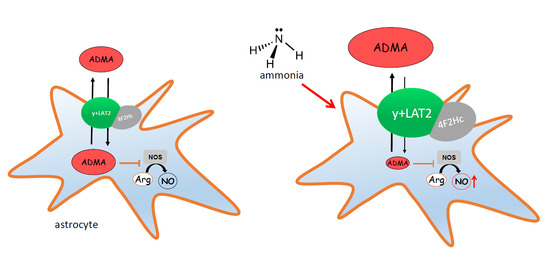
Ammonia Reduces Intracellular Asymmetric Dimethylarginine in Cultured Astrocytes Stimulating Its y+LAT2 Carrier-Mediated Loss
Abstract
:1. Introduction
2. Results
2.1. The Effect of Ammonia and Slc7a6 Silencing on ADMA and SDMA Intra/Extracellular Concentration in Cultured Astrocytes
2.2. The Expression of ADMA-Metabolizing Enzymes
2.3. The Nitrite/Nitrate Production in Cultured Astrocytes
2.4. The Effect of ADMA on [3H]Arginine Uptake to Cultured Astrocytes
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Cell Culture and Treatments
4.3. Silencing of the Gene Coding for y+LAT2 Transporter
4.4. Dimethylarginines and Arginine Determination in Cell Homogenates and Culture Media
4.5. l-[3H]Arginine Uptake Assay
4.6. Real-Time PCR Analysis
4.7. Protein Isolation and Western Blot Analysis
4.8. Determination of Nitric Oxide (NO) Concentration
4.9. Protein Determination
4.10. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lockwood, A.H. Blood ammonia levels and hepatic encephalopathy. Metab. Brain Dis. 2004, 19, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Giguere, J.F.; Butterworth, R.F. Amino acid changes in regions of the CNS in relation to function in experimental portal-systemic encephalopathy. Neurochem. Res. 1984, 9, 1309–1321. [Google Scholar] [CrossRef] [PubMed]
- Parekh, P.J.; Balart, L.A. Ammonia and its role in the pathogenesis of hepatic encephalopathy. Clin. Liver. Dis. 2015, 19, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Dzoljic, E.; Grbatinic, I.; Kostic, V. Why is nitric oxide important for our brain? Funct. Neurol. 2015, 30, 159–163. [Google Scholar] [PubMed]
- Nauser, T.; Koppenol, W.H. The rate constant of the reaction of superoxide with nitrogen monoxide: Approaching the diffusion limit. J. Phys. Chem. A 2002, 106, 4084–4086. [Google Scholar] [CrossRef]
- Gorg, B.; Qvartskhava, N.; Keitel, V.; Bidmon, H.J.; Selbach, O.; Schliess, F.; Haussinger, D. Ammonia induces RNA oxidation in cultured astrocytes and brain in vivo. Hepatology 2008, 48, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Kruczek, C.; Gorg, B.; Keitel, V.; Bidmon, H.J.; Schliess, F.; Haussinger, D. Ammonia increases nitric oxide, free Zn2+, and metallothionein mRNA expression in cultured rat astrocytes. Biol. Chem. 2011, 392, 1155–1165. [Google Scholar] [CrossRef] [PubMed]
- Murthy, C.R.; Rama Rao, K.V.; Bai, G.; Norenberg, M.D. Ammonia-induced production of free radicals in primary cultures of rat astrocytes. J. Neurosci. Res. 2001, 66, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Bobermin, L.D.; Wartchow, K.M.; Flores, M.P.; Leite, M.C.; Quincozes-Santos, A.; Goncalves, C.A. Ammonia-induced oxidative damage in neurons is prevented by resveratrol and lipoic acid with participation of heme oxygenase 1. Neurotoxicology 2015, 49, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Skowrońska, M.; Zielińska, M.; Wójcik-Stanaszek, L.; Ruszkiewicz, J.; Milatovic, D.; Aschner, M.; Albrecht, J. Ammonia increases paracellular permeability of rat brain endothelial cells by a mechanism encompassing oxidative/nitrosative stress and activation of matrix metalloproteinases. J. Neurochem. 2012, 121, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Llansola, M.; Rodrigo, R.; Monfort, P.; Montoliu, C.; Kosenko, E.; Cauli, O.; Piedrafita, B.; el Mlili, N.; Felipo, V. NMDA receptors in hyperammonemia and hepatic encephalopathy. Metab. Brain Dis. 2007, 22, 321–335. [Google Scholar] [CrossRef] [PubMed]
- Hazell, A.S.; Norenberg, M.D. Ammonia and manganese increase arginine uptake in cultured astrocytes. Neurochem. Res. 1998, 23, 869–873. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, M.; Skowronska, M.; Fresko, I.; Albrecht, J. Upregulation of the heteromeric y(+)LAT2 transporter contributes to ammonia-induced increase of arginine uptake in rat cerebral cortical astrocytes. Neurochem. Int. 2012, 61, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, M.; Ruszkiewicz, J.; Hilgier, W.; Fresko, I.; Albrecht, J. Hyperammonemia increases the expression and activity of the glutamine/arginine transporter y+ LAT2 in rat cerebral cortex: Implications for the nitric oxide/cGMP pathway. Neurochem. Int. 2011, 58, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Zielinska, M.; Milewski, K.; Skowronska, M.; Gajos, A.; Zieminska, E.; Beresewicz, A.; Albrecht, J. Induction of inducible nitric oxide synthase expression in ammonia-exposed cultured astrocytes is coupled to increased arginine transport by upregulated y(+)LAT2 transporter. J. Neurochem. 2015, 135, 1272–1281. [Google Scholar] [CrossRef] [PubMed]
- Teerlink, T. ADMA metabolism and clearance. Vasc. Med. 2005, 10, S73–S81. [Google Scholar] [CrossRef] [PubMed]
- Leiper, J.; Vallance, P. Biological significance of endogenous methylarginines that inhibit nitric oxide synthases. Cardiovasc. Res. 1999, 43, 542–548. [Google Scholar] [CrossRef]
- Teerlink, T.; Luo, Z.; Palm, F.; Wilcox, C.S. Cellular ADMA: Regulation and action. Pharmacol. Res. 2009, 60, 448–460. [Google Scholar] [CrossRef] [PubMed]
- Bode-Boger, S.M.; Scalera, F.; Ignarro, L.J. The l-arginine paradox: Importance of the l-arginine/asymmetrical dimethylarginine ratio. Pharmacol. Ther. 2007, 114, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Davids, M.; Richir, M.C.; Visser, M.; Ellger, B.; van den Berghe, G.; van Leeuwen, P.A.; Teerlink, T. Role of dimethylarginine dimethylaminohydrolase activity in regulation of tissue and plasma concentrations of asymmetric dimethylarginine in an animal model of prolonged critical illness. Metabolism 2012, 61, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Tran, C.T.; Fox, M.F.; Vallance, P.; Leiper, J.M. Chromosomal localization, gene structure, and expression pattern of DDAH1: Comparison with DDAH2 and implications for evolutionary origins. Genomics 2000, 68, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Davids, M.; van Hell, A.J.; Visser, M.; Nijveldt, R.J.; van Leeuwen, P.A.; Teerlink, T. Role of the human erythrocyte in generation and storage of asymmetric dimethylarginine. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H1762–H1770. [Google Scholar] [CrossRef] [PubMed]
- Milewski, K.; Hilgier, W.; Albrecht, J.; Zielinska, M. The dimethylarginine (ADMA)/nitric oxide pathway in the brain and periphery of rats with thioacetamide-induced acute liver failure: Modulation by histidine. Neurochem. Int. 2015, 88, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Czarnecka, A.; Milewski, K.; Jazwiec, R.; Zielinska, M. Intracerebral administration of s-adenosylhomocysteine or s-adenosylmethionine attenuates the increases in the cortical extracellular levels of dimethylarginines without affecting cGMP level in rats with acute liver failure. Neurotox. Res. 2017, 31, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Skowrońska, M.; Albrecht, J. Oxidative and nitrosative stress in ammonia neurotoxicity. Neurochem. Int. 2013, 62, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, A.R.; Murthy, Ch.R.K.; Norenberg, M.D. Oxidative stress and mitogen-activated protein kinase phosphorylation mediate ammonia-induced cell swelling and glutamate uptake inhibition in cultured astrocytes. J. Neurosci. 2006, 26, 4774–4784. [Google Scholar] [CrossRef] [PubMed]
- Schliess, F.; Gorg, B.; Fischer, R.; Desjardins, P.; Bidmon, H.J.; Herrmann, A.; Butterworth, R.F.; Zilles, K.; Haussinger, D. Ammonia induces MK-801-sensitive nitration and phosphorylation of protein tyrosine residues in rat astrocytes. FASEB J. 2002, 16, 739–741. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hou, L.; Xu, D.; Chen, A.; Yang, L.; Zhuang, Y.; Xu, Y.; Fassett, J.T.; Chen, Y. Effect of asymmetric dimethylarginine (ADMA) on heart failure development. Nitric Oxide 2016, 54, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Lluch, P.; Segarra, G.; Medina, P. Asymmetric dimethylarginine as a mediator of vascular dysfunction in cirrhosis. World J. Gastroenterol. 2015, 21, 9466–9475. [Google Scholar] [CrossRef] [PubMed]
- Tousoulis, D.; Georgakis, M.K.; Oikonomou, E.; Papageorgiou, N.; Zaromitidou, M.; Latsios, G.; Papaioannou, S.; Siasos, G. Asymmetric dimethylarginine: Clinical significance and novel therapeutic approaches. Curr. Med. Chem. 2015, 22, 2871–2901. [Google Scholar] [CrossRef] [PubMed]
- Mookerjee, R.P.; Malaki, M.; Davies, N.A.; Hodges, S.J.; Dalton, R.N.; Turner, C.; Sen, S.; Williams, R.; Leiper, J.; Vallance, P.; et al. Increasing dimethylarginine levels are associated with adverse clinical outcome in severe alcoholic hepatitis. Hepatology 2007, 45, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, J.S.; Ahluwalia, V.; Wade, J.B.; Sanyal, A.J.; White, M.B.; Noble, N.A.; Monteith, P.; Fuchs, M.; Sterling, R.K.; Luketic, V.; et al. Asymmetric dimethylarginine is strongly associated with cognitive dysfunction and brain MR spectroscopic abnormalities in cirrhosis. J. Hepatol. 2013, 58, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Balasubramaniyan, V.; Wright, G.; Sharma, V.; Davies, N.A.; Sharifi, Y.; Habtesion, A.; Mookerjee, R.P.; Jalan, R. Ammonia reduction with ornithine phenylacetate restores brain eNOS activity via the DDAH-ADMA pathway in bile duct-ligated cirrhotic rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G145–G152. [Google Scholar] [CrossRef] [PubMed]
- Bekpinar, S.; Vardagli, D.; Unlucerci, Y.; Can, A.; Uysal, M.; Gurdol, F. Effect of rosiglitazone on asymmetric dimethylarginine metabolism in thioacetamide-induced acute liver injury. Pathophysiology 2015, 22, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Wiencken, A.E.; Casagrande, V.A. Endothelial nitric oxide synthetase (eNOS) in astrocytes: Another source of nitric oxide in neocortex. Glia 1999, 26, 280–290. [Google Scholar] [CrossRef]
- Ueda, S.; Kato, S.; Matsuoka, H.; Kimoto, M.; Okuda, S.; Morimatsu, M.; Imaizumi, T. Regulation of cytokine-induced nitric oxide synthesis by asymmetric dimethylarginine: Role of dimethylarginine dimethylaminohydrolase. Circ. Res. 2003, 92, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Strobel, J.; Mieth, M.; Endress, B.; Auge, D.; Konig, J.; Fromm, M.F.; Maas, R. Interaction of the cardiovascular risk marker asymmetric dimethylarginine (ADMA) with the human cationic amino acid transporter 1 (CAT1). J. Mol. Cell. Cardiol. 2012, 53, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Strobel, J.; Muller, F.; Zolk, O.; Endress, B.; Konig, J.; Fromm, M.F.; Maas, R. Transport of asymmetric dimethylarginine (ADMA) by cationic amino acid transporter 2 (CAT2), organic cation transporter 2 (OCT2) and multidrug and toxin extrusion protein 1 (MATE1). Amino Acids 2013, 45, 989–1002. [Google Scholar] [CrossRef] [PubMed]
- Closs, E.I.; Ostad, M.A.; Simon, A.; Warnholtz, A.; Jabs, A.; Habermeier, A.; Daiber, A.; Forstermann, U.; Munzel, T. Impairment of the extrusion transporter for asymmetric dimethyl-l-arginine: A novel mechanism underlying vasospastic angina. Biochem. Biophys. Res. Commun. 2012, 423, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Closs, E.I.; Basha, F.Z.; Habermeier, A.; Forstermann, U. Interference of l-arginine analogues with l-arginine transport mediated by the y+ carrier hCAT-2B. Nitric. Oxide. 1997, 1, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, M. Badanie Zmian Aktywności i/lub Ekspresji Enzymów Metabolizujących Dwumetyloargininę (ADMA) w Korze Czołowej w Szczurzych Modelach Encefalopatii Wątrobowej. Master’s Thesis, The Warsaw Medical University, Warsaw, Poland, 2012. [Google Scholar]
- Tain, Y.L.; Kao, Y.H.; Hsieh, C.S.; Chen, C.C.; Sheen, J.M.; Lin, I.C.; Huang, L.T. Melatonin blocks oxidative stress-induced increased asymmetric dimethylarginine. Free Radic. Biol. Med. 2010, 49, 1088–1098. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.C.; Tran, C.T.; Leiper, J.M.; Hingorani, A.D.; Vallance, P. Common genetic variation in a basal promoter element alters DDAH2 expression in endothelial cells. Biochem. Biophys. Res. Commun. 2003, 310, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Valkonen, V.P.; Tuomainen, T.P.; Laaksonen, R. DDAH gene and cardiovascular risk. Vasc. Med. 2005, 10, S45–S48. [Google Scholar] [PubMed]
- Pi, J.; Kumagai, Y.; Sun, G.; Shimojo, N. Improved method for simultaneous determination of l-arginine and its mono- and dimethylated metabolites in biological samples by high-performance liquid chromatography. J. Chromatogr. B Biomed. Sci. Appl. 2000, 742, 199–203. [Google Scholar] [CrossRef]
- Martens-Lobenhoffer, J.; Bode-Boger, S.M. Quantification of l-arginine, asymmetric dimethylarginine and symmetric dimethylarginine in human plasma: A step improvement in precision by stable isotope dilution mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2012, 904, 140–143. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt Method. Methods 2001, 25, 402–408. [Google Scholar] [PubMed]






| Intracellular Concentration | Control | Ammonia | Mock Control | Ammonia + y+LAT2 siRNA |
|---|---|---|---|---|
| ADMA (µM) | 7.3 ± 1.01 | 4.38 ± 1.80 * | 6.91 ± 1.47 | 8.29 ± 0.84 # |
| SDMA (µM) | 5.82 ± 1.32 | 3.27 ± 1.18 * | 5.29 ± 1.02 | 5.6 ± 1.31 |
| Arg (µM) | 155.83 ± 50.4 | 176.33 ± 48.26 | 183.92 ± 37.83 | 155.35 ± 46.71 |
| Arg/ADMA | 24.47 ± 4.16 | 41.84 ± 6.73 * | 26.21 ± 4.05 | 21.11 ± 3.75 |
| ADMA/SDMA | 1.23 ± 0.23 | 1.42 ± 0.37 | 1.26 ± 0.27 | 1.45 ± 0.33 |
| Extracellular Concentration | Control | Ammonia | Mock Control | Ammonia + y+LAT2 siRNA |
|---|---|---|---|---|
| ADMA (µM) | 0.365 ± 0.03 | 0.410 ± 0.04 * | 0.358 ± 0.03 | 0.368 ± 0.05 |
| SDMA (µM) | 0.864 ± 0.24 | 1.378 ± 0.16 * | 1.04 ± 0.09 | 1.189 ± 0.12 |
| Arg (mM) | 0.306 ± 0.12 | 0.312 ± 0.05 | 0.309 ± 0.04 | 0.301 ± 0.09 |
| Arg/ADMA | 864.24 ± 18.34 | 756.59 ± 9.66 * | 872.33 ± 14.9 | 813.71 ± 12.28 |
| ADMA/SDMA | 0.44 ± 0.05 | 0.29 ± 0.06 | 0.36 ± 0.1 | 0.3 ± 0.04 |
| Intracellular Concentration | RBE-4 | |
|---|---|---|
| Control | Ammonia | |
| ADMA (µM) | 14.27 ± 2.23 | 8.29 ± 0.84 * |
| SDMA (µM) | 4.29 ± 0.53 | 2.85 ± 0.31 * |
| Arg (µM) | 134.39 ± 19.13 | 102.5 ± 11.18 |
| Arg/ADMA | 11.20 ± 3.05 | 14.33 ± 3.80 |
| ADMA/SDMA | 3.18 ± 0.25 | 2.97 ± 0.19 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milewski, K.; Bogacińska-Karaś, M.; Fręśko, I.; Hilgier, W.; Jaźwiec, R.; Albrecht, J.; Zielińska, M. Ammonia Reduces Intracellular Asymmetric Dimethylarginine in Cultured Astrocytes Stimulating Its y+LAT2 Carrier-Mediated Loss. Int. J. Mol. Sci. 2017, 18, 2308. https://doi.org/10.3390/ijms18112308
Milewski K, Bogacińska-Karaś M, Fręśko I, Hilgier W, Jaźwiec R, Albrecht J, Zielińska M. Ammonia Reduces Intracellular Asymmetric Dimethylarginine in Cultured Astrocytes Stimulating Its y+LAT2 Carrier-Mediated Loss. International Journal of Molecular Sciences. 2017; 18(11):2308. https://doi.org/10.3390/ijms18112308
Chicago/Turabian StyleMilewski, Krzysztof, Małgorzata Bogacińska-Karaś, Inez Fręśko, Wojciech Hilgier, Radosław Jaźwiec, Jan Albrecht, and Magdalena Zielińska. 2017. "Ammonia Reduces Intracellular Asymmetric Dimethylarginine in Cultured Astrocytes Stimulating Its y+LAT2 Carrier-Mediated Loss" International Journal of Molecular Sciences 18, no. 11: 2308. https://doi.org/10.3390/ijms18112308
APA StyleMilewski, K., Bogacińska-Karaś, M., Fręśko, I., Hilgier, W., Jaźwiec, R., Albrecht, J., & Zielińska, M. (2017). Ammonia Reduces Intracellular Asymmetric Dimethylarginine in Cultured Astrocytes Stimulating Its y+LAT2 Carrier-Mediated Loss. International Journal of Molecular Sciences, 18(11), 2308. https://doi.org/10.3390/ijms18112308