Hyaluronan Production by Renomedullary Interstitial Cells: Influence of Endothelin, Angiotensin II and Vasopressin
Abstract
:1. Introduction
2. Results
2.1. Hyaluronan (HA) in Supernatant
2.2. Hyaluronidase (Hyal) Activity in Supernatant
2.3. Hyaluronan Synthase (HAS) and Hyaluronidase mRNA in Renomedullary Interstitial Cells in Culture (RMICS)
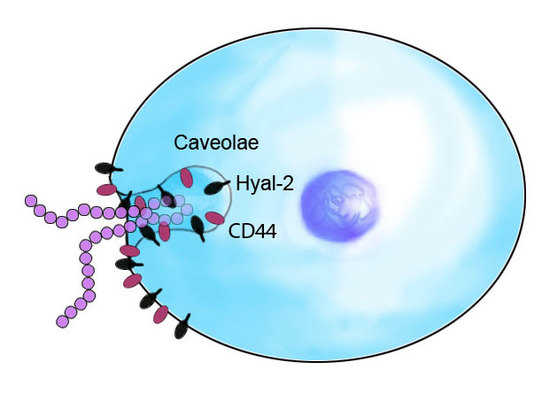
2.4. CD44 on RMIC Surface
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Culture of RMICs
4.3. Experimental Protocol—In Vitro
4.4. Analysis of HA
4.5. Hyaluronidase Activity in Supernatants
4.6. CD44 Analysis
4.7. Gene Expression Analysis
4.8. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| HA | Hyaluronan |
| HAS | Hyaluronan synthase |
| Hyal | Hyaluronidase |
| Ang II | Angiotensin II |
| RMICs | Renomedullary interstitial cells |
| ET-1 | Endothelin-1 |
References
- Laurent, T.C.; Fraser, J.R.E. Hyaluronan. FASEB J. 1992, 6, 2397–2404. [Google Scholar] [PubMed]
- Göransson, V.; Johnsson, C.; Nylander, O.; Hansell, P. Renomedullary and intestinal hyaluronan content during body water excess: A comparative study in rats and gerbils. J. Physiol. 2002, 542, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Hansell, P.; Göransson, V.; Odlind, C.; Gerdin, B.; Hällgren, R. Hyaluronan content in the kidney in different states of body hydration. Kidney Int. 2000, 58, 2061–2068. [Google Scholar] [CrossRef] [PubMed]
- Johnsson, C.; Tufveson, G.; Wahlberg, J.; Hällgren, R. Experimentally-induced warm renal ischemia induces cortical accumulation of hyaluronan in the kidney. Kidney Int. 1996, 50, 1224–1229. [Google Scholar] [CrossRef] [PubMed]
- Wells, A.F.; Larsson, E.; Tengblad, A.; Fellstrom, B.; Tufveson, G.; Klareskog, L.; Laurent, T.C. The localization of hyaluronan in normal and rejected human kidneys. Transplantation 1990, 50, 240–243. [Google Scholar] [CrossRef] [PubMed]
- Stridh, S.; Palm, F.; Hansell, P. Renal interstitial hyaluronan: Functional aspects during normal and pathological conditions. Am. J. Physiol. Regul. Physiol. 2012, 302, R1235–R1249. [Google Scholar] [CrossRef] [PubMed]
- Ginetzinsky, A.G. Role of hyaluronidase in the re-absorption of water in renal tubules: The mechanism of action of the antidiuretic hormone. Nature 1958, 182, 1218–1219. [Google Scholar] [CrossRef] [PubMed]
- Göransson, V.; Johnsson, C.; Jacobson, A.; Heldin, P.; Hallgren, R.; Hansell, P. Renal hyaluronan accumulation and hyaluronan synthase expression after ischaemia-reperfusion injury in the rat. Nephrol. Dial. Transplant. 2004, 19, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, A.B.M.; Johnsson, C.; Friberg, P.; Hansell, P. Renal cortical accumulation of hyaluronan in adult rats neonatally exposed to ACE inhibition. Acta Physiol. Scand. 2001, 173, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Colombaro, V.; Declèves, A.E.; Jadot, I.; Voisin, V.; Giordano, L.; Habsch, I.; Nonclercq, D.; Flamion, B.; Caron, N. Inhibition of hyaluronan is protective against renal ischaemia-reperfusion injury. Nephrol. Dial. Transplant. 2013, 28, 2484–2493. [Google Scholar] [CrossRef] [PubMed]
- Göransson, V.; Hansell, P.; Moss, S.; Alcorn, D.; Johnsson, C.; Hällgren, R.; Maric, C. Renomedullary interstitial cells in culture; the osmolality and oxygen tension influence the extracellular amounts of hyaluronan and cellular surface expression of CD44. Matrix Biol. 2001, 20, 129–136. [Google Scholar] [CrossRef]
- Hansell, P.; Maric, C.; Alcorn, D.; Göransson, V.; Johnsson, C.; Hällgren, R. Renomedullary interstitial cells regulate hyaluronan turnover depending on growth media osmolality suggesting a role in renal water handling. Acta Physiol. Scand. 1999, 165, 115–116. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, J.L. Renomedullary interstitial cells: A target for endocrine and paracrine actions of vasoactive peptides in the renal medulla. Clin. Exp. Pharmacol. Physiol. 2000, 27, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Culty, M.; Nguyen, H.A.; Underhill, C.B. The hyaluronan receptor (CD44) participates in the uptake and degradation of hyaluronan. J. Cell Biol. 1992, 116, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Aruffo, A.; Stamenkovic, I.; Melnick, M.; Underhill, C.B.; Seed, B. CD44 is the principal cell surface receptor for hyaluronate. Cell 1990, 61, 1303–1313. [Google Scholar] [CrossRef]
- Rügheimer, L.; Olerud, J.; Johnsson, C.; Takahashi, T.; Shimizu, K.; Hansell, P. Hyaluronan synthases and hyaluronidases in the kidney during changes in hydration status. Matrix Biol. 2009, 28, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Colombaro, V.; Jadot, I.; Declèves, A.E.; Voisin, V.; Giordano, L.; Habsch, I.; Malaisse, J.; Flamion, B.; Caron, N. Lack of hyaluronidases exacerbates renal post-ischemic injury, inflammation, and fibrosis. Kidney Int. 2015, 88, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Albeiroti, S.; Ayasoufi, K.; Hill, D.R.; Shen, B.; de la Motte, C.A. Platelet hyaluronidase-2: An enzyme that translocates to the surface upon activation to function in extracellular matrix degradation. Blood 2015, 125, 1460–1469. [Google Scholar] [CrossRef] [PubMed]
- Harada, H.; Takahashi, M. CD44-dependent intracellular and extracellular catabolism of hyaluronic acid by hyaluronidase-1 and -2. J. Biol. Chem. 2007, 282, 5597–5607. [Google Scholar] [CrossRef] [PubMed]
- Formby, B.; Stern, R. Lactate-sensitive response elements in genes involved in hyaluronan catabolism. Biochem. Biophys. Res. Commun. 2003, 305, 203–208. [Google Scholar] [CrossRef]
- Hua, Q.; Knudson, C.B.; Knudson, W. Internalization of hyaluronan by chondrocytes occurs via receptor-mediated endocytosis. J. Cell Sci. 1993, 106, 365–375. [Google Scholar] [PubMed]
- Asteriou, T.; Vincent, J.C.; Tranchepain, F.; Deschrevel, B. Inhibition of hyaluronan hydrolysis catalysed by hyaluronidase at high substrate concentration and low ionic strength. Matrix Biol. 2006, 25, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Edwards, R.M.; Pullen, M.; Nambi, P. Activation of endothelin ETB receptors increases glomerular cGMP via an L-arginine-dependent pathway. Am. J. Physiol. Ren. Physiol. 1992, 263, F1020–F1025. [Google Scholar]
- Harris, P.J.; Zhuo, J.; Mendelsohn, F.A.; Skinner, S.L. Haemodynamic and renal tubular effects of low doses of endothelin in anaesthetized rats. J. Physiol. 1991, 433, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Chenevier-Gobeaux, C.; Morin-Robinet, S.; Lemarechal, H.; Poiraudeau, S.; Ekindjian, J.C.; Borderie, D. Effects of pro- and anti-inflammatory cytokines and nitric oxide donors on hyaluronic acid synthesis by synovial cells from patients with rheumatoid arthritis. Clin. Sci. 2004, 107, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Deliu, E.; Brailoiu, G.C.; Mallilankaraman, K.; Wang, H.; Madesh, M.; Undieh, A.S.; Koch, W.J.; Brailoiu, E. Intracellular endothelin type B receptor-driven Ca2+ signal elicits nitric oxide production in endothelial cells. J. Biol. Chem. 2012, 287, 41023–41031. [Google Scholar] [CrossRef] [PubMed]
- Rügheimer, L.; Johnsson, C.; Hansell, P. Nitric Oxide and Prostaglandins Influence the Renomedullary Hyaluronan Content. In Hyaluronan—Its Structure, Metabolism, Biological Activities and Therapeutic Applications; Balazs, E.A., Hascall, V.C., Eds.; Winmar Enterprises: Edgewater, NJ, USA, 2005; Volume II, pp. 773–776. ISBN 0-9771359-0. [Google Scholar]
- De Nucci, G.; Thomas, R.; D’Orleans-Juste, P.; Antunes, E.; Walder, C.; Warner, T.D.; Vane, J.R. Pressor effects of circulating endothelin are limited by its removal in the pulmonary circulation and by the release of prostacyclin and endothelium-derived relaxing factor. Proc. Natl. Acad. Sci. USA 1988, 85, 9797–9800. [Google Scholar] [CrossRef] [PubMed]
- Warner, T.D.; Mitchell, J.A.; de Nucci, G.; Vane, J.R. Endothelin-1 and endothelin-3 release EDRF from isolated perfused arterial vessels of the rat and rabbit. J. Cardiovasc. Pharmacol. 1989, 13, S85–S88. [Google Scholar] [CrossRef] [PubMed]
- Honda, A.; Sekiguchi, Y.; Mori, Y. Prostaglandin E2 stimulates cyclic AMP-mediated hyaluronan synthesis in rabbit pericardial mesothelial cells. Biochem. J. 1993, 292, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Mahadevan, P.; Larkins, R.G.; Fraser, J.R.; Fosang, A.J.; Dunlop, M.E. Increased hyaluronan production in the glomeruli from diabetic rats: A link between glucose-induced prostaglandin production and reduced sulphated proteoglycan. Diabetologia 1995, 38, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Makiyama, Y.; Mitsui, Y. Endothelin-1 is involved in the growth promotion of vascular smooth muscle cells by hyaluronic acid. Int. J. Cardiol. 2000, 76, 39–47. [Google Scholar] [CrossRef]
- Hyndman, K.A.; Pollock, J.S. Nitric oxide and the A and B of endothelin of sodium homeostasis. Curr. Opin. Nephrol. Hypertens. 2013, 22, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Rügheimer, L.; Johnsson, C.; Maric, C.; Hansell, P. Hormonal regulation of renomedullary hyaluronan. Acta Physiol. (Oxford) 2008, 193, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, L.N.; Goryunova, T.E. Mechanism of the renal hyaluronate hydrolases activation in response to ADH. In Proceedings of the 28th International Congress of Physiological Sciences, Budapest, Hungary, 13–19 July 1980, Kidney and Body Fluids; Takacs, L., Ed.; Elsevier: Amsterdam, The Netherlands, 1981; pp. 587–591. ISBN 9781483153803. [Google Scholar]
- Ivanova, L.N.; Goryunova, T.E.; Nikiforovskaya, L.F.; Tishchenko, N.I. Hyaluronate hydrolase activity and glycosaminoglycans in the Brattleboro rat kidney. Ann. N. Y. Acad. Sci. 1982, 394, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Law, R.O.; Rowen, D. The influence of hyaluronidase on urinary and renal medullary composition following antidiuretic stimulus in the rat. J. Physiol. 1981, 311, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, L.N.; Babina, A.V.; Baturina, G.; Katkova, L.E. The effect of vasopressin on the expression of genes of key enzymes of the interstitial hyaluronan turnover and concentration ability in WAG rat kidneys. Russ. J. Genet. Appl. Res. 2017, 7, 249–257. [Google Scholar] [CrossRef]
- Stridh, S.; Kerjaschki, D.; Chen, Y.; Rügheimer, L.; Astrand, A.B.; Johnsson, C.; Friberg, P.; Olerud, J.; Palm, F.; Takahashi, T.; et al. Angiotensin converting enzyme inhibition blocks interstitial hyaluronan dissipation in the neonatal rat kidney via hyaluronan synthase 2 and hyaluronidase 1. Matrix Biol. 2011, 30, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Maric, C.; Aldred, G.P.; Antoine, A.M.; Eitle, E.; Dean, R.G.; Williams, D.A.; Harris, P.J.; Alcorn, D. Actions of endothelin-1 on cultured rat renomedullary interstitial cells are modulated by nitric oxide. Clin. Exp. Pharmacol. Physiol. 1999, 26, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Maric, C.; Zheng, W.; Walther, T. Interactions between Angiotensin II and Atrial Natriuretic Peptide in Renomedullary Interstitial Cells: The Role of Neutral Endopeptidase. Nephron Physiol. 2006, 103, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Spicer, A.P. Hyaluronan: A multifunctional, megaDalton, stealth molecule. Curr. Opin. Cell Biol. 2000, 12, 581–586. [Google Scholar] [CrossRef]
- Botzki, A.; Rigden, D.J.; Braun, S.; Nukui, M.; Salmen, S.; Hoechstetter, J.; Bernhardt, G.; Dove, S.; Jedrzejas, M.J.; Buschauer, A. L-Ascorbic acid 6-hexadecanoate, a potent hyaluronidase inhibitor. X-ray structure and molecular modeling of enzyme-inhibitor complexes. J. Biol. Chem. 2004, 279, 45990–45997. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, T.M.; Banks, S.A.; Alonso-Galicia, M.; Cockrell, K.; Carroll, J.F.; Bigler, S.A.; Hall, J.E. Distribution of renal medullary hyaluronan in lean and obese rabbits. Kidney Int. 2000, 58, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Rosinger, A.Y.; Lawman, H.G.; Akinbami, L.J.; Ogden, C.L. The role of obesity in the relation between total water intake and urine osmolality in US adults, 2009–2012. Am. J. Clin. Nutr. 2016, 104, 1554–1561. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, J.; Dean, R.; Maric, C.; Aldred, P.G.; Harris, P.; Alcorn, D.; Mendelsohn, F.A. Localization and interactions of vasoactive peptide receptors in renomedullary interstitial cells of the kidney. Kidney Int. Suppl. 1998, 67, S22–S28. [Google Scholar] [CrossRef] [PubMed]
- Fontoura, B.M.A.; Nussenzveig, D.R.; Pelton, K.M.; Maack, T. Atrial natriuretic factor receptors in cultured renomedullary interstitial cells. Am. J. Physiol. Cell Physiol. 1990, 258, C692–C699. [Google Scholar]
- Maric, C.; Aldered, G.P.; Antoine, A.M.; Dean, R.G.; Eitle, E.; Mendelsohn, F.A.O.; Williams, D.A.; Harris, P.J.; Alcorn, D. Effects of angiotensin II on cultured rat renomedullary interstitial cells are mediated by AT1A receptors. Am. J. Physiol. Ren. Physiol. 1996, 271, F1020–F1028. [Google Scholar]
- Ikegami-Kawai, M.; Okuda, R.; Nemoto, T.; Inada, N.; Takahashi, T. Enhanced activity of serum and urinary hyaluronidases in streptozotocin-induced diabetic Wistar and GK rats. Glycobiology 2004, 14, 65–72. [Google Scholar] [CrossRef] [PubMed]









| Gene | Gene Accession | Forward Primer | Reverse Primer |
|---|---|---|---|
| G6PDH | NM_017006.2 | GTCATGCAGAACCACCTCCT | ACATACTGGCCAAGGACCAC |
| AktB | NM_031144.3 | GCCCTGGCTCCTAGCACC | CCACCAATCCACACAGAGTACTTG |
| TBP | NM_001004198.1 | ACCCTTCACCAATGACTCCTATG | ATGATGACTGCAGCAAATCGC |
| HAS2 | NM_013153.1 | GTGACTGCACCAGTTCCGCTAA | CATGTCTAATGGGACTGCACACAAG |
| Hyal1 | NM_207616.1 | TCGGACCCTTTATCCTGAAC | TTCTTACACCACTCTCCACTC |
| Hyal2 | NM_172040.2 | CGTTACGTCAAGGCAGTCAG | AGGTACACGGAGGGAAAGAG |
| Gene | Ct Average NOsm (n = 8) | Ct Average LOsm (n = 8) | Primer Efficiency (%) |
|---|---|---|---|
| G6PDH | 25.2 ± 0.2 | 25.5 ± 0.2 | 96.6 |
| AKTB | 24.4 ± 0.2 | 22.5 ± 0.2 | 98.3 |
| TBP | 17.1 ± 0.1 | 17.0 ± 0.2 | 120.1 |
| HAS2 | 27.3 ± 0.2 | 29.0 ± 0.3 | 104.0 |
| Hyal 1 | 27.3 ± 0.4 | 26.7 ± 0.4 | 124.5 |
| Hyal 2 | 23.6 ± 0.2 | 24.3 ± 0.2 | 125.1 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stridh, S.; Palm, F.; Takahashi, T.; Ikegami-Kawai, M.; Friederich-Persson, M.; Hansell, P. Hyaluronan Production by Renomedullary Interstitial Cells: Influence of Endothelin, Angiotensin II and Vasopressin. Int. J. Mol. Sci. 2017, 18, 2701. https://doi.org/10.3390/ijms18122701
Stridh S, Palm F, Takahashi T, Ikegami-Kawai M, Friederich-Persson M, Hansell P. Hyaluronan Production by Renomedullary Interstitial Cells: Influence of Endothelin, Angiotensin II and Vasopressin. International Journal of Molecular Sciences. 2017; 18(12):2701. https://doi.org/10.3390/ijms18122701
Chicago/Turabian StyleStridh, Sara, Fredrik Palm, Tomoko Takahashi, Mayumi Ikegami-Kawai, Malou Friederich-Persson, and Peter Hansell. 2017. "Hyaluronan Production by Renomedullary Interstitial Cells: Influence of Endothelin, Angiotensin II and Vasopressin" International Journal of Molecular Sciences 18, no. 12: 2701. https://doi.org/10.3390/ijms18122701
APA StyleStridh, S., Palm, F., Takahashi, T., Ikegami-Kawai, M., Friederich-Persson, M., & Hansell, P. (2017). Hyaluronan Production by Renomedullary Interstitial Cells: Influence of Endothelin, Angiotensin II and Vasopressin. International Journal of Molecular Sciences, 18(12), 2701. https://doi.org/10.3390/ijms18122701