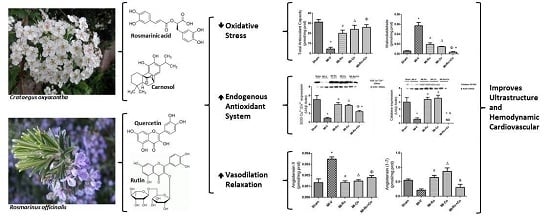
Extracts of Crataegus oxyacantha and Rosmarinus officinalis Attenuate Ischemic Myocardial Damage by Decreasing Oxidative Stress and Regulating the Production of Cardiac Vasoactive Agents
Abstract
:1. Introduction
2. Results
2.1. Qualitative and Quantitative Analysis of the Extracts from Ro and Co
2.2. Effect of Ro, Co, and Their Combination in Myocardial Oxidative Stress
2.3. Effect of Natural Compound Administration on Vasoconstrictor Agents Production
2.4. Effect of Natural Compound Administration on Ang-(1–7), Bradykinin, and the eNOS Pathway
2.5. Ultraestructural Analysis
2.6. Effect of Ro, Co, and Their Combination (Ro+Co) on Cardiac Function
3. Discussion
3.1. Effect of Ro, Co, and Their Combination in Myocardial Oxidative Stress
3.2. Effect of Natural Compound Administration on Vasoconstrictor Agents
3.3. Effect of Ro and Co, Compounds Administration on Vasodilators
3.4. Effect of Ro and Co Administration on Myocardial Structure and Function
4. Materials and Methods
4.1. Preparation of Extracts of Crataegus oxyacantha and Rosmarinus officinalis
4.2. High-Performance Liquid Chromatography (HPLC) Analysis
4.3. Animals
4.4. Myocardial Infarction (MI)
4.5. Isolated Heart Perfused by the Langendorff Method
4.6. Structural Analysis by Electron Microscopy
4.7. Measurement of Total Antioxidant Capacity
4.8. Determination of Oxidative Stress Markers, and Vasoconstrictor and Vasorelaxant Agents
4.9. Western Blotting of SOD-Cu2+/Zn2+, SOD-Mn2+, Catalase, NOX4, p22phox and eNOS
4.10. Data Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hearse, D.J. Myocardial protection during ischemia and reperfusion. Mol. Cell. Biochem. 1998, 186, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Ryter, S.W.; Kim, H.P.; Hoetzel, A.; Park, J.W.; Nakahira, K.; Wang, X.; Choi, A.M. Mechanism of cell death in oxidative stress. Antioxid. Redox Signal. 2007, 9, 49–89. [Google Scholar] [CrossRef] [PubMed]
- Droge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T. Signal transduction by reactive oxygen species in non phagocytic cells. J. Leukoc. Biol. 1999, 65, 337–340. [Google Scholar] [PubMed]
- Sorescu, D.; Griendling, K.K. Reactive oxygen species, mitochondria, and NAD(P)H oxidases in the development and progression of heart failure. Congest. Heart Fail. 2002, 8, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Guo, H.; Lou, H. Grape seed polyphenols protect cardiac cells from apoptosis via induction of endogenous antioxidant enzymes. J. Agric. Food Chem. 2007, 55, 1695–1701. [Google Scholar] [CrossRef] [PubMed]
- Zweier, J.; Flaherty, J.T.; Weisfeldt, M.L. Direct measurement of free radical generation following reperfusion of ischemic myocardium. Proc. Natl. Acad. Sci. USA 1987, 84, 1404–1407. [Google Scholar] [CrossRef] [PubMed]
- Stewart, D.; Kubac, G.; Costello, K.B.; Cernacek, P. Increased plasma endothelin-1 in the early hours of acute myocardial infarction. J. Am. Coll. Cardiol. 1991, 18, 38–43. [Google Scholar] [CrossRef]
- Yamagishi, H.; Kim, S.; Takeuchi, K.; Takeda, T. Contribution of cardiac renin-angiotensin system to ventricular remodelling in myocardial-infacted rats. J. Mol. Cell. Cardiol. 1993, 25, 1369–1380. [Google Scholar] [CrossRef] [PubMed]
- Browe, D.; Baumgarten, C.M. Angiotensin II (AT1) receptors and NADPH oxidase regulates Cl-current elicited by B1 integrin stretch bin rabbit ventricular myocytes. J. Gen. Physiol. 2004, 124, 273–287. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.M.; Randhawa, P.K.; Singh, N.; Jaggi, A.S. Bradykinin in ischemic conditioning-induced tissue protection: Evidences and possible mechanisms. Eur. J. Pharmacol. 2015, 768, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Ferrario, C.; Chapell, M.; Tallant, E.A.; Brosnihan, K.B.; Diz, D.I. Counterregulatory actions of angiotensin (1–7). Hypertension 1997, 30, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Muller, A.; Linke, W.; Klaus, W. Crataegus extract blocks potassium currents in guinea pig ventricular cardiac myocytes. Planta Med. 1999, 65, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Rakotoarison, D.; Gressier, B.; Trotin, F.; Brunet, C.; Dine, T.; Luyckx, M.; Vasseur, J.; Cazin, M.; Cazin, J.C.; Pinkas, M. Antioxidant activities of phenolic extracts from flowers, in vitro callus and cell suspension cultures of crataegus monogyna. Pharmazie 1997, 52, 60–64. [Google Scholar] [PubMed]
- Jalakshmi, R.; Niranjali Devaraj, S. Cardioprotective effect of tincture of crataegus on isoproterenol-induced myocardial infarction in rats. J. Pharm. Pharmacol. 2004, 56, 921–926. [Google Scholar] [CrossRef] [PubMed]
- Long, S.; Care, R.A.; Crofoot, K.M.; Proteau, P.J.; Filtz, T.M. Effect of hawthorn (Crataegus oxycantha) crude extract and chromatographic fractions on multiple activities in a culture cardiomyocyte assay. Phytomedicine 2006, 13, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Elango, C.; Jayachandaran, K.S.; Niranjali Devaraj, S. Hawthorn extrat reduces infarct volume and improves neurological score by reducing oxidative stress in rat brain following middle cerebral artery oclussion. Int. J. Dev. Neurosci. 2009, 27, 799–803. [Google Scholar] [CrossRef] [PubMed]
- Haraguchi, H.; Saito, T.; Okamura, N.; Yagi, A. Inhibition of lipid peroxidation and superoxide generation by dipertenoids from Rosmarinus Officinalis. Planta Med. 1995, 61, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Frankel, E.N.; Huang, S.; Aeschbach, R.; Prior, E. Evaluation of antioxidant activity of Rosmary extracts carnosol and carnosic acid in bulk vegetable oil and fish oil and their emulsions. J. Sci. Food Agric. 1996, 72, 201–208. [Google Scholar] [CrossRef]
- Aruoma, O.I.; Halliwell, B.; Aescbach, R.; Löligers, J. Antioxidant and pro-oxidant properties of active Rosmary constituents: Carnosol and carnosic acid. Xenobiotica 1992, 22, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Maksimovic, Z.; Malenic, D.; Kovacevic, N. Polyphenol contents and antioxidant activity of Maydis stigma extracts. Bioresour. Technol. 2005, 96, 873–877. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, R.; Ceconi, C.; Curello, S.; Visioli, O. Myocardial damage during ischemia and reperfusion. Eur. Heart J. 1993, 14, 25–30. [Google Scholar] [PubMed]
- Swaminathan, J.K.; Khan, M.; Mohan, I.K.; Selvendiran, K.; Niranjali Devaraj, S.; Rivera, B.K.; Kuppusamy, P. Cardioprotective propiertes of Crataegus Oxycantha against ischemia-reperfusion injury. Phytomedicine 2010, 17, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, R.; Alvarado, J.L.; Presno, M.; Pérez-Veyna, O.; Serrano, C.J.; Yahuaca, P. Oxidative stress modulation by Rosmarinus officinalis in CCL4-induced liver Cirrhosis. Phytother. Res. 2010, 24, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Woo, Y.; Zhang, J.C.; Vijayasarathy, C.; Zwacka, R.M.; Englehardt, J.F.; Gardner, T.J.; Sweeeney, H.L. Recombinant adenovirus mediated cardiac gene transfer of superoxide dismutase and catalase attenuates postischemic contractile dysfunction. Circulation 1998, 98, 2281–2289. [Google Scholar]
- Hill, M.; Singal, P.K. Antioxidant and oxidative stress changes during heart failure subsequent to myocardial infarction in rats. Am. J. Pathol. 1996, 148, 291–300. [Google Scholar] [PubMed]
- Wang, Y.; Yang, Q.; Yan, J.T.; Zhao, C.; Cianflone, K.; Wang, D.W. Effects of bezafibrate on the expression of endothelial nitric oxide synthase gene and its mechanism in cultured bovine endothelial cells. Atherosclerosis 2006, 187, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Pryor, W.; Squadrito, G.L. The chemistry of peroxynitrite: A product from the reaction of nitric oxide with superoxide. Am. J. Physiol. 1995, 268, L699–L722. [Google Scholar] [PubMed]
- Chen, Z.; Siu, B.; Ho, Y.S.; Vincent, R.; Chua, C.C.; Hamdy, R.C.; Chua, B.H. Overexpression of MnSOD protecs against myocardial ischemia/reperfusion injury in transgenic mice. Mol. Cell. Cardiol. 1998, 30, 2281–2289. [Google Scholar] [CrossRef] [PubMed]
- Iwata, T.; Konno, N.; Yanagishita, T.; Katagiri, T. The role of catalase as a defence mechanism against reperfusion injury. J. Mol. Cell. 1993, 25, S104. [Google Scholar]
- Zou, X.; Ratti, B.A.; O’Brien, J.G.; Lautenschlager, S.O.; Gius, D.R.; Bonini, M.G.; Zhu, Y. Manganese superoxide dismutase (SOD2): Is there a center in the universe of mitochondrial redox signaling? J. Bioenerg. Biomembr. 2017, 14, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hart, P.C.; Mao, M.; de Abreu, A.L.; Ansenberger-Fricano, K.; Ekoue, D.N.; Ganini, D.; Kajdacsy-Balla, A.; Diamond, A.M.; Minshall, R.D.; Consolaro, M.E.; et al. MnSOD upregulation sustains theWarburg effect via mitochondrial ROS and AMPK-dependent signalling in cancer. Nat. Commun. 2015, 6, 653. [Google Scholar] [CrossRef] [PubMed]
- Corder, R.; Warburton, R.C.; Khan, N.Q.; Brown, R.E.; Wood, E.G.; Lees, D.M. The procyanidin-induced pseudo laminar shear stress response: A new concept for the reversal of endothelial dysfunction. Clin. Sci. 2004, 107, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Alici, O.; Kavakli, H.S.; Koca, C.; Altintas, N.D.; Aydin, M.; Alici, S. Value of caffeic acid phenethyl ester pretreatment in experimental sepsis model in rats. Mediat. Inflamm. 2015, 2015, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ibarra-Lara, L.; Hong, E.; Soria-Castro, E.; Torres-Narvaéz, J.C.; Pérez-Severiano, F.; del Valle-Mondragón, L.; Cervantes-Pérez, L.G.; Ramirez-Ortega, M.; Pastelín-Hernández, G.S.; Sánchez-Mendoza, A. Clofibrate PPARa activation reduced oxidative stress and improves ultrastructure and ventricular hemodynamics in no-flow myocardial ischemia. J. Cardiovasc. Pharmacol. 2012, 60, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Ibarra-Lara, L.; Sánchez-Aguilar, M.; Sánchez-Mendoza, A.; del Valle-Mondragón, L.; Soria-Castro, E.; Carreón-Torres, E.; Díaz-Díaz, E.; Vázquez-Meza, H.; Guarner-Lans, V.; Rubio-Ruiz, M.E. Fenofibrate therapy restores antioxidant protection and improves myocardial insulin resistance in a rat model of metabolic syndrome and myocardial ischemia: The role of Angiotensin II. Molecules 2016, 22, 31. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Tian, J.; Xu, Y.; Li, C.; Meng, X.; Fu, F. Protective effect of RA on myocardial infarction-induced cardiac fibrosis via AT1R/p38 MAPK pathway signaling and modulation of the ACE2/ACE ratio. J. Agric. Food Chem. 2016, 64, 6716–6722. [Google Scholar] [CrossRef] [PubMed]
- Orhan, I.E. Phytochemical and pharmacological activity profile of Crataegus oxycantha L. (hawthorn)—A cardiotonic herb. Curr. Med. Chem. 2017, 24. [Google Scholar] [CrossRef]
- Oudot, A.; Vargely, C.; Ecarnot-Laubriet, A.; Rochette, L. Angiotensin II activates NADPH oxidase in isolated rat hearts subject ti ischemia-reperfusion. Eur. J. Pharmacol. 2003, 462, 145–154. [Google Scholar] [CrossRef]
- Loot, A.; Roks, A.J.; Henning, R.H.; Tio, R.H. Angiotensin-(1–7) attenuates the development of heart failure after myocardial infarction in rats. Circulation 2002, 105, 1548–1550. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Guo, R.X.; Ma, H.; Wang, L.C.; Chen, Z.H.; Yang, C.T.; Feng, J.Q. Effects of angiotensin-(1–7) on oxidative stress and functional changes of isolated rat hearts induced by ischemia-reperfusion. Nan Fang Yi Ke Da Xue Bao 2008, 28, 1345–1348. [Google Scholar]
- Benter, I.; Yousif, M.H.; Dhaunsi, G.S.; Kaur, J.; Chappell, M.C.; Diz, D.I. Angiotensin-(1–7) prevents activation of NADPH oxidase and renal vascular dysfunction in diabetic hypertensive rats. Am. J. Nephrol. 2008, 28, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Giraldez, R.; Panda, A.; Xia, Y.; Sanders, S.; Zweier, J. Decreased nitric-oxide synthase activity causes impaired endothelium dependent relaxation in the postischemic heart. J. Biol. Chem. 1997, 272, 21420–21426. [Google Scholar] [CrossRef] [PubMed]
- Oidor-Chan, V.H.; Hong, E.; Pérez-Severiano, F.; Montes, S.; Torres-Narváez, J.C.; Del Valle-Mondragón, L.; Pastelín-Hernández, G.; Sánchez-Mendoza, A. Fenofibrate plus metformin produces cardioprotection in a Type 2 diabetes and acute myocardial infarction model. PPAR Res. 2016, 2016, 8237264. [Google Scholar] [CrossRef] [PubMed]
- Vasquez-Vivar, J.; Kalynaraman, B.; Martasek, P.; Hogg, N.; Masters, B.S.; Karoui, H.; Tordo, P.; Pritchard, K.A., Jr. Superoxide generation by endothelial nitric oxide synthesis: The influence of cofactors. Proc. Natl. Acad. Sci. USA 1998, 95, 9220–9225. [Google Scholar] [CrossRef] [PubMed]
- Bulhak, A.A.; Sjoquist, P.O.; Xu, C.B.; Edvinsson, L.; Pernow, J. Protection against myocardial ischaemia/reperfusion injury by PPAR-α activation is related to production of nitric oxide and endothelin-1. Basic Res. Cardiol. 2006, 101, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.; Canty, J.M. Modulation of coronary autoregulatory response by nitric oxide. Evidence for flow-dependent resistance adjustments in conscious dogs. Circ. Res. 1992, 73, 232–240. [Google Scholar] [CrossRef]
- Offstad, P.; Naess, P.A.; Aksnes, G.; Tønnessen, T.; Ilebekk, A.; Kirkebøen, K.A. Nitric oxide regulates coronary blood flow at various coronary arterial pressures in intact porcine hearts. Acta Physiol. 1995, 154, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Tamargo, J.; Caballero, R.; Gomez, R.; Núñez, L.; Vaquero, M.; Delpón, E. Efectos del oxido nitrico sobre la función cardíaca. Rev. Esp. Cardiol. 2006, 6, 3–20. [Google Scholar] [CrossRef]
- Kaul, N.; Siveski-Iliskovic, N.; Hill, M.; Slezak, J.; Singal, P.K. Free radicals and the heart. J. Pharmacol. Toxicol. Methods 1993, 30, 55–67. [Google Scholar] [CrossRef]
- Misra, M.K.; Sarwat, M.; Bhakuni, P.; Tuteja, R.; Tuteja, N. Oxidative stress and ischemic myocardial syndromes. Med. Sci. Monit. 2009, 15, RA209–RA219. [Google Scholar] [PubMed]
- Ruiz Meana, M.; García-Dorado, D.I. Fisiopatologia del daño miocardico por isquemia-reperfusión: Nuevas oportunidades terapeúticas en el infarto agudo al miocardio. Rev. Esp. Cardiol. 2009, 62, 199–209. [Google Scholar] [CrossRef]
- Hertelendi, Z.; Tóth, A.; Borbély, A.; Galajda, Z.; van der Velden, J.; Stienen, G.J.; Edes, I. Oxidation of myofilament protein sulfhydryl groups reduces the contractile force and its Ca2+ sensitivity in human cardiomyocytes. Antioxid. Redox Signal. 2008, 10, 1175–1184. [Google Scholar] [CrossRef] [PubMed]
- Brennan, J.P.; Bardswell, S.C.; Burgoyne, J.R.; Fuller, W.; Schröder, E.; Wait, R.; Begum, S.; Kentish, J.C.; Eaton, P. Oxidant-induced activation of type I protein kinase A is mediated by RI subunit interprotein disulfide bond formation. J. Biol. Chem. 2006, 281, 21827–21836. [Google Scholar] [CrossRef] [PubMed]
- Murino, R.B.P.; Portugal, S.P.; de Freitas, G.A.; Henrique, F.A.A.; Okoshi, K.; Chiuso-Minicucci, F.; Azevedo, P.S.; Mamed, Z.L.A.; Ferreira, M.M.; Wang, X.-D.; et al. Rosemary supplementation (Rosmarinus oficinallis L.) attenuates cardiac remodeling after myocardial infarction in rats. PLoS ONE 2017, 12, e0177521. [Google Scholar] [CrossRef]
- Veveris, M.; Koch, E.; Chatterjee, S.S. Crataegus special extract WS 1442 improves cardiac function and reduces infarct size in a rat model of prolonged coronary ischemia and reperfusion. Life Sci. 2004, 1945–1955. [Google Scholar] [CrossRef] [PubMed]
- Goldhaber, J.; Ji, S.; Lamp, S.T.; Weiss, J.N. Effect of exogenous free radicals in electromechanical function and metabolism in isolated rebbit and guine pig ventricle. Implications for ischemia and reperfusion injury. J. Clin. Investig. 1989, 83, 1800–1809. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Lee, M.H.; Ho, C.T.; Chang, S.S. Elucidation of the chemical structure of natural antioxidants isolated from rosmary. J. Am. Oil Chem. Soc. 1982, 59, 339–345. [Google Scholar] [CrossRef]
- Hussain, M.H. Fast high performance liquid chromatography and ultraviolet method for determination of phenolic antioxidants in fresh Rosemary leaves. J. Nat. Sci. Res. 2015, 5, 89–92. [Google Scholar]
- Zhang, Z.; Chang, Q.; Zhu, M.; Huang, Y.; Ho, W.K.; Chen, Z. Characterization of antioxidants present in hawthorn fruits. J. Nutr. Biochem. 2001, 12. [Google Scholar] [CrossRef]
- Ibarra-Lara, L.; del Valle-Mondragón, L.; Soria-Castro, E.; Torres-Narváez, J.C.; Pérez-Severiano, F.; Sánchez-Aguilar, M.; Ramírez-Ortega, M.; Cervantes-Pérez, L.G.; Pastelín-Hernández, G.S.; Oidor-Chan, V.H.; et al. Peroxisome proliferator-activated receptor-α stimulation by clofibrate favors an antioxidant and vasodilator environment in a stressed left ventricle. Pharmacol. Rep. 2016, 68, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Kumarathasan, P.; Goegan, P.; Vincen, R. An automated high-performance liquid chromatography fluorescence method for the analyses of endothelins in plasma samples. Anal. Biochem. 2001, 299, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Kvasnicová, V.; Samcová, E.; Jursová, A.; Jelínek, I. Determination of 8-hydroxy-2′-deoxyguanosine in untreated urine by capillary electrophoresis with UV detection. J. Chromatogr. A 2003, 985, 513–517. [Google Scholar] [CrossRef]
- Tůma, P.; Samcová, E.; Kvasnicová, V. Improved detection limit for a direct determination of 8-hydroxy-2′-deoxyguanosine in untreated urine samples by capillary electrophoresis with optical detection. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2004, 813, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Ibarra-Lara, L.; Sánchez-Aguilar, M.; Hong, E.; del Valle-Mondragón, L.; Soria-Castro, E.; Pérez-Severiano, F.; Torres-Narváez, J.C.; Ramírez-Ortega, M.; Pastelín-Hernández, G.S.; Cervantes-Pérez, L.G.; et al. PPARα stimulation modulates myocardial ischemia-induced activation of renin-angiotensin system. J. Cardiovasc. Pharmacol. 2015, 65, 430–437. [Google Scholar] [CrossRef] [PubMed]







 ); band I (→) and * showed swealling mitochondria. Magnification 12,000× for sarcomere (a–e), and the scale bar represents 500 nm; 20,000× for mitochondria and nuclei, and the scale bar represents 2 µm (f–o).
); band I (→) and * showed swealling mitochondria. Magnification 12,000× for sarcomere (a–e), and the scale bar represents 500 nm; 20,000× for mitochondria and nuclei, and the scale bar represents 2 µm (f–o).
 ); band I (→) and * showed swealling mitochondria. Magnification 12,000× for sarcomere (a–e), and the scale bar represents 500 nm; 20,000× for mitochondria and nuclei, and the scale bar represents 2 µm (f–o).
); band I (→) and * showed swealling mitochondria. Magnification 12,000× for sarcomere (a–e), and the scale bar represents 500 nm; 20,000× for mitochondria and nuclei, and the scale bar represents 2 µm (f–o).
| Extract | Compound | Migration Time (min) | Concentration ± SD (ng/mL) |
|---|---|---|---|
| Medeeha Method 280 nm | |||
| Rosmarinus officinalis | Rosmarinic acid | 6.112 | 5.214 ± 0.103 |
| Carnosol | 8.193 | 4.758 ± 0.086 | |
| Zhang Method 360 nm | |||
| Crataegus oxyacantha | Rutin | 24.075 | 6.265 ± 0.093 |
| Quercetin | 56.708 | 11.041 ± 0.118 | |
| Sham | MI-V | MI-Ro | MI-Co | MI-Ro+Co | |
|---|---|---|---|---|---|
| Left ventricular pressure (mmHg) | 74.0 ± 1.3 | 56.8 ± 3.8* | 69.2 ± 2.7 | 74.0 ± 1.3 Δ | 70.0 ± 1.6 |
| Coronary perfusion pressure (mmHg) | 66.6 ± 5.4 | 88.0 ± 4.6 | 70.2 ± 4.6 | 73.8 ± 5.6 | 76.0 ± 8.7 |
| Cardiac mechanical work (beats/min × mmHg) | 24,808.0 ± 482.4 | 19,085.0 ± 1281.2 * | 23,251.0 ± 922.6 | 24,864.0 ± 438.1 Δ | 23,520.0 ± 541.8 |
| Coronary vascular resistance (mmHg/mL/min) | 6.7 ± 0.5 | 8.8 ± 0.5 | 7.0 ± 0.5 | 7.4 ± 0.6 | 7.6 ± 0.9 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuevas-Durán, R.E.; Medrano-Rodríguez, J.C.; Sánchez-Aguilar, M.; Soria-Castro, E.; Rubio-Ruíz, M.E.; Del Valle-Mondragón, L.; Sánchez-Mendoza, A.; Torres-Narvaéz, J.C.; Pastelín-Hernández, G.; Ibarra-Lara, L. Extracts of Crataegus oxyacantha and Rosmarinus officinalis Attenuate Ischemic Myocardial Damage by Decreasing Oxidative Stress and Regulating the Production of Cardiac Vasoactive Agents. Int. J. Mol. Sci. 2017, 18, 2412. https://doi.org/10.3390/ijms18112412
Cuevas-Durán RE, Medrano-Rodríguez JC, Sánchez-Aguilar M, Soria-Castro E, Rubio-Ruíz ME, Del Valle-Mondragón L, Sánchez-Mendoza A, Torres-Narvaéz JC, Pastelín-Hernández G, Ibarra-Lara L. Extracts of Crataegus oxyacantha and Rosmarinus officinalis Attenuate Ischemic Myocardial Damage by Decreasing Oxidative Stress and Regulating the Production of Cardiac Vasoactive Agents. International Journal of Molecular Sciences. 2017; 18(11):2412. https://doi.org/10.3390/ijms18112412
Chicago/Turabian StyleCuevas-Durán, Raúl Enrique, Juan Carlos Medrano-Rodríguez, María Sánchez-Aguilar, Elizabeth Soria-Castro, María Esther Rubio-Ruíz, Leonardo Del Valle-Mondragón, Alicia Sánchez-Mendoza, Juan Carlos Torres-Narvaéz, Gustavo Pastelín-Hernández, and Luz Ibarra-Lara. 2017. "Extracts of Crataegus oxyacantha and Rosmarinus officinalis Attenuate Ischemic Myocardial Damage by Decreasing Oxidative Stress and Regulating the Production of Cardiac Vasoactive Agents" International Journal of Molecular Sciences 18, no. 11: 2412. https://doi.org/10.3390/ijms18112412
APA StyleCuevas-Durán, R. E., Medrano-Rodríguez, J. C., Sánchez-Aguilar, M., Soria-Castro, E., Rubio-Ruíz, M. E., Del Valle-Mondragón, L., Sánchez-Mendoza, A., Torres-Narvaéz, J. C., Pastelín-Hernández, G., & Ibarra-Lara, L. (2017). Extracts of Crataegus oxyacantha and Rosmarinus officinalis Attenuate Ischemic Myocardial Damage by Decreasing Oxidative Stress and Regulating the Production of Cardiac Vasoactive Agents. International Journal of Molecular Sciences, 18(11), 2412. https://doi.org/10.3390/ijms18112412