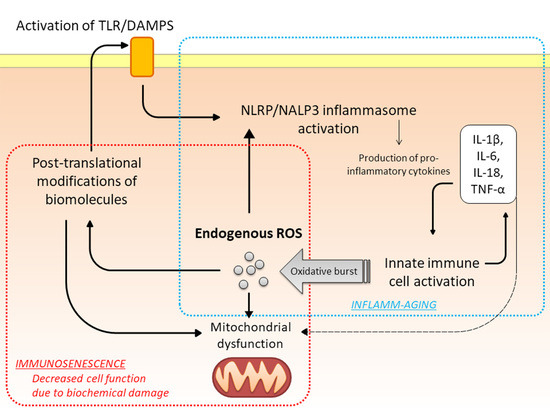
The Contribution of Oxidative Stress and Inflamm-Aging in Human and Equine Asthma
Abstract
:1. Introduction
2. Theories of Aging
3. Oxidative Stress
4. Oxidative Stress and Asthma
5. Age-Associated Changes in Pulmonary Structure and (Immune) Function
6. Asthma in the Elderly: A Different Disease? [12]
7. Severe Equine Asthma: A Model of Oxi-Inflamm-Aging
8. Unanswered Questions in Geriatric Asthma and Possible Contribution of the Equine Asthma Model to Knowledge Advancements
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Vaupel, J.W. Biodemography of human ageing. Nature 2010, 464, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.E.; Sheaff, M.T. The pathology of ageingoncepts and mechanisms. J. Pathol. 2007, 211, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Walford, R.L. The Immunologic Theory of Aging. Gerontologist 1964, 4, 195–197. [Google Scholar] [CrossRef] [PubMed]
- Fulop, T.; Dupuis, G.; Witkowski, J.M.; Larbi, A. The Role of Immunosenescence in the Development of Age-Related Diseases. Rev. Investig. Clin. 2016, 68, 84–91. [Google Scholar]
- Franceschi, C.; Bonafe, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Boe, D.M.; Boule, L.A.; Kovacs, E.J. Innate immune responses in the ageing lung. Clin. Exp. Immunol. 2017, 187, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Lowery, E.M.; Brubaker, A.L.; Kuhlmann, E.; Kovacs, E.J. The aging lung. Clin. Interv. Aging 2013, 8, 1489–1496. [Google Scholar] [PubMed]
- Davalli, P.; Mitic, T.; Caporali, A.; Lauriola, A.; D’Arca, D. ROS, Cell Senescence, and Novel Molecular Mechanisms in Aging and Age-Related Diseases. Oxid. Med. Cell. Longev. 2016, 2016, 3565127. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, E.J.; Boe, D.M.; Boule, L.A.; Curtis, B.J. Inflammaging and the Lung. Clin. Geriatr. Med. 2017, 33, 459–471. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, M.; Miquel, J. An update of the oxidation-inflammation theory of aginghe involvement of the immune system in oxi-inflamm-aging. Curr. Pharm. Des. 2009, 15, 3003–3026. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.H. Mitochondrial Dysfunction and Oxidative Stress in Asthma: Implications for Mitochondria-Targeted Antioxidant Therapeutics. Pharmaceuticals 2011, 4, 429–456. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, S.; Benfante, A.; Spatafora, M.; Scichilone, N. Asthma in the elderly different disease? Breathe Sheff 2016, 12, 18–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Busse, P.J.; Mathur, S.K. Age-related changes in immune functionffect on airway inflammation. J. Allergy Clin. Immunol. 2010, 126, 690–699. [Google Scholar] [CrossRef] [PubMed]
- Dunn, R.M.; Busse, P.J.; Wechsler, M.E. Asthma in the elderly and late-onset adult asthma. Allergy 2017. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Kim, S.R.; Lee, Y.C. Impact of oxidative stress on lung diseases. Respirology 2009, 14, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Boulet, L.P. Asthma in the elderly patient. Asthma Res. Pract. 2016, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Kleniewska, P.; Pawliczak, R. The participation of oxidative stress in the pathogenesis of bronchial asthma. Biomed. Pharmacother. 2017, 94, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, H.; Ichinose, M. Role of Oxidative Stress in Aggravation of Asthma. Arerugi 2017, 66, 931–935. [Google Scholar] [PubMed]
- Bullone, M.; Lavoie, J.P. Asthma “of horses and men”-How can equine heaves help us better understand human asthma immunopathology and its functional consequences? Mol. Immunol. 2015, 66, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Partridge, L. Some highlights of research on aging with invertebrates, 2010. Aging Cell 2011, 10, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, A.; Idelchik, M.; Melendez, J.A. Redox control of senescence and age-related disease. Redox Biol. 2017, 11, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Cannizzo, E.S.; Clement, C.C.; Sahu, R.; Follo, C.; Santambrogio, L. Oxidative stress, inflamm-aging and immunosenescence. J. Proteom. 2011, 74, 2313–2323. [Google Scholar] [CrossRef] [PubMed]
- Gerschman, R.; Gilbert, D.L.; Nye, S.W.; Dwyer, P.; Fenn, W.O. Oxygen poisoning and x-irradiation mechanism in common. Science 1954, 119, 623–626. [Google Scholar] [CrossRef] [PubMed]
- Gerschman, R.; Gilbert, D.L.; Nye, S.W.; Fenn, W.O. Influence of x-irradiation on oxygen poisoning in mice. Proc. Soc. Exp. Biol. Med. 1954, 86, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Harman, D. Aging theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef] [PubMed]
- Harman, D. The biologic clockhe mitochondria? J. Am. Geriatr. Soc. 1972, 20, 145–147. [Google Scholar] [CrossRef]
- Muller, F.L.; Lustgarten, M.S.; Jang, Y.; Richardson, A.; Van Remmen, H. Trends in oxidative aging theories. Free Radic. Biol. Med. 2007, 43, 477–503. [Google Scholar] [CrossRef] [PubMed]
- Chandel, N.S. Mitochondrial regulation of oxygen sensing. Adv. Exp. Med. Biol. 2010, 661, 339–354. [Google Scholar] [PubMed]
- Chance, B.; Sies, H.; Boveris, A. Hydroperoxide metabolism in mammalian organs. Physiol. Rev. 1979, 59, 527–605. [Google Scholar] [PubMed]
- Ye, J.; Keller, J.N. Regulation of energy metabolism by inflammation feedback response in obesity and calorie restriction. Aging 2010, 2, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Thannickal, V.J.; Fanburg, B.L. Reactive oxygen species in cell signaling. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000, 279, L1005–L1028. [Google Scholar] [PubMed]
- Rahman, I.; Biswas, S.K.; Kode, A. Oxidant and antioxidant balance in the airways and airway diseases. Eur. J. Pharmacol. 2006, 533, 222–239. [Google Scholar] [CrossRef] [PubMed]
- Ricciardolo, F.L.; Sterk, P.J.; Gaston, B.; Folkerts, G. Nitric oxide in health and disease of the respiratory system. Physiol. Rev. 2004, 84, 731–765. [Google Scholar] [CrossRef] [PubMed]
- Erzurum, S.C. New Insights in Oxidant Biology in Asthma. Ann. Am. Thorac. Soc. 2016, 13 (Suppl. 1), S35–S39. [Google Scholar] [PubMed]
- Fulop, T.; Larbi, A.; Douziech, N.; Fortin, C.; Guerard, K.P.; Lesur, O.; Khalil, A.; Dupuis, G. Signal transduction and functional changes in neutrophils with aging. Aging Cell 2004, 3, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Ishigatsubo, Y.; Aoki, I. Pathology of asthma. Front. Microbiol. 2013, 4, 263. [Google Scholar] [CrossRef] [PubMed]
- Alam, R.; Good, J.; Rollins, D.; Verma, M.; Chu, H.; Pham, T.H.; Martin, R.J. Airway and serum biochemical correlates of refractory neutrophilic asthma. J. Allergy Clin. Immunol. 2017, 140, 1004–1014. [Google Scholar] [CrossRef] [PubMed]
- Carr, T.F.; Zeki, A.A.; Kraft, M. Eosinophilic and Non-Eosinophilic Asthma. Am. J. Respir. Crit. Care Med. 2017. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.S.; Lee, T.H.; Jun, J.A.; Baek, A.R.; Park, J.S.; Koo, S.M.; Kim, Y.K.; Lee, H.S.; Park, C.S. Neutrophilic inflammation in asthmaechanisms and therapeutic considerations. Expert Rev. Respir. Med. 2017, 11, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.F. Neutrophilic asthma distinct target for treatment? Lancet Respir. Med. 2016, 4, 765–767. [Google Scholar] [CrossRef]
- Panettieri, R.A., Jr. Neutrophilic and Pauci-immune Phenotypes in Severe Asthma. Immunol. Allergy Clin. N. Am. 2016, 36, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Kolls, J.K. Neutrophilic Inflammation in Asthma and Association with Disease Severity. Trends Immunol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Bishopp, A.; Sathyamurthy, R.; Manney, S.; Webbster, C.; Krishna, M.T.; Mansur, A.H. Biomarkers of oxidative stress and antioxidants in severe asthma: A Prospective Case-Control Study. Ann. Allergy Asthma Immunol. 2017, 118, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, H.; Ichinose, M. Oxidative and nitrative stress in bronchial asthma. Antioxid. Redox Signal. 2008, 10, 785–797. [Google Scholar] [CrossRef] [PubMed]
- To, M.; Kono, Y.; Ogura, N.; Mikami, S.; Honda, N.; Hitani, A.; Kano, I.; Haruki, K.; To, Y. Obesity-related systemic oxidative stress: An important factor of poor asthma control. Allergol. Int. 2017. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Matsukura, S.; Takeuchi, H.; Kawaguchi, M.; Ieki, K.; Odaka, M.; Watanabe, S.; Homma, T.; Dohi, K.; Aruga, T.; et al. Increase in reactive oxygen metabolite level in acute exacerbations of asthma. Int. Arch. Allergy Immunol. 2008, 146 (Suppl. 1), 67–72. [Google Scholar] [CrossRef] [PubMed]
- Sideleva, O.; Black, K.; Dixon, A.E. Effects of obesity and weight loss on airway physiology and inflammation in asthma. Pulm. Pharmacol. Ther. 2013, 26, 455–458. [Google Scholar] [CrossRef] [PubMed]
- Maneechotesuwan, K.; Essilfie-Quaye, S.; Kharitonov, S.A.; Adcock, I.M.; Barnes, P.J. Loss of control of asthma following inhaled corticosteroid withdrawal is associated with increased sputum interleukin-8 and neutrophils. Chest 2007, 132, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.; Ward, C.; Stenton, C.S.; Bird, G.; Hendrick, D.J.; Walters, E.H. Number and activity of inflammatory cells in bronchoalveolar lavage fluid in asthma and their relation to airway responsiveness. Thorax 1998, 43, 684–692. [Google Scholar] [CrossRef]
- Calhoun, W.J.; Reed, H.E.; Moest, D.R.; Stevens, C.A. Enhanced superoxide production by alveolar macrophages and air-space cells, airway inflammation, and alveolar macrophage density changes after segmental antigen bronchoprovocation in allergic subjects. Am. Rev. Respir. Dis. 1992, 145, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Simpson, J.L.; Phipps, S.; Baines, K.J.; Oreo, K.M.; Gunawardhana, L.; Gibson, P.G. Elevated expression of the NLRP3 inflammasome in neutrophilic asthma. Eur. Respir. J. 2014, 43, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Lugrin, J.; Rosenblatt-Velin, N.; Parapanov, R.; Liaudet, L. The role of oxidative stress during inflammatory processes. Biol. Chem. 2014, 395, 203–230. [Google Scholar] [CrossRef] [PubMed]
- Bergeron, C.; Tulic, M.K.; Hamid, Q. Airway remodelling in asthmarom benchside to clinical practice. Can. Respir. J. 2014, 17, e85–e93. [Google Scholar] [CrossRef]
- Chan, T.K.; Tan, W.S.D.; Peh, H.Y.; Wong, W.S.F. Aeroallergens Induce Reactive Oxygen Species Production and DNA Damage and Dampen Antioxidant Responses in Bronchial Epithelial Cells. J. Immunol. 2017, 199, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Pattnaik, B.; Bodas, M.; Bhatraju, N.K.; Ahmad, T.; Pant, R.; Guleria, R.; Ghosh, B.; Agrawal, A. IL-4 promotes asymmetric dimethylarginine accumulation, oxo-nitrative stress, and hypoxic response-induced mitochondrial loss in airway epithelial cells. J. Allergy Clin. Immunol. 2016, 138, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Bauer, V.; Sotnikova, R.; Machova, J.; Matyas, S.; Pucovsky, V.; Stefek, M. Reactive oxygen species induced smooth muscle responses in the intestine, vessels and airways and the effect of antioxidants. Life Sci. 1999, 65, 1909–1917. [Google Scholar] [CrossRef]
- Katsumata, U.; Miura, M.; Ichinose, M.; Kimura, K.; Takahashi, T.; Inoue, H.; Takishima, T. Oxygen radicals produce airway constriction and hyperresponsiveness in anesthetized cats. Am. Rev. Respir. Dis. 1990, 141, 1158–1161. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Clanton, T.L. Reactive oxygen species formation in the transition to hypoxia in skeletal muscle. Am. J. Physiol. Cell. Physiol. 2005, 289, C207–C216. [Google Scholar] [CrossRef] [PubMed]
- Wiegman, C.H.; Michaeloudes, C.; Haji, G.; Narang, P.; Clarke, C.J.; Russell, K.E.; Bao, W.; Pavlidis, S.; Barnes, P.J.; Kanerva, J.; et al. Oxidative stress-induced mitochondrial dysfunction drives inflammation and airway smooth muscle remodeling in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2015, 136, 769–780. [Google Scholar] [CrossRef] [PubMed]
- Tuo, Q.R.; Ma, Y.F.; Chen, W.; Luo, X.J.; Shen, J.; Guo, D.; Zheng, Y.M.; Wang, Y.X.; Ji, G.; Liu, Q.H. Reactive oxygen species induce a Ca2+-spark increase in sensitized murine airway smooth muscle cells. Biochem. Biophys. Res. Commun. 2013, 434, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Poli, G.; Parola, M. Oxidative damage and fibrogenesis. Free Radic. Biol. Med. 1997, 22, 287–305. [Google Scholar] [CrossRef]
- Sutcliffe, A.; Hollins, F.; Gomez, E.; Saunders, R.; Doe, C.; Cooke, M.; Challiss, R.A.; Brightling, C.E. Increased nicotinamide adenine dinucleotide phosphate oxidase 4 expression mediates intrinsic airway smooth muscle hypercontractility in asthma. Am. J. Respir. Crit. Care Med. 2012, 185, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Prakash, Y.S.; Pabelick, C.M.; Sieck, G.C. Mitochondrial Dysfunction in Airway Disease. Chest 2017, 152, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Gansauge, S.; Gansauge, F.; Nussler, A.K.; Rau, B.; Poch, B.; Schoenberg, M.H.; Beger, H.G. Exogenous, but not endogenous, nitric oxide increases proliferation rates in senescent human fibroblasts. FEBS Lett. 1997, 410, 160–164. [Google Scholar] [CrossRef]
- Dasgupta, J.; Kar, S.; Liu, R.; Joseph, J.; Kalyanaraman, B.; Remington, S.J.; Chen, C.; Melendez, J.A. Reactive oxygen species control senescence-associated matrix metalloproteinase-1 through c-Jun-N-terminal kinase. J. Cell. Physiol. 2010, 225, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Doelman, C.J.; Bast, A. Oxygen radicals in lung pathology. Free Radic. Biol. Med. 1990, 9, 381–400. [Google Scholar] [CrossRef]
- Gillissen, A.; Nowak, D. Characterization of N-acetylcysteine and ambroxol in anti-oxidant therapy. Respir. Med. 1998, 92, 609–623. [Google Scholar] [CrossRef]
- Henricks, P.A.; Nijkamp, F.P. Reactive oxygen species as mediators in asthma. Pulm. Pharmacol. Ther. 2001, 14, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, A.J.; Altman, L.C.; Wight, T.N.; Luchtel, D.L. Ozone alters the distribution of beta1 integrins in cultured primate bronchial epithelial cells. Am. J. Respir. Cell Mol. Biol. 1998, 19, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Jaffer, O.A.; Carter, A.B.; Sanders, P.N.; Dibbern, M.E.; Winters, C.J.; Murthy, S.; Ryan, A.J.; Rokita, A.G.; Prasad, A.M.; Zabner, J.; et al. Mitochondrial-targeted antioxidant therapy decreases transforming growth factor-beta-mediated collagen production in a murine asthma model. Am. J. Respir. Cell Mol. Biol. 2015, 52, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Uchida, M.; Anderson, E.L.; Squillace, D.L.; Patil, N.; Maniak, P.J.; Iijima, K.; Kita, H.; O’Grady, S.M. Oxidative stress serves as a key checkpoint for IL-33 release by airway epithelium. Allergy 2017, 72, 1521–1531. [Google Scholar] [CrossRef] [PubMed]
- Wan, W.Y.; Hollins, F.; Haste, L.; Woodman, L.; Hirst, R.A.; Bolton, S.; Gomez, E.; Sutcliffe, A.; Desai, D.; Chachi, L.; et al. NADPH Oxidase-4 Overexpression Is Associated With Epithelial Ciliary Dysfunction in Neutrophilic Asthma. Chest 2016, 149, 1445–1459. [Google Scholar] [CrossRef] [PubMed]
- Rada, B.; Boudreau, H.E.; Park, J.J.; Leto, T.L. Histamine stimulates hydrogen peroxide production by bronchial epithelial cells via histamine H1 receptor and dual oxidase. Am. J. Respir. Cell Mol. Biol. 2014, 50, 125–134. [Google Scholar] [PubMed]
- Heiss, L.N.; Lancaster, J.R., Jr.; Corbett, J.A.; Goldman, W.E. Epithelial autotoxicity of nitric oxideole in the respiratory cytopathology of pertussis. Proc. Natl. Acad. Sci. USA 1994, 91, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Naylor, B. The shedding of the mucosa of the bronchial tree in asthma. Thorax 1962, 17, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Folkerts, G.; Kloek, J.; Muijsers, R.B.; Nijkamp, F.P. Reactive nitrogen and oxygen species in airway inflammation. Eur. J. Pharmacol. 2001, 429, 251–262. [Google Scholar] [CrossRef]
- Nagata, M. Inflammatory cells and oxygen radicals. Curr. Drug Targets Inflamm. Allergy 2005, 4, 503–504. [Google Scholar] [CrossRef] [PubMed]
- Comhair, S.A.; Bhathena, P.R.; Dweik, R.A.; Kavuru, M.; Erzurum, S.C. Rapid loss of superoxide dismutase activity during antigen-induced asthmatic response. Lancet 2000, 355, 624. [Google Scholar] [CrossRef]
- Comhair, S.A.; Xu, W.; Ghosh, S.; Thunnissen, F.B.; Almasan, A.; Calhoun, W.J.; Janocha, A.J.; Zheng, L.; Hazen, S.L.; Erzurum, S.C. Superoxide dismutase inactivation in pathophysiology of asthmatic airway remodeling and reactivity. Am. J. Pathol. 2005, 166, 663–674. [Google Scholar] [CrossRef]
- Ghosh, S.; Janocha, A.J.; Aronica, M.A.; Swaidani, S.; Comhair, S.A.; Xu, W.; Zheng, L.; Kaveti, S.; Kinter, M.; Hazen, S.L.; et al. Nitrotyrosine proteome survey in asthma identifies oxidative mechanism of catalase inactivation. J. Immunol. 2006, 176, 5587–5597. [Google Scholar] [CrossRef] [PubMed]
- Varshavskii, B.; Trubnikov, G.V.; Galaktipmpva, L.P.; Koreniak, N.A.; Koledeznaia, I.L.; Oberemok, A.N. [Oxidant-antioxidant status of patients with bronchial asthma during inhalation and systemic glucocorticoid therapy]. Terapevticheskii Arkhiv 2003, 75, 21–24. [Google Scholar] [PubMed]
- Smith, L.J.; Shamsuddin, M.; Sporn, P.H.; Denenberg, M.; Anderson, J. Reduced superoxide dismutase in lung cells of patients with asthma. Free Radic. Biol. Med. 1997, 22, 1301–1307. [Google Scholar] [CrossRef]
- Kinnula, V.L.; Crapo, J.D. Superoxide dismutases in the lung and human lung diseases. Am. J. Respir. Crit. Care Med. 2003, 167, 1600–1619. [Google Scholar] [CrossRef] [PubMed]
- Comhair, S.A.; Ricci, K.S.; Arroliga, M.; Lara, A.R.; Dweik, R.A.; Song, W.; Hazen, S.L.; Bleecker, E.R.; Busse, W.W.; Chung, K.F.; et al. Correlation of systemic superoxide dismutase deficiency to airflow obstruction in asthma. Am. J. Respir. Crit. Care Med. 2005, 172, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Burbank, A.J.; Duran, C.G.; Pan, Y.; Burns, P.; Jones, S.; Jiang, Q.; Yang, C.; Jenkins, S.; Wells, H.; Alexis, N.; et al. Gamma tocopherol-enriched supplement reduces sputum eosinophilia and endotoxin-induced sputum neutrophilia in volunteers with asthma. J. Allergy Clin. Immunol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Lang, M.R.; Fiaux, G.W.; Gillooly, M.; Stewart, J.A.; Hulmes, D.J.; Lamb, D. Collagen content of alveolar wall tissue in emphysematous and non-emphysematous lungs. Thorax 1994, 49, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Janssens, J.P.; Pache, J.C.; Nicod, L.P. Physiological changes in respiratory function associated with ageing. Eur. Respir. J. 1999, 13, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Verbeken, E.K.; Cauberghs, M.; Mertens, I.; Clement, J.; Lauweryns, J.M.; Van de Woestijne, K.P. The senile lung. Comparison with normal and emphysematous lungs. 1. Structural aspects. Chest 1992, 101, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Dyer, C. The interaction of ageing and lung disease. Chronic Respir. Dis. 2012, 9, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.R. Structural and physiological age-associated changes in aging lungs. Semin. Respir. Crit. Care Med. 2010, 31, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Niewoehner, D.E.; Kleinerman, J. Morphologic basis of pulmonary resistance in the human lung and effects of aging. J. Appl. Physiol. 1974, 36, 412–418. [Google Scholar] [PubMed]
- Kim, J.; Heise, R.L.; Reynolds, A.M.; Pidaparti, R.M. Aging effects on airflow dynamics and lung function in human bronchioles. PLoS ONE 2017, 12, e0183654. [Google Scholar] [CrossRef] [PubMed]
- Childs, B.G.; Durik, M.; Baker, D.J.; van Deursen, J.M. Cellular senescence in aging and age-related diseaserom mechanisms to therapy. Nat. Med. 2015, 21, 1424–1435. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Hales, C.N.; Ozanne, S.E. DNA damage, cellular senescence and organismal ageingausal or correlative? Nucleic Acids. Res. 2007, 35, 7417–7428. [Google Scholar] [CrossRef] [PubMed]
- Ochs, M.; Weibel, E.R. Functional design of the human lung for gas exchange. In Fishman’s Pulmonary Disease and Disorders, 4th ed.; Fishman, A.P., Ed.; McGraw Hill: New York, NY, USA, 2008; pp. 23–70. [Google Scholar]
- Pignatti, P.; Ragnoli, B.; Radaeli, A.; Moscato, G.; Malerba, M. Age-related increase of airway neutrophils in older healthy nonsmoking subjects. Rejuvenation Res. 2011, 14, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.C.; Rosenthal, N.S.; Soergel, P.; Peterson, K. Neutrophils and low-grade inflammation in the seemingly normal aging human lung. Mech. Ageing Dev. 1998, 104, 169–181. [Google Scholar] [CrossRef]
- Carpagnano, G.E.; Turchiarelli, V.; Spanevello, A.; Palladino, G.P.; Barbaro, M.P. Aging and airway inflammation. Aging Clin. Exp. Res. 2013, 25, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Spanevello, A.; Confalonieri, M.; Sulotto, F.; Romano, F.; Balzano, G.; Migliori, G.B.; Bianchi, A.; Michetti, G. Induced sputum cellularity. Reference values and distribution in normal volunteers. Am. J. Respir. Crit. Care Med. 2000, 162, 1172–1174. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.A.; Green, R.H.; Brightling, C.E.; Birring, S.S.; Parker, D.; Wardlaw, A.J.; Pavord, I.D. The influence of age on induced sputum differential cell counts in normal subjects. Chest 2004, 126, 1811–1814. [Google Scholar] [CrossRef]
- Fulop, T., Jr.; Fouquet, C.; Allaire, P.; Perrin, N.; Lacombe, G.; Stankova, J.; Rola-Pleszczynski, M.; Gagne, D.; Wagner, J.R.; Khalil, A.; et al. Changes in apoptosis of human polymorphonuclear granulocytes with aging. Mech. Ageing Dev. 1997, 96, 15–34. [Google Scholar] [CrossRef]
- Fortin, C.F.; Lesur, O.; Fulop, T., Jr. Effects of aging on triggering receptor expressed on myeloid cells (TREM)-1-induced PMN functions. FEBS Lett. 2007, 581, 1173–1178. [Google Scholar] [CrossRef] [PubMed]
- Tortorella, C.; Simone, O.; Piazzolla, G.; Stella, I.; Cappiello, V.; Antonaci, S. Role of phosphoinositide 3-kinase and extracellular signal-regulated kinase pathways in granulocyte macrophage-colony-stimulating factor failure to delay fas-induced neutrophil apoptosis in elderly humans. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- Hearps, A.C.; Martin, G.E.; Angelovich, T.A.; Cheng, W.J.; Maisa, A.; Landay, A.L.; Jaworowski, A.; Crowe, S.M. Aging is associated with chronic innate immune activation and dysregulation of monocyte phenotype and function. Aging Cell 2012, 11, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Plowden, J.; Renshaw-Hoelscher, M.; Engleman, C.; Katz, J.; Sambhara, S. Innate immunity in agingmpact on macrophage function. Aging Cell 2004, 3, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Plackett, T.P.; Boehmer, E.D.; Faunce, D.E.; Kovacs, E.J. Aging and innate immune cells. J. Leukoc. Biol. 2004, 76, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Esparza, B.; Sanchez, H.; Ruiz, M.; Barranquero, M.; Sabino, E.; Merino, F. Neutrophil function in elderly persons assessed by flow cytometry. Immunol. Investig. 1996, 25, 185–190. [Google Scholar] [CrossRef]
- Nogueira-Neto, J.; Cardoso, A.S.; Monteiro, H.P.; Fonseca, F.L.; Ramos, L.R.; Junqueira, V.B.; Simon, K.A. Basal neutrophil function in human aging: Implications in endothelial cell adhesion. Cell Biol. Int. 2016, 40, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Hazeldine, J.; Harris, P.; Chapple, I.L.; Grant, M.; Greenwood, H.; Livesey, A.; Sapey, E.; Lord, J.M. Impaired neutrophil extracellular trap formation novel defect in the innate immune system of aged individuals. Aging Cell 2014, 13, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Wessels, I.; Jansen, J.; Rink, L.; Uciechowski, P. Immunosenescence of polymorphonuclear neutrophils. Sci. World J. 2010, 10, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, G.; Balistreri, C.R.; Candore, G.; Cigna, D.; Colombo, A.; Romano, G.C.; Colucci, A.T.; Gervasi, F.; Listi, F.; Potestio, M.; et al. Granulocyte and natural killer activity in the elderly. Mech. Ageing Dev. 1999, 108, 25–38. [Google Scholar] [CrossRef]
- Kovalenko, E.I.; Boyko, A.A.; Semenkov, V.F.; Lutsenko, G.V.; Grechikhina, M.V.; Kanevskiy, L.M.; Azhikina, T.L.; Telford, W.G.; Sapozhnikov, A.M. ROS production, intracellular HSP70 levels and their relationship in human neutrophilsffects of age. Oncotarget 2014, 5, 11800–11812. [Google Scholar] [CrossRef] [PubMed]
- Castelo-Branco, C.; Soveral, I. The immune system and aging review. Gynecol. Endocrinol. 2014, 30, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.J.; Nelson, C.E.; Brodie, E.L.; Desantis, T.Z.; Baek, M.S.; Liu, J.; Woyke, T.; Allgaier, M.; Bristow, J.; Wiener-Kronish, J.P.; et al. Airway microbiota and bronchial hyperresponsiveness in patients with suboptimally controlled asthma. J. Allergy Clin. Immunol. 2011, 127, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Chotirmall, S.H.; Burke, C.M. Aging and the microbiomemplications for asthma in the elderly? Expert Rev. Respir. Med. 2015, 9, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.A.; Chotirmall, S.H. The Impact of Immunosenescence on Pulmonary Disease. Med. Inflamm. 2015, 2015, 692546. [Google Scholar] [CrossRef] [PubMed]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarizationn vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Nyugen, J.; Agrawal, S.; Gollapudi, S.; Gupta, S. Impaired functions of peripheral blood monocyte subpopulations in aged humans. J. Clin. Immunol. 2010, 30, 806–813. [Google Scholar] [CrossRef] [PubMed]
- Mahbub, S.; Deburghgraeve, C.R.; Kovacs, E.J. Advanced age impairs macrophage polarization. J. Interferon Cytokine Res. 2012, 32, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Brandenberger, C.; Muhlfeld, C. Mechanisms of lung aging. Cell Tissue Res. 2017, 367, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Van Duin, D.; Mohanty, S.; Thomas, V.; Ginter, S.; Montgomery, R.R.; Fikrig, E.; Allore, H.G.; Medzhitov, R.; Shaw, A.C. Age-associated defect in human TLR-1/2 function. J. Immunol. 2007, 178, 970–975. [Google Scholar] [CrossRef] [PubMed]
- Kong, K.F.; Delroux, K.; Wang, X.; Qian, F.; Arjona, A.; Malawista, S.E.; Fikrig, E.; Montgomery, R.R. Dysregulation of TLR3 impairs the innate immune response to West Nile virus in the elderly. J. Virol. 2008, 82, 7613–7623. [Google Scholar] [CrossRef] [PubMed]
- Van Duin, D.; Allore, H.G.; Mohanty, S.; Ginter, S.; Newman, F.K.; Belshe, R.B.; Medzhitov, R.; Shaw, A.C. Prevaccine determination of the expression of costimulatory B7 molecules in activated monocytes predicts influenza vaccine responses in young and older adults. J. Infect. Dis. 2007, 195, 1590–1597. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, J.L.; Solana, R.; Alonso, M.C.; Pena, J. Changes in the expression of HLA-class II antigens on peripheral blood monocytes from aged humans. Dis. Markers 1990, 8, 85–91. [Google Scholar] [PubMed]
- Gayoso, I.; Sanchez-Correa, B.; Campos, C.; Alonso, C.; Pera, A.; Casado, J.G.; Morgado, S.; Tarazona, R.; Solana, R. Immunosenescence of human natural killer cells. J. Innate Immun. 2011, 3, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Campos, C.; Pera, A.; Lopez-Fernandez, I.; Alonso, C.; Tarazona, R.; Solana, R. Proinflammatory status influences NK cells subsets in the elderly. Immunol. Lett. 2014, 162, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Campos, C.; Pera, A.; Sanchez-Correa, B.; Alonso, C.; Lopez-Fernandez, I.; Morgado, S.; Tarazona, R.; Solana, R. Effect of age and CMV on NK cell subpopulations. Exp. Gerontol. 2014, 54, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Beli, E.; Clinthorne, J.F.; Duriancik, D.M.; Hwang, I.; Kim, S.; Gardner, E.M. Natural killer cell function is altered during the primary response of aged mice to influenza infection. Mech. Ageing Dev. 2011, 132, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.C.; Chan, K.N.; Hu, W.H.; Lam, W.K.; Zheng, L.; Tipoe, G.L.; Sun, J.; Leung, R.; Tsang, K.W. The effect of aging on nasal mucociliary clearance, beat frequency, and ultrastructure of respiratory cilia. Am. J. Respir. Crit. Care Med. 2001, 163, 983–988. [Google Scholar] [CrossRef] [PubMed]
- Svartengren, M.; Falk, R.; Philipson, K. Long-term clearance from small airways decreases with age. Eur. Respir. J. 2005, 26, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Bailey, K.L.; Bonasera, S.J.; Wilderdyke, M.; Hanisch, B.W.; Pavlik, J.A.; DeVasure, J.; Robinson, J.E.; Sisson, J.H.; Wyatt, T.A. Aging causes a slowing in ciliary beat frequency, mediated by PKCepsilon. Am. J. Physiol. Lung Cell Mol. Physiol. 2014, 306, L584–L589. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Martinez, M.; Rodriguez-Flores, L.E.; Ancer-Arellano, A.; Cerda-Flores, R.M.; de-la-Garza-Gonzalez, C.; Ancer-Rodriguez, J.; Jaramillo-Rangel, G. Analysis of Cell Turnover in the Bronchiolar Epithelium Through the Normal Aging Process. Lung 2016, 194, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Zheng, D.; Limmon, G.V.; Leung, N.H.; Xu, S.; Rajapakse, J.C.; Yu, H.; Chow, V.T.; Chen, J. Aging exacerbates damage and delays repair of alveolar epithelia following influenza viral pneumonia. Respir. Res. 2014, 15, 116. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhang, H.; Davies, K.J.A.; Forman, H.J. Aging-related decline in the induction of Nrf2-regulated antioxidant genes in human bronchial epithelial cells. Redox Biol. 2017, 14, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Alder, J.K.; Barkauskas, C.E.; Limjunyawong, N.; Stanley, S.E.; Kembou, F.; Tuder, R.M.; Hogan, B.L.; Mitzner, W.; Armanios, M. Telomere dysfunction causes alveolar stem cell failure. Proc. Natl. Acad. Sci. USA 2015, 112, 5099–5104. [Google Scholar] [CrossRef] [PubMed]
- Mason, R.J.; Greene, K.; Voelker, D.R. Surfactant protein A and surfactant protein D in health and disease. Am. J. Physiol. 1998, 275, L1–L13. [Google Scholar] [PubMed]
- Whitsett, J.A. Surfactant proteins in innate host defense of the lung. Biol. Neonate 2005, 88, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Moliva, J.I.; Rajaram, M.V.; Sidiki, S.; Sasindran, S.J.; Guirado, E.; Pan, X.J.; Wang, S.H.; Ross, P., Jr.; Lafuse, W.P.; Schlesinger, L.S.; et al. Molecular composition of the alveolar lining fluid in the aging lung. AGE 2014, 36, 9633. [Google Scholar] [CrossRef] [PubMed]
- Ilumets, H.; Mazur, W.; Toljamo, T.; Louhelainen, N.; Nieminen, P.; Kobayashi, H.; Ishikawa, N.; Kinnula, V.L. Ageing and smoking contribute to plasma surfactant proteins and protease imbalance with correlations to airway obstruction. BMC Pulm. Med. 2011, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Agrawal, S.; Cao, J.N.; Su, H.; Osann, K.; Gupta, S. Altered innate immune functioning of dendritic cells in elderly humans role of phosphoinositide 3-kinase-signaling pathway. J. Immunol. 2007, 178, 6912–6922. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhao, J.; Legge, K.; Perlman, S. Age-related increases in PGD(2) expression impair respiratory DC migration, resulting in diminished T cell responses upon respiratory virus infection in mice. J. Clin. Investig. 2011, 121, 4921–4930. [Google Scholar] [CrossRef] [PubMed]
- Chougnet, C.A.; Thacker, R.I.; Shehata, H.M.; Hennies, C.M.; Lehn, M.A.; Lages, C.S.; Janssen, E.M. Loss of Phagocytic and Antigen Cross-Presenting Capacity in Aging Dendritic Cells Is Associated with Mitochondrial Dysfunction. J. Immunol. 2015, 195, 2624–2632. [Google Scholar] [CrossRef]
- Prakash, S.; Agrawal, S.; Vahed, H.; Ngyuen, M.; BenMohamed, L.; Gupta, S.; Agrawal, A. Dendritic cells from aged subjects contribute to chronic airway inflammation by activating bronchial epithelial cells under steady state. Mucosal Immunol. 2014, 7, 1386–1394. [Google Scholar] [CrossRef]
- Agrawal, A.; Tay, J.; Ton, S.; Agrawal, S.; Gupta, S. Increased reactivity of dendritic cells from aged subjects to self-antigen, the human DNA. J. Immunol. 2009, 182, 1138–1145. [Google Scholar] [CrossRef]
- Panda, A.; Qian, F.; Mohanty, S.; van Duin, D.; Newman, F.K.; Zhang, L.; Chen, S.; Towle, V.; Belshe, R.B.; Fikrig, E.; et al. Age-associated decrease in TLR function in primary human dendritic cells predicts influenza vaccine response. J. Immunol. 2010, 184, 2518–2527. [Google Scholar] [CrossRef]
- Ademokun, A.; Wu, Y.C.; Dunn-Walters, D. The ageing B cell populationomposition and function. Biogerontology 2010, 11, 125–137. [Google Scholar] [CrossRef]
- Chong, Y.; Ikematsu, H.; Yamaji, K.; Nishimura, M.; Nabeshima, S.; Kashiwagi, S.; Hayashi, J. CD27(+) (memory) B cell decrease and apoptosis-resistant CD27(-) (naive) B cell increase in aged humansmplications for age-related peripheral B cell developmental disturbances. Int. Immunol. 2005, 17, 383–390. [Google Scholar] [CrossRef]
- Koch, S.; Larbi, A.; Derhovanessian, E.; Ozcelik, D.; Naumova, E.; Pawelec, G. Multiparameter flow cytometric analysis of CD4 and CD8 T cell subsets in young and old people. Immun. Ageing 2008, 5, 6. [Google Scholar] [CrossRef]
- Larbi, A.; Dupuis, G.; Douziech, N.; Khalil, A.; Fulop, T., Jr. Low-grade inflammation with aging has consequences for T-lymphocyte signaling. Ann. N. Y. Acad. Sci. 2004, 1030, 125–133. [Google Scholar] [CrossRef]
- Douek, D.C.; McFarland, R.D.; Keiser, P.H.; Gage, E.A.; Massey, J.M.; Haynes, B.F.; Polis, M.A.; Haase, A.T.; Feinberg, M.B.; Sullivan, J.L.; et al. Changes in thymic function with age and during the treatment of HIV infection. Nature 1998, 396, 690–695. [Google Scholar] [CrossRef]
- Fagnoni, F.F.; Vescovini, R.; Passeri, G.; Bologna, G.; Pedrazzoni, M.; Lavagetto, G.; Casti, A.; Franceschi, C.; Passeri, M.; Sansoni, P. Shortage of circulating naive CD8(+) T cells provides new insights on immunodeficiency in aging. Blood 2000, 95, 2860–2868. [Google Scholar]
- Gupta, S.; Bi, R.; Su, K.; Yel, L.; Chiplunkar, S.; Gollapudi, S. Characterization of naive, memory and effector CD8+ T cellsffect of age. Exp. Gerontol. 2004, 39, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Doe, C.; Bafadhel, M.; Siddiqui, S.; Desai, D.; Mistry, V.; Rugman, P.; McCormick, M.; Woods, J.; May, R.; Sleeman, M.A.; et al. Expression of the T helper 17-associated cytokines IL-17A and IL-17F in asthma and COPD. Chest 2010, 138, 1140–1147. [Google Scholar] [CrossRef] [PubMed]
- Vanaudenaerde, B.M.; Verleden, S.E.; Vos, R.; De Vleeschauwer, S.I.; Willems-Widyastuti, A.; Geenens, R.; Van Raemdonck, D.E.; Dupont, L.J.; Verbeken, E.K.; Meyts, I. Innate and adaptive interleukin-17-producing lymphocytes in chronic inflammatory lung disorders. Am. J. Respir. Crit. Care Med. 2011, 183, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Brandenberger, C.; Li, N.; Jackson-Humbles, D.N.; Rockwell, C.E.; Wagner, J.G.; Harkema, J.R. Enhanced allergic airway disease in old mice is associated with a Th17 response. Clin. Exp. Allergy 2014, 44, 1282–1292. [Google Scholar] [CrossRef] [PubMed]
- Diller, M.L.; Kudchadkar, R.R.; Delman, K.A.; Lawson, D.H.; Ford, M.L. Balancing Inflammation: The Link between Th17 and Regulatory T Cells. Med. Inflamm. 2016, 2016, 6309219. [Google Scholar] [CrossRef] [PubMed]
- Kinnula, V.L.; Chang, L.; Everitt, J.I.; Crapo, J.D. Oxidants and antioxidants in alveolar epithelial type II cellsn situ, freshly isolated, and cultured cells. Am. J. Physiol. 1992, 262, L69–L77. [Google Scholar] [PubMed]
- Kinnula, V.L.; Crapo, J.D.; Raivio, K.O. Generation and disposal of reactive oxygen metabolites in the lung. Lab. Investig. 1995, 73, 3–19. [Google Scholar] [PubMed]
- Kinnula, V.L.; Whorton, A.R.; Chang, L.Y.; Crapo, J.D. Regulation of hydrogen peroxide generation in cultured endothelial cells. Am. J. Respir. Cell Mol. Biol. 1992, 6, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, A.; Siddiqui, N.; Alharbi, N.O.; Alharbi, M.M. Airway and systemic oxidant-antioxidant dysregulation in asthma possible scenario of oxidants spill over from lung into blood. Pulm. Pharmacol. Ther. 2014, 29, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Dunn, R.; Wechsler, M.E. Reducing asthma attacks in patients with severe asthma: The role of bronchial thermoplasty. Allergy Asthma Proc. 2015, 36, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Oraka, E.; Kim, H.J.; King, M.E.; Callahan, D.B. Asthma prevalence among US elderly by age groupsge still matters. J. Asthma 2012, 49, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Hanania, N.A.; King, M.J.; Braman, S.S.; Saltoun, C.; Wise, R.A.; Enright, P.; Falsey, A.R.; Mathur, S.K.; Ramsdell, J.W.; Rogers, L.; et al. Asthma in the elderly: Current understanding and future research needs--a report of a National Institute on Aging (NIA) workshop. J. Allergy Clin. Immunol. 2011, 128, S4–S24. [Google Scholar] [CrossRef] [PubMed]
- Park, H.W.; Song, W.J.; Kim, S.H.; Park, H.K.; Kim, S.H.; Kwon, Y.E.; Kwon, H.S.; Kim, T.B.; Chang, Y.S.; Cho, Y.S.; et al. Classification and implementation of asthma phenotypes in elderly patients. Ann. Allergy Asthma Immunol. 2015, 114, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Busse, P.J.; Birmingham, J.M.; Calatroni, A.; Manzi, J.; Goryachokovsky, A.; Fontela, G.; Federman, A.D.; Wisnivesky, J.P. Effect of aging on sputum inflammation and asthma control. J. Allergy Clin. Immunol. 2017, 139, 1808–1818. [Google Scholar] [CrossRef] [PubMed]
- Ducharme, M.E.; Prince, P.; Hassan, N.; Nair, P.; Boulet, L.P. Expiratory flows and airway inflammation in elderly asthmatic patients. Respir. Med. 2011, 105, 1284–1289. [Google Scholar] [CrossRef] [PubMed]
- Nyenhuis, S.M.; Schwantes, E.A.; Evans, M.D.; Mathur, S.K. Airway neutrophil inflammatory phenotype in older subjects with asthma. J. Allergy Clin. Immunol. 2010, 125, 1163–1165. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Linderholm, A.; Franzi, L.; Kenyon, N.; Grasberger, H.; Harper, R. Dual oxidase regulates neutrophil recruitment in allergic airways. Free Radic. Biol. Med. 2013, 65, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Summers, C.; Rankin, S.M.; Condliffe, A.M.; Singh, N.; Peters, A.M.; Chilvers, E.R. Neutrophil kinetics in health and disease. Trends Immunol. 2010, 31, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Bai, T.R.; Cooper, J.; Koelmeyer, T.; Pare, P.D.; Weir, T.D. The effect of age and duration of disease on airway structure in fatal asthma. Am. J. Respir. Crit. Care Med. 2000, 162, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Inoue, H.; Niimi, A.; Takeda, T.; Matsumoto, H.; Ito, I.; Matsuoka, H.; Jinnai, M.; Otsuka, K.; Oguma, T.; Nakaji, H.; et al. Pathophysiological characteristics of asthma in the elderly comprehensive study. Ann. Allergy Asthma Immunol. 2014, 113, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Leclere, M.; Lavoie-Lamoureux, A.; Lavoie, J.P. Heaves, an asthma-like disease of horses. Respirology 2011, 16, 1027–1046. [Google Scholar] [CrossRef] [PubMed]
- Horohov, D.W. The equine immune responses to infectious and allergic disease model for humans? Mol. Immunol. 2015, 66, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Couetil, L.L.; Cardwell, J.M.; Gerber, V.; Lavoie, J.P.; Leguillette, R.; Richard, E.A. Inflammatory Airway Disease of Horses-Revised Consensus Statement. J. Vet. Intern. Med. 2016, 30, 503–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexander, A.F. Chronic alveolar emphysema in the horse. Am. Rev. Respir. Dis. 1959, 80, 141–146. [Google Scholar] [PubMed]
- Bosshard, S.; Gerber, V. Evaluation of coughing and nasal discharge as early indicators for an increased risk to develop equine recurrent airway obstruction (RAO). J. Vet. Intern. Med. 2014, 28, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, J.W.; Reid, S.W.; Christley, R.M. A survey of horse owners in Great Britain regarding horses in their care. Part 2: Risk factors for recurrent airway obstruction. Equine Vet. J. 2007, 39, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Hansen, S.; Baptiste, K.E.; Fjeldborg, J.; Horohov, D.W. A review of the equine age-related changes in the immune systemomparisons between human and equine aging, with focus on lung-specific immune-aging. Ageing Res. Rev. 2015, 20, 11–23. [Google Scholar] [CrossRef] [PubMed]
- McFarlane, D. Immune Dysfunction in Aged Horses. Vet. Clin. N. Am. Equine Pract. 2016, 32, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Adams, A.A.; Breathnach, C.C.; Katepalli, M.P.; Kohler, K.; Horohov, D.W. Advanced age in horses affects divisional history of T cells and inflammatory cytokine production. Mech. Ageing Dev. 2008, 129, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Adams, A.A.; Katepalli, M.P.; Kohler, K.; Reedy, S.E.; Stilz, J.P.; Vick, M.M.; Fitzgerald, B.P.; Lawrence, L.M.; Horohov, D.W. Effect of body condition, body weight and adiposity on inflammatory cytokine responses in old horses. Vet. Immunol. Immunopathol. 2009, 127, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Hansen, S.; Sun, L.; Baptiste, K.E.; Fjeldborg, J.; Horohov, D.W. Age-related changes in intracellular expression of IFN-gamma and TNF-alpha in equine lymphocytes measured in bronchoalveolar lavage and peripheral blood. Dev. Comp. Immunol. 2013, 39, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Lavoie-Lamoureux, A.; Leclere, M.; Lemos, K.; Wagner, B.; Lavoie, J.P. Markers of Systemic Inflammation in Horses with Heaves. J. Vet. Intern. Med. 2012, 26, 1419–1426. [Google Scholar] [CrossRef] [PubMed]
- Leclere, M.; Bedard, C.; Cortes-Dubly, M.L.; Lavoie, J.P. Blood hypercoagulability and systemic inflammation in horses with heaves. Vet. J. 2015, 206, 105–107. [Google Scholar] [CrossRef] [PubMed]
- Debrue, M.; Hamilton, E.; Joubert, P.; Lajoie-Kadoch, S.; Lavoie, J.P. Chronic exacerbation of equine heaves is associated with an increased expression of interleukin-17 mRNA in bronchoalveolar lavage cells. Vet. Immunol. Immunopathol. 2005, 105, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Cordeau, M.E.; Joubert, P.; Dewachi, O.; Hamid, Q.; Lavoie, J.P. IL-4, IL-5 and IFN-gamma mRNA expression in pulmonary lymphocytes in equine heaves. Vet. Immunol. Immunopathol. 2004, 97, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, J.P.; Maghni, K.; Desnoyers, M.; Taha, R.; Martin, J.G.; Hamid, Q.A. Neutrophilic airway inflammation in horses with heaves is characterized by a Th2-type cytokine profile. Am. J. Respir. Crit. Care Med. 2001, 164, 1410–1413. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, D.M.; Grunig, G.; Matychak, M.B.; Young, J.; Wagner, B.; Erb, H.N.; Antczak, D.F. Recurrent airway obstruction (RAO) in horses is characterized by IFN-gamma and IL-8 production in bronchoalveolar lavage cells. Vet. Immunol. Immunopathol. 2003, 96, 83–91. [Google Scholar] [CrossRef]
- Ainsworth, D.M.; Wagner, B.; Erb, H.N.; Young, J.C.; Retallick, D.E. Effects of in vitro exposure to hay dust on expression of interleukin-17, -23, -8, and -1beta and chemokine (C-X-C motif) ligand 2 by pulmonary mononuclear cells isolated from horses chronically affected with recurrent airway disease. Am. J. Vet. Res. 2007, 68, 1361–1369. [Google Scholar] [CrossRef] [PubMed]
- McFarlane, D.; Hill, K.; Anton, J. Neutrophil function in healthy aged horses and horses with pituitary dysfunction. Vet. Immunol. Immunopathol. 2015, 165, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Herteman, N.; Vargas, A.; Lavoie, J.P. Characterization of Circulating Low-Density Neutrophils Intrinsic Properties in Healthy and Asthmatic Horses. Sci. Rep. 2017, 7, 7743. [Google Scholar] [CrossRef] [PubMed]
- Christmann, U.; Hite, R.D.; Witonsky, S.G.; Elvinger, F.; Werre, S.R.; Thatcher, C.D.; Tan, R.H.; Buechner-Maxwell, V.A. Influence of age on surfactant isolated from healthy horses maintained on pasture. J. Vet. Intern. Med. 2009, 23, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Gerber, V.; Robinson, N.E.; Luethi, S.; Marti, E.; Wampfler, B.; Straub, R. Airway inflammation and mucus in two age groups of asymptomatic well-performing sport horses. Equine Vet. J. 2003, 35, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Christmann, U.; Hite, R.D.; Tan, R.H.; Thatcher, C.D.; Witonsky, S.G.; Werre, S.R.; Buechner-Maxwell, V.A. Surfactant alterations in horses with recurrent airway obstruction at various clinical stages. Am. J. Vet. Res. 2010, 71, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Christmann, U.; Welles, E.G.; Waldridge, B.M.; Schumacher, J.; Grier, B.L.; Hite, R.D. Abnormalities in lung surfactant in horses clinically affected with recurrent airway obstruction (RAO). J. Vet. Intern. Med. 2008, 22, 1452–1455. [Google Scholar] [CrossRef] [PubMed]
- Andersson, S.; Kheiter, A.; Merritt, T.A. Oxidative inactivation of surfactants. Lung 1999, 177, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Aguilera-Tejero, E.; Estepa, J.C.; Lopez, I.; Mayer-Valor, R.; Rodriguez, M. Arterial blood gases and acid-base balance in healthy young and aged horses. Equine Vet. J. 1998, 30, 352–354. [Google Scholar] [CrossRef] [PubMed]
- Horohov, D.W.; Adams, A.A.; Chambers, T.M. Immunosenescence of the equine immune system. J. Comp. Pathol. 2010, 142 (Suppl. 1), S78–S84. [Google Scholar] [CrossRef] [PubMed]
- Lavoie-Lamoureux, A.; Beauchamp, G.; Quessy, S.; Martin, J.G.; Lavoie, J.P. Systemic inflammation and priming of peripheral blood leukocytes persist during clinical remission in horses with heaves. Vet. Immunol. Immunopathol. 2012, 146, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Katepalli, M.P.; Adams, A.A.; Lear, T.L.; Horohov, D.W. The effect of age and telomere length on immune function in the horse. Dev. Comp. Immunol. 2008, 32, 1409–1415. [Google Scholar] [CrossRef] [PubMed]
- Wnuk, M.; Bugno-Poniewierska, M.; Lewinska, A.; Oklejewicz, B.; Zabek, T.; Bartosz, G.; Slota, E. Age-related changes in genomic stability of horses. Mech. Ageing Dev. 2011, 132, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Marlin, D.J.; Johnson, L.; Kingston, D.A.; Smith, N.C.; Deaton, C.M.; Mann, S.; Heaton, P.; Van Vugt, F.; Saunders, K.; Kydd, J.; et al. Application of the comet assay for investigation of oxidative DNA damage in equine peripheral blood mononuclear cells. J. Nutr. 2004, 134, 2133S–2140S. [Google Scholar] [PubMed]
- Williams, C.A.; Gordon, M.E.; Betros, C.L.; McKeever, K.H. Apoptosis and antioxidant status are influenced by age and exercise training in horses. J. Anim. Sci. 2008, 86, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Deaton, C.M. The role of oxidative stress in an equine model of human asthma. Redox Rep. 2006, 11, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Deaton, C.M.; Marlin, D.J.; Smith, N.C.; Harris, P.A.; Roberts, C.A.; Schroter, R.C.; Kelly, F.J. Pulmonary epithelial lining fluid and plasma ascorbic acid concentrations in horses affected by recurrent airway obstruction. Am. J. Vet. Res. 2004, 65, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Kirschvink, N.; Bureau, F.; Art, T.; Lekeux, P. Bronchoconstrictive properties of inhaled 8-epi-PGF2alpha in healthy and heaves-susceptible horses. Vet. Res. 2001, 32, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Art, T.; Kirschvink, N.; Smith, N.; Lekeux, P. Indices of oxidative stress in blood and pulmonary epithelium lining fluid in horses suffering from recurrent airway obstruction. Equine Vet. J. 1999, 31, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Niedzwiedz, A.; Jaworski, Z. Oxidant-antioxidant status in the blood of horses with symptomatic recurrent airway obstruction (RAO). J. Vet. Intern. Med. 2014, 28, 1845–1852. [Google Scholar] [CrossRef] [PubMed]
- Deaton, C.M.; Marlin, D.J.; Smith, N.C.; Smith, K.C.; Newton, R.J.; Gower, S.M.; Cade, S.M.; Roberts, C.A.; Harris, P.A.; Schroter, R.C.; et al. Breath condensate hydrogen peroxide correlates with both airway cytology and epithelial lining fluid ascorbic acid concentration in the horse. Free Radic. Res. 2004, 38, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Deaton, C.M.; Marlin, D.J.; Smith, N.C.; Harris, P.A.; Dagleish, M.P.; Schroter, R.C.; Kelly, F.J. Effect of acute airway inflammation on the pulmonary antioxidant status. Exp. Lung Res. 2005, 31, 653–670. [Google Scholar] [CrossRef] [PubMed]
- Deaton, C.M.; Marlin, D.J.; Smith, N.C.; Harris, P.A.; Schroter, R.C.; Kelly, F.J. Antioxidant supplementation in horses affected by recurrent airway obstruction. J. Nutr. 2004, 134, 2065S–2067S. [Google Scholar]
- Kirschvink, N.; Fievez, L.; Bougnet, V.; Art, T.; Degand, G.; Smith, N.; Marlin, D.; Roberts, C.; Harris, P.; Lekeux, P. Effect of nutritional antioxidant supplementation on systemic and pulmonary antioxidant status, airway inflammation and lung function in heaves-affected horses. Equine Vet. J. 2002, 34, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Nogradi, N.; Couetil, L.L.; Messick, J.; Stochelski, M.A.; Burgess, J.R. Omega-3 fatty acid supplementation provides an additional benefit to a low-dust diet in the management of horses with chronic lower airway inflammatory disease. J. Vet. Intern. Med. 2015, 29, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.A. The effect of oxidative stress during exercise in the horse. J. Anim. Sci. 2016, 94, 4067–4075. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Pannell, B.K.; Liu, Z. Characterization and redox mechanism of asthma in the elderly. Oncotarget 2016, 7, 25010–25021. [Google Scholar] [CrossRef] [PubMed]
- Connolly, M.J.; Crowley, J.J.; Charan, N.B.; Nielson, C.P.; Vestal, R.E. Reduced subjective awareness of bronchoconstriction provoked by methacholine in elderly asthmatic and normal subjects as measured on a simple awareness scale. Thorax 1992, 47, 410–413. [Google Scholar] [CrossRef] [PubMed]
- Braman, S.S. Asthma in the Elderly. Clin. Geriatr. Med. 2017, 33, 523–537. [Google Scholar] [CrossRef] [PubMed]



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bullone, M.; Lavoie, J.-P. The Contribution of Oxidative Stress and Inflamm-Aging in Human and Equine Asthma. Int. J. Mol. Sci. 2017, 18, 2612. https://doi.org/10.3390/ijms18122612
Bullone M, Lavoie J-P. The Contribution of Oxidative Stress and Inflamm-Aging in Human and Equine Asthma. International Journal of Molecular Sciences. 2017; 18(12):2612. https://doi.org/10.3390/ijms18122612
Chicago/Turabian StyleBullone, Michela, and Jean-Pierre Lavoie. 2017. "The Contribution of Oxidative Stress and Inflamm-Aging in Human and Equine Asthma" International Journal of Molecular Sciences 18, no. 12: 2612. https://doi.org/10.3390/ijms18122612
APA StyleBullone, M., & Lavoie, J. -P. (2017). The Contribution of Oxidative Stress and Inflamm-Aging in Human and Equine Asthma. International Journal of Molecular Sciences, 18(12), 2612. https://doi.org/10.3390/ijms18122612