kinoshita, L.S.; Spina, A.P.; Castello, A.C.D. Apocynaceae in Lista de Espécies da Flora do Brasil. Jardim Botânico do Rio de Janeiro. Available online: http://floradobrasil.jbrj.gov.br/jabot/floradobrasil/FB15551 (accessed on 6 June 2017).
- Kaur, M. Evaluation of Anti-inflammatory activity of Ethanol Extract of Bark of Aspidosperma quebraco-blanco. Res. J. Pharm. Technol. 2013, 6, 575–576. [Google Scholar]
- Singh, N.; Jain, N.K.; Kannojia, P.; Garud, N.; Pathak, K.A.; Mehta, S.C. Scholars Research Library. Der Pharma Chem. 2010, 2, 95–100. [Google Scholar]
- Aquino, A.B.; Cavalcante-Silva, L.H.A.; Matta, C.B.B.; Epifânio, W.A.D.N.; Aquino, P.G.V.; Santana, A.E.G.; Alexandre-Moreira, M.S.; De Araújo-Júnior, J.X. The antinociceptive and anti-inflammatory activities of Aspidosperma tomentosum (Apocynaceae). Sci. World J. 2013, 2013, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Harborne, J.B.; Baxter, H. The Handbook of Natural Flavonoids; John Wiley: Chichester, UK, 1999; p. 1800. ISBN 978-0-471-95893-2. [Google Scholar]
- Middleton, E.; Kandaswami, C.; Theoharides, T.C. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000, 52, 673–751. [Google Scholar] [PubMed]
- Pathak, D.; Pathak, K.; Singla, A.K. Flavonoids as medicinal agents: Recent advances. Fitoterapia 1991, 557, 371–389. [Google Scholar]
- Marcarini, J.C.; Tsuboy, M.F.S.; Luiz, R.C.; Ribeiro, L.R.; Hoffmann-Campo, C.B.; Mantovani, M.S. Investigation of cytotoxic, apoptosis-inducing, genotoxic and protective effects of the flavonoid rutin in HTC hepatic cells. Exp. Toxicol. Pathol. 2011, 63, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Torres-Rêgo, M.; Furtado, A.A.; Bitencourt, M.A.; Lima, M.C.; Andrade, R.C.; Azevedo, E.P.; Soares Tda, C.; Tomaz, J.C.; Lopes, N.P.; da Silva-Júnior, A.A.; et al. Anti-inflammatory activity of aqueous extract and bioactive compounds identified from the fruits of Hancornia speciosa Gomes (Apocynaceae). BMC Complement. Altern. Med. 2016, 16, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Furtado, A.A.; Torres-Rêgo, M.; Lima, M.C.J.S.; Bitencourt, M.A.O.; Estrela, A.B.; Souza da Silva, N.; da Silva Siqueira, E.M.; Tomaz, J.C.; Lopes, N.P.; Silva-Júnior, A.A.; et al. Aqueous extract from Ipomoea asarifolia (Convolvulaceae) leaves and its phenolic compounds have anti-inflammatory activity in murine models of edema, peritonitis and air-pouch inflammation. J. Ethnopharmacol. 2016, 192, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Naegele, E. Determination of Chlorogenic Acid in Coffee Products According to DIN 10767; Food Testing and Agriculture-Food Authenticity; Agilent Technologies: Waldbronn, Germany, 2016; pp. 1–8. [Google Scholar]
- Merfort, I.; Wendisch, D. Flavonoidglycoside acids from flowers of Arnica montana und Arnica chamissonis. Planta Med. 1987, 53, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Craveiro, A.A.; Matos, F.J.A.; Serur, L.M. Alkaloids of Aspidosperma pyrifolium. Phytochemistry 1983, 22, 1526–1528. [Google Scholar] [CrossRef]
- Mousinho, K.C.; Oliveira Cde, C.; Ferreira, J.R.; Carvalho, A.A.; Magalhães, H.I.; Bezerra, D.P.; Alves, A.P.; Costa-Lotufo, L.V.; Pessoa, C.; De Matos, M.P.; et al. Antitumor effect of laticifer proteins of Himatanthus drasticus (Mart.) Plumel—Apocynaceae. J. Ethnopharmacol. 2011, 137, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Longhi-Balbinot, D.T.; Lanznaster, D.; Baggio, C.H.; Silva, M.D.; Cabrera, C.H.; Facundo, V.A.; Santos, A.R. Anti-inflammatory effect of triterpene 3β, 6β, 16β-trihydroxylup-20(29)-ene obtained from Combretum leprosum Mart & Eich in mice. J. Ethnopharmacol. 2012, 142, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Luster, A.D.; Alon, R.; von Andrian, U.H. Immune cell migration in inflammation: Present and future therapeutic targets. Nat. Immunol. 2005, 6, 1182–1190. [Google Scholar] [CrossRef] [PubMed]
- Spiller, F.; Alves, M.K.; Vieira, S.M.; Carvalho, T.A.; Leite, C.E.; Lunardelli, A.; Poloni, J.A.; Cunha, F.Q.; de Oliveira, J.R. Anti-inflammatory effects of red pepper (Capsicum baccatum) on carrageenan- and antigen-induced inflammation. J. Pharm. Pharmacol. 2008, 60, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Syam, S.; Bustamam, A.; Abdullah, R.; Sukari, M.A.; Hashim, N.M.; Mohan, S.; Looi, C.Y.; Wong, W.F.; Yahayu, M.A.; Abdelwahab, S.I. β Mangostin suppress LPS-induced inflammatory response in RAW 264.7 macrophages in vitro and carrageenan-induced peritonitis in vivo. J. Ethnopharmacol. 2014, 153, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Dawson, J.; Sedgwick, A.D.; Edwards, J.C.; Lees, P. A comparative study of the cellular, exudative and histological responses to carrageenan, dextran and zymosan in the mouse. Int. J. Tissue React. 1991, 13, 171–185. [Google Scholar] [PubMed]
- Lee, C.W.; Park, S.M.; Kim, Y.S.; Jegal, K.H.; Lee, J.R.; Cho, I.J.; Ku, S.K.; Lee, J.Y.; Ahn, Y.T.; Son, Y.; et al. Biomolecular evidence of anti-inflammatory effects by Clematis mandshurica Ruprecht root extract in rodent cells. J. Ethnopharmacol. 2014, 155, 1141–1155. [Google Scholar] [CrossRef] [PubMed]
- Erharuyi, O.; Falodun, A.; Langer, P. Medicinal uses, phytochemistry and pharmacology of Picralima nitida (Apocynaceae) in tropical diseases: A review. Asian Pac. J. Trop. Med. 2014, 7, 1–8. [Google Scholar] [CrossRef]
- Bucaretchi, F.; Fernandes, L.C.; Fernandes, C.B.; Branco, M.M.; Prado, C.C.; Vieira, R.J.; De Capitani, E.M.; Hyslop, S. Clinical consequences of Tityus bahiensis and Tityus serrulatus scorpion stings in the region of Campinas, southeastern Brazil. Toxicon 2014, 89, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, M.M.; Pereira, M.E.; Amaral, C.F.; Rezende, N.A.; Campolina, D.; Bucaretchi, F.; Gazzinelli, R.T.; Cunha-Melo, J.R. Serum levels of cytokines in patients envenomed by Tityus serrulatus scorpion sting. Toxicon 1999, 37, 1155–1164. [Google Scholar] [CrossRef]
- Harborne, J.B.; Williams, C.A. Advances in flavonoids research since 1992. Phytochemistry 2000, 55, 481–504. [Google Scholar] [CrossRef]
- Almeida-Lima, J.; Costa, L.S.; Silva, N.B.; Melo-Silveira, R.F.; Silva, F.V.; Cansanção Felipe, M.B.; Batistuzzo Medeiros, S.R.; Leite, E.L.; Rocha, H.A. Evaluating the possible genotoxic, mutagenic and tumor cell proliferation-inhibition effects of a non-anticoagulant, but antithrombotic algal heterofucan. J. Appl. Toxicol. 2010, 30, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Pessini, A.C.; De Souza, A.M.; Faccioli, L.H.; Gregório, Z.M.; Arantes, E.C. Time course of acute-phase response induced by Tityus serrulatus venom and TsTX-I in mice. Int. Immunopharmacol. 2003, 3, 765–774. [Google Scholar] [CrossRef]






| Groups | Dose (mg/kg) | Peritoneal Cell Migration (×106 cells/mL) | Inhibition (%) | |
|---|---|---|---|---|
| Carrageenan 1% | saline | - | 19.80 ± 2.189 | |
| dexa | 0.5 | 7.500 ± 0.5701 *** | 62.1 | |
| AE | 2 | 10.80 ± 0.9028 * | 45.4 | |
| AE | 10 | 10.60 ± 4.529 * | 46.4 | |
| AE | 40 | 7.900 ± 2.731 ** | 60.1 | |
| Carrageenan 1% | saline | - | 23.70 ± 0.752 | |
| dexa | 0.5 | 7.500 ± 0.5701 *** | 62.1 | |
| rutin | 2 | 7.000 ± 2.133 *** | 70.4 | |
| rutin | 2.5 | 11.10 ± 1.470 *** | 53.1 | |
| rutin | 5 | 9.000 ± 0.6892 *** | 62 | |
| Groups | Dose (mg/kg) | Peritoneal Cell Migration (×106 cells/mL) | Inhibition (%) | |
|---|---|---|---|---|
| T. serrulatus venom (7.5 μg/animal) | saline | - | 19.10 ± 1.145 | |
| SAAV | - | 5.875 ± 1.638 *** | 69.6 | |
| AE | 40 | 9.083 ± 1.393 *** | 52.8 | |
| AE | 50 | 8.417 ± 1.207 *** | 56.0 | |
| AE | 60 | 5.250 ± 1.055 *** | 72.7 | |
| T. serrulatus venom (7.5 μg/animal) | saline | - | 19.10 ± 1.145 | |
| SAAV | - | 5.875 ± 1.638 *** | 69.6 | |
| rutin | 2 | 9.300 ± 1.758 *** | 51.3 | |
| rutin | 2.5 | 5.700 ± 0.5612 *** | 70.1 | |
| rutin | 5 | 6.400 ± 1.198 *** | 66.4 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souza Lima, M.C.J.d.; Oliveira Bitencourt, M.A.; Furtado, A.A.; Torres-Rêgo, M.; Siqueira, E.M.d.S.; Oliveira, R.M.; Oliveira Rocha, H.A.; Ferreira Rocha, K.B.; Silva-Júnior, A.A.d.; Zucolotto, S.M.; et al. Aspidosperma pyrifolium Has Anti-Inflammatory Properties: An Experimental Study in Mice with Peritonitis Induced by Tityus serrulatus Venom or Carrageenan. Int. J. Mol. Sci. 2017, 18, 2248. https://doi.org/10.3390/ijms18112248
Souza Lima MCJd, Oliveira Bitencourt MA, Furtado AA, Torres-Rêgo M, Siqueira EMdS, Oliveira RM, Oliveira Rocha HA, Ferreira Rocha KB, Silva-Júnior AAd, Zucolotto SM, et al. Aspidosperma pyrifolium Has Anti-Inflammatory Properties: An Experimental Study in Mice with Peritonitis Induced by Tityus serrulatus Venom or Carrageenan. International Journal of Molecular Sciences. 2017; 18(11):2248. https://doi.org/10.3390/ijms18112248
Chicago/Turabian StyleSouza Lima, Maíra Conceição Jerônimo de, Mariana Angélica Oliveira Bitencourt, Allanny Alves Furtado, Manoela Torres-Rêgo, Emerson Michell da Silva Siqueira, Ruth Medeiros Oliveira, Hugo Alexandre Oliveira Rocha, Keyla Borges Ferreira Rocha, Arnóbio Antônio da Silva-Júnior, Silvana Maria Zucolotto, and et al. 2017. "Aspidosperma pyrifolium Has Anti-Inflammatory Properties: An Experimental Study in Mice with Peritonitis Induced by Tityus serrulatus Venom or Carrageenan" International Journal of Molecular Sciences 18, no. 11: 2248. https://doi.org/10.3390/ijms18112248
APA StyleSouza Lima, M. C. J. d., Oliveira Bitencourt, M. A., Furtado, A. A., Torres-Rêgo, M., Siqueira, E. M. d. S., Oliveira, R. M., Oliveira Rocha, H. A., Ferreira Rocha, K. B., Silva-Júnior, A. A. d., Zucolotto, S. M., & Fernandes-Pedrosa, M. D. F. (2017). Aspidosperma pyrifolium Has Anti-Inflammatory Properties: An Experimental Study in Mice with Peritonitis Induced by Tityus serrulatus Venom or Carrageenan. International Journal of Molecular Sciences, 18(11), 2248. https://doi.org/10.3390/ijms18112248