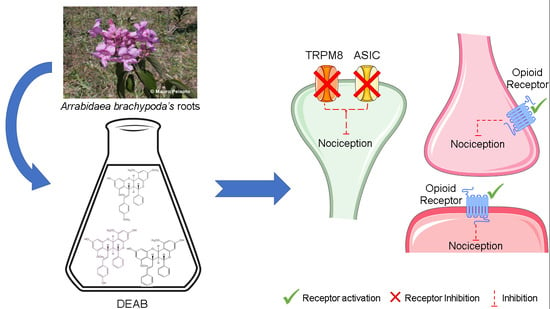
Involvement of Opioid System, TRPM8, and ASIC Receptors in Antinociceptive Effect of Arrabidaea brachypoda (DC) Bureau
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Collection and Identification of Plant Samples
3.3. Preparation of the Fraction and Isolation
3.4. Animals
3.5. Locomotor Performance
3.6. Formalin-Induced Nociception
3.7. Hot Plate Test
3.8. Involvement of Transient Receptor Potential Cation Channel Subfamily V Member 1, A Member 1, and M Member 8 (TRPV1, TRPA1, and TRPM8, Respectively) and Acid-Sensing Ion Channel (ASIC)
3.9. Involvement of Glutamatergic System
3.10. Involvement of the Opioid System
3.11. Statistical Analyses
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gurib-Fakim, A. Medicinal plants: Traditions of yesterday and drugs of tomorrow. Mol. Asp. Med. 2006, 27, 1–93. [Google Scholar] [CrossRef] [PubMed]
- Rishton, G.M. Natural Products as a Robust Source of New Drugs and Drug Leads: Past Successes and Present Day Issues. Am. J. Cardiol. 2008, 101. [Google Scholar] [CrossRef] [PubMed]
- Novaes, P.; Molinillo, J.M.G.; Varela, R.M.; Macías, F.A. Ecological phytochemistry of Cerrado (Brazilian savanna) plants. Phytochem. Rev. 2013, 12, 839–855. [Google Scholar] [CrossRef]
- Rodrigues, E.; Mendes, F.R.; Negri, G. Plants indicated by Brazilian Indians to central nervous system disturbances: A bibliographical approach. Curr. Med. Chem. 2006, 211–244. [Google Scholar] [CrossRef]
- Da Rocha, C.Q.; Vilela, F.C.; Cavalcante, G.P.; Santa-Cecília, F.V.; Santos-e-Silva, L.; dos Santos, M.H.; Giusti-Paiva, A. Anti-inflammatory and antinociceptive effects of Arrabidaea brachypoda (DC.) Bureau roots. J. Ethnopharmacol. 2011, 133, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Da Rocha, C.Q.; de-Faria, F.M.; Marcourt, L.; Ebrahimi, S.N.; Kitano, B.T.; Ghilardi, A.F.; Luiz Ferreira, A.; de Almeida, A.C.A.; Dunder, R.J.; Souza-Brito, A.R.M.; et al. Gastroprotective effects of hydroethanolic root extract of Arrabidaea brachypoda: Evidences of cytoprotection and isolation of unusual glycosylated polyphenols. Phytochemistry 2017, 135, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Da Rocha, C.Q.; Queiroz, E.F.; Meira, C.S.; Moreira, D.R.M.; Soares, M.B.P.; Marcourt, L.; Vilegas, W.; Wolfender, J.-L. Dimeric Flavonoids from Arrabidaea brachypoda and Assessment of Their Anti-Trypanosoma cruzi Activity. J. Nat. Prod. 2014, 77, 1345–1350. [Google Scholar] [CrossRef] [PubMed]
- Le Bars, D.; Gozariu, M.; Cadden, S.W. Animal models of nociception. Pharmacol. Rev. 2001, 53, 597–652. [Google Scholar] [CrossRef] [PubMed]
- Barrot, M. Tests and models of nociception and pain in rodents. Neuroscience 2012, 211, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Tjølsen, A.; Berge, O.-G.; Hunskaar, S.; Rosland, J.H.; Hole, K. The formalin test: An evaluation of the method. Pain 1992, 51, 5–17. [Google Scholar] [CrossRef]
- Hunskaar, S.; Hole, K. The formalin test in mice: Dissociation between inflammatory and non-inflammatory pain. Pain 1987, 30, 103–114. [Google Scholar] [CrossRef]
- Coderre, T.J.; Yashpal, K. Intracellular Messengers Contributing to Persistent Nociception and Hyperalgesia Induced by L-glutamate and Substance P in the Rat Formalin Pain Model. Eur. J. Neurosci. 1994, 6, 1328–1334. [Google Scholar] [CrossRef] [PubMed]
- McNamara, C.R.; Mandel-Brehm, J.; Bautista, D.M.; Siemens, J.; Deranian, K.L.; Zhao, M.; Hayward, N.J.; Chong, J.A.; Julius, D.; Moran, M.M.; et al. TRPA1 mediates formalin-induced pain. Proc. Natl. Acad. Sci. USA 2007, 104, 13525–13530. [Google Scholar] [CrossRef] [PubMed]
- Trevisani, M.; Siemens, J.; Materazzi, S.; Bautista, D.M.; Nassini, R.; Campi, B.; Imamachi, N.; Andre, E.; Patacchini, R.; Cottrell, G.S.; et al. 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc. Natl. Acad. Sci. USA 2007, 104, 13519–13524. [Google Scholar] [CrossRef] [PubMed]
- Gunn, A.; Bobeck, E.N.; Weber, C.; Morgan, M.M. The influence of non-nociceptive factors on hot-plate latency in rats. J. Pain 2011, 12, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Numazaki, M.; Tominaga, M. Nociception and TRP Channels. Curr. Drug Targets CNS Neurol. Disord. 2004, 3, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Nilius, B.; Owsianik, G. The transient receptor potential family of ion channels. Genome Biol. 2011, 12, 218. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y. TRPs and pain. Semin. Immunopathol. 2016, 38, 277–291. [Google Scholar] [CrossRef] [PubMed]
- Laing, R.J.; Dhaka, A. ThermoTRPs and Pain. Neuroscience 2016, 22, 171–187. [Google Scholar] [CrossRef] [PubMed]
- Stucky, C.L.; Dubin, A.E.; Jeske, N.A.; Malin, S.A.; McKemy, D.D.; Story, G.M. Roles of transient receptor potential channels in pain. Brain Res. Rev. 2009, 60, 2–23. [Google Scholar] [CrossRef] [PubMed]
- Deval, E.; Lingueglia, E. Acid-Sensing Ion Channels and nociception in the peripheral and central nervous systems. Neuropharmacology 2015, 94, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Wemmie, J.A.; Taugher, R.J.; Kreple, C.J. Acid-sensing ion channels in pain and disease. Nat. Rev. Neurosci. 2013, 14, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, M.-G.; Vanek, M.; Guerini, D.; Gasser, J.A.; Jones, C.E.; Junker, U.; Hofstetter, H.; Wolf, R.M.; Seuwen, K. Proton-sensing G-protein-coupled receptors. Nature 2003, 425, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.H.; Chen, C.C. Roles of Proton-Sensing Receptors in the Transition from Acute to Chronic Pain. J. Dent. Res. 2016, 95, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.-P.; Huang, Y.-H.; Chang, C.-J.; Huang, Y.-F.; Hsieh, W.-S.; Tabata, Y.; Ishii, S.; Sun, W.-H. TDAG8 involved in initiating inflammatory hyperalgesia and establishing hyperalgesic priming in mice. Sci. Rep. 2017, 7, 41415. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-Y.; Dai, S.-P.; Chang, Y.-C.; Sun, W.-H. Acidosis Mediates the Switching of Gs-PKA and Gi-PKCε Dependence in Prolonged Hyperalgesia Induced by Inflammation. PLoS ONE 2015, 10, e0125022. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-J.; Huang, C.-W.; Lin, C.-S.; Chang, W.-H.; Sun, W.-H. Expression and Function of Proton-Sensing G-Protein-Coupled Receptors in Inflammatory Pain. Mol. Pain 2009, 5. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Fan, L.; Balakrishna, S.; Sui, A.; Morris, J.B.; Jordt, S.-E. TRPM8 is the Principal Mediator of Menthol-induced Analgesia of Acute and Inflammatory Pain. Pain 2013, 154, 2169–2177. [Google Scholar] [CrossRef] [PubMed]
- Andersson, D.A.; Nash, M.; Bevan, S. Modulation of the Cold-Activated Channel TRPM8 by Lysophospholipids and Polyunsaturated Fatty Acids. J. Neurosci. 2007, 27, 3347–3355. [Google Scholar] [CrossRef] [PubMed]
- Al-Hasani, R.; Bruchas, M.R. Molecular Mechanisms of Opioid Receptor-Dependent Signalling and Behaviour. Anesthesiology 2011, 115, 1363–1381. [Google Scholar] [CrossRef] [PubMed]
- Tyers, M.B. A classification of opiate receptors that mediate antinociception in animals. Br. J. Pharmacol. 1980, 69, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Endoh, T.; Matsuura, H.; Tajima, A.; Izumimoto, N.; Tajima, C.; Suzuki, T.; Saitoh, A.; Suzuki, T.; Narita, M.; Tseng, L.; et al. Potent antinociceptive effects of TRK-820, a novel κ-opioid receptor agonist. Life Sci. 1999, 65, 1685–1694. [Google Scholar] [CrossRef]
- Dunham, N.W.; Miya, T.S. A note on a simple apparatus for detecting neurological deficit in rats and mice. J. Am. Pharm. Assoc. Am. Pharm. Assoc. 1957, 46, 208–209. [Google Scholar] [CrossRef] [PubMed]
- Eddy, N.B.; Leimbach, D. Synthetic analgesics. II. Dithienylbutenyl- and dithienylbutylamines. J. Pharmacol. Exp. Ther. 1953, 107, 385–393. [Google Scholar] [PubMed]
- Baggio, C.H.; Freitas, C.S.; Marcon, R.; Werner, M.F.; Rae, G.A.; Smiderle, F.R.; Sassaki, G.L.; Iacomini, M.; Marques, M.C.A.; Santos, A.R.S. Antinociception of β-d-glucan from Pleurotus pulmonarius is possibly related to protein kinase C inhibition. Int. J. Biol. Macromol. 2012, 50, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Sakurada, T.; Katsumata, K.; Tan-No, K.; Sakurada, S.; Kisara, K. The capsaicin test in mice for evaluating tachykinin antagonists in the spinal cord. Neuropharmacology 1992, 31, 1279–1285. [Google Scholar] [CrossRef]
- Beirith, A.; Santos, A.R.S.; Calixto, J.B.; Calixtó, J.B. Mechanisms underlying the nociception and paw oedema caused by injection of glutamate into the mouse paw. Brain Res. 2002, 924, 219–228. [Google Scholar] [CrossRef]
- Santos, A.R.; Miguel, O.G.; Yunes, R.A.; Calixto, J.B. Antinociceptive properties of the new alkaloid, cis-8, 10-di-N-propyllobelidiol hydrochloride dihydrate isolated from Siphocampylus verticillatus: Evidence for the mechanism of action. J. Pharmacol. Exp. Ther. 1999, 289, 417–426. [Google Scholar] [PubMed]
- Santos, A.R.S.; Gadotti, V.M.; Oliveira, G.L.; Tibola, D.; Paszcuk, A.F.; Neto, A.; Spindola, H.M.; Souza, M.M.; Rodrigues, A.L.S.; Calixto, J.B. Mechanisms involved in the antinociception caused by agmatine in mice. Neuropharmacology 2005, 48, 1021–1034. [Google Scholar] [CrossRef] [PubMed]







© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, V.P.; Rocha, C.Q.d.; Périco, L.L.; Santos, R.D.C.d.; Ohara, R.; Nishijima, C.M.; Ferreira Queiroz, E.; Wolfender, J.-L.; Rocha, L.R.M.d.; Santos, A.R.S.; et al. Involvement of Opioid System, TRPM8, and ASIC Receptors in Antinociceptive Effect of Arrabidaea brachypoda (DC) Bureau. Int. J. Mol. Sci. 2017, 18, 2304. https://doi.org/10.3390/ijms18112304
Rodrigues VP, Rocha CQd, Périco LL, Santos RDCd, Ohara R, Nishijima CM, Ferreira Queiroz E, Wolfender J-L, Rocha LRMd, Santos ARS, et al. Involvement of Opioid System, TRPM8, and ASIC Receptors in Antinociceptive Effect of Arrabidaea brachypoda (DC) Bureau. International Journal of Molecular Sciences. 2017; 18(11):2304. https://doi.org/10.3390/ijms18112304
Chicago/Turabian StyleRodrigues, Vinícius Peixoto, Cláudia Quintino da Rocha, Larissa Lucena Périco, Raquel De Cássia dos Santos, Rie Ohara, Catarine Massucato Nishijima, Emerson Ferreira Queiroz, Jean-Luc Wolfender, Lúcia Regina Machado da Rocha, Adair Roberto Soares Santos, and et al. 2017. "Involvement of Opioid System, TRPM8, and ASIC Receptors in Antinociceptive Effect of Arrabidaea brachypoda (DC) Bureau" International Journal of Molecular Sciences 18, no. 11: 2304. https://doi.org/10.3390/ijms18112304
APA StyleRodrigues, V. P., Rocha, C. Q. d., Périco, L. L., Santos, R. D. C. d., Ohara, R., Nishijima, C. M., Ferreira Queiroz, E., Wolfender, J. -L., Rocha, L. R. M. d., Santos, A. R. S., Vilegas, W., & Hiruma-Lima, C. A. (2017). Involvement of Opioid System, TRPM8, and ASIC Receptors in Antinociceptive Effect of Arrabidaea brachypoda (DC) Bureau. International Journal of Molecular Sciences, 18(11), 2304. https://doi.org/10.3390/ijms18112304