1. Introduction
Prenatal development of humans and animals requires adequate placental supply of oxygen and nutrients [
1,
2], which, in turn, requires adequate maternal nutrition and placental function. Inadequate placental nutrient supply leads to intrauterine growth restriction (IUGR), resulting in newborns that are small for their gestational age. Such offspring may be predisposed to perinatal morbidity and mortality [
3] as well as lifelong chronic non-communicable disorders such as obesity, type II diabetes, hypertension and cardiovascular diseases [
4,
5,
6,
7].
Among humans, IUGR due to maternal malnutrition shows a much higher incidence in resource-challenged areas (15%) than in developed areas (6%) [
8,
9]. In recent years, incidence has been increasing in developed countries because of maternal eating disorders, voluntary intake restriction for aesthetic reasons [
10] and abnormal placental development leading to placental insufficiency [
11]. This abnormal placental development has been associated with postponement of childbearing, inadequate lifestyle and maternal and gestational factors [
8,
12,
13].
Previous studies from our group using a swine model of IUGR have highlighted the importance of maternal nutrition for proper foetal development. Maternal malnutrition compromises foetal metabolism and development, causing growth retardation and triggering adaptive changes in the foetus to increase likelihood of survival [
14]. Work from other groups has shown that the maternal lipid profile and placental efficiency are strongly related to cholesterol and triglyceride availability for human and other animal foetuses, which affects their viability and growth [
15,
16,
17,
18,
19,
20]. The developing foetus requires substantial amounts of lipids, mainly polyunsaturated fatty acids (PUFA), the availability of which is determined largely by maternal circulating levels and placental transfer [
21]. Impaired placental transfer of PUFA causes or exacerbates IUGR [
21,
22,
23] and may be caused by lipid peroxidation or other forms of oxidative stress [
24,
25].
Extensive research in humans and animal models has shown that the sex of the offspring can affect pre- and postnatal development in compromised pregnancies such as IUGR [
14,
26,
27,
28,
29,
30,
31]. Less clear is whether this sex effect extends to lipid homeorhesis. Therefore the present study exploited the power of the pig as a translational model [
32] to investigate sex-related differences in lipid availability and fatty acid composition during foetal development. The pig, like humans, is omnivorous and prone to sedentary behaviour and obesity, and the metabolism, cardiovascular system and relative organ sizes in pigs are similar to those in humans [
33,
34,
35,
36]. A further advantage of studying swine is that insights will impact not only research but also farm productivity. IUGR occurs often in swine because of inadequate maternal nutrition or placental insufficiency [
2,
37,
38], and it limits farm profitability [
14].
2. Results
Foetuses were obtained from five sows on Gestational Day (GD) 70 (n = 33) or from four sows on GD 90 (n = 23). The average number of foetuses per sow was 6.8 ± 3.8 on GD 70 and 5.8 ± 3.6 on GD 90. Of the 56 foetuses, 23 were female, of which 13 were recovered on GD 70 and 10 on GD 90; among the 33 male foetuses, 20 were recovered on GD 70 and 13 on GD 90. Hence, the sex ratio was close to 1:1, with males accounting for 57.7% of GD 70 foetuses and 56.5% of GD 90 foetuses. Evidence of IUGR was found in eight pregnancies, affecting four GD 70 foetuses (11%) and four GD 90 foetuses (17.4%).
2.1. Effects of Maternal Metabolic Status on Foetal Development
The size and weight of normal (non-IUGR) foetuses increased with gestational age (
Table 1), as did development of the placenta, liver, kidney and intestine, based on histology (all
p < 0.0001).
In these normal foetuses, body development correlated strongly with maternal lipid profile depicted in
Figure 1. Specifically, lower maternal plasma cholesterol concentrations correlated with lower foetal body weight (
r = 0.777,
p < 0.05) and corpulence in terms of trunk length (
r = 0.832), thoracic circumference (
r = 0.808) and abdominal circumference (
r = 0.958) (all
p < 0.01). Lower maternal plasma cholesterol concentrations also correlated with higher ratios of organ-to-body weight for brain (
r = −0.774), heart (
r = −0.752), liver (
r = −0.679), and spleen (
r = −0.712) (all
p < 0.01); these correlations reflect changes to improve foetal viability. Conversely, lower maternal plasma concentrations of high-density lipoprotein cholesterol (HDL-c) correlated with lower ratios of heart-to-body weight (
r = 0.677,
p < 0.05). Adaptive foetal growth mechanisms were observed already on GD 70, when lower maternal plasma cholesterol concentrations correlated with higher ratios of head-to-body weight (
r = −0.987,
p < 0.005) and heart-to-body weight (
r = −0.883,
p < 0.05). At the same time, maternal HDL-c concentrations correlated with higher ratios of brain-to-body weight (
r = −0.923) and spleen-to-body weight (
r = −0.891, both
p < 0.05).
Maternal plasma concentrations of glucose and fructosamine did not affect weight of normal foetuses on GD 70, but they did show an influence on GD 90. Lower plasma fructosamine concentrations correlated with lower foetal body weight (r = 0.964) as well as higher ratios of head-to-body weight (r = −0.962) and brain-to-head weight (r = −0.969, all p < 0.05). Similarly, lower maternal glucose levels correlated with higher ratios of head-to-body weight (r = −0.959) and brain-to-head weight (r = −0.963, both p < 0.05).
2.2. Effects of Foetal Sex on Developmental Trajectories during Pregnancy
No significant association was observed between offspring sex and foetal weight in normal foetuses (
Table 1), although among male foetuses collected on GD 90, we observed a trend toward higher total body weight (
p = 0.09) and carcass weight (
p = 0.06) compared to female foetuses. Fat content of the carcass, the
longissimus dorsi muscle or the liver did not vary significantly as a function of foetal sex or gestational age (
Table 2).
Male and female normal foetuses collected on GD 70 did not differ significantly in the relative weights or maturation states of several structures and organs. On GD 90, normal female foetuses showed a significantly higher degree of placental development (
p < 0.05) and significantly higher ratios of head-to-body weight and brain-to-body weight (both
p < 0.05;
Figure 2).
The comparison, on both GD 70 and 90, of foetuses with severe growth restriction and normal foetuses, showed smaller values than their littermates for body weight (GD 70, 126.5 ± 14.3 g vs. 183.4 ± 4.1 g,
p < 0.05; GD 90, 404.5 ± 30.9 g vs. 621.8 ± 14.5 g,
p < 0.005), body length (GD 70, 17.6 ± 0.9 cm vs. 20.5 ± 0.2 cm; GD 90, 27.0 ± 0.9 cm vs. 29.8 ± 0.4 cm; both
p < 0.05), head weight (GD 70, 35.3 ± 3.2 g vs. 48.2 ± 1 g,
p < 0.05; GD 90, 103.5 ± 6.3 g vs. 141.9 ± 3.5 g,
p < 0.005), carcass weight (GD 70, 66.2 ± 8.9 g vs. 100 ± 2.9 g,
p < 0.05; GD 90, 222.3 ± 18.7 g vs. 364.1 ± 9.6 g,
p < 0.005) and total weight of viscerae (GD 70, 18.2 ± 2 g vs. 28.3 ± 0.9 g,
p < 0.01; GD 90, 64.3 ± 7.3 g vs. 98 ± 2.3 g,
p < 0.05). However, the ratio of brain-to-body weight was significantly higher in foetuses with severe growth restriction than in littermates on GD 70 (0.048 ± 0.004 vs. 0.036 ± 0.001) and GD 90 (0.045 ± 0.004 vs. 0.033 ± 0.001; both
p < 0.01). This was also observed when only female foetuses were examined (
p < 0.0005 at both ages;
Figure 3). On GD 90, the ratios of brain-to-body weight and brain-to-carcass weight were significantly higher in foetuses with severe growth restriction (both
p < 0.0005), as were the ratios of liver- and spleen-to-body weight (both
p < 0.05;
Figure 3).
2.3. Changes in Foetal Metabolism during Pregnancy and Sex-Related Effects
Table 3 shows measurements of indicators of glucose and lipid metabolism based on markers in plasma, allantoic and amniotic fluids from normal foetuses. Comparison of measurements on GDs 70 and 90 shows that the availability of foetal triglycerides significantly decreased in foetal blood (
p < 0.05) and allantoic fluid (
p < 0.005) during pregnancy. Over the same period, the availability of foetal triglycerides in amniotic fluid increased (
p < 0.05). Low-density lipoprotein cholesterol (LDL-c) in foetal plasma decreased during pregnancy (
p < 0.005), while HDL-c increased (
p < 0.001). Total cholesterol decreased in amniotic fluid (
p < 0.0001) but increased in allantoic fluid (
p < 0.005). In both these compartments, HDL-c and LDL-c concentrations remained unchanged during pregnancy. Similar results were obtained for the two foetal sexes, except that LDL-c in blood decreased to a greater extent in females than males (
p < 0.05).
Similar changes to these described in normal foetuses were observed in the subset of foetuses showing severe IUGR, and no sex effects were observed in this case. Foetuses with severe IUGR showed lower plasma LDL-c concentrations than littermates on GD 90 (29.7 ± 2.5 mg/dL vs. 35.8 ± 1.03 mg/dL, p < 0.05).
2.4. Changes in Foetal Antioxidant/Oxidative Status during Pregnancy and Sex-Related Effects
Ferric reducing antioxidant power (FRAP), an index of antioxidant capacity, decreased from GD 70 (10.8 ± 1.3 µmol/mL) to GD 90 (9.9 ± 1.6 µmol/mL,
p < 0.005) in samples from normal foetuses, yet the concentration of malondialdehyde (MDA) in plasma, an index of total lipid oxidation, decreased during the same period from 20.6 ± 0.4 mmol/mL to 15.8 ± 0.6 mmol/mL (
p < 0.005). Nevertheless, the ratio of MDA to cholesterol, which takes into account lipid availability, indicated less relative oxidation on GD 70 than GD 90 (2.8 ± 0.9 vs. 3.5 ± 0.7). Similar results were obtained for the ratio of MDA to LDL-c (4.1 ± 1.5 vs. 6.1 ± 1.2) and the ratio of MDA to triglycerides (5.1 ± 1.9 vs. 8.0 ± 2.8) (all
p < 0.05;
Figure 4).
2.5. Changes in Foetal Muscle Fatty Acid Composition during Pregnancy and Sex-Related Effects
The neutral and polar fatty acid fractions of the
longissimus dorsi muscle in normal foetuses varied significantly with gestational age and foetal sex (
Table 4 and
Table 5). On GD 70, the neutral fraction of female normal foetuses contained significantly more α-linolenic fatty acid (C18:3n-3) and total saturated fatty acids (SFA) than the neutral fraction of male normal foetuses (both
p < 0.05), as well as less cis-vaccenic fatty acid (C18:1n-7) and lower ratios of ∑n-6/∑n-3 (
p < 0.01) and monounsaturated fatty acids (MUFA) to SFA (
p < 0.05). All these sex-related differences disappeared by GD 90.
During pregnancy, content of palmitoleic acid (C16:1n-7) and homo-γ-linolenic acid (C20:3n-6) increased in female normal foetuses (both p < 0.001), as did the ratios of ∑n-6/∑n-3 (p < 0.01) and C18:1/C18:0 (p < 0.005). Similar results were observed for male normal foetuses (∑n-6/∑n-3 p < 0.001 and C18:1/C18:0 p < 0.01, respectively). The content of several fatty acids decreased significantly during pregnancy, including cis-7 hexadecenoic acid (C16:1n-9), heptadecanoic acid (C17:1) and eicosapentaenoic acid (C20:5n-3) (all p < 0.001 in males and p < 0.05 in females), as well as stearic acid (C18:0) and erucic acid (C22:1n-9) (all p < 0.05 in males and females).
During pregnancy, male normal foetuses showed increases in myristic acid (C14:0, p < 0.05), palmitic acid (C16:0, p < 0.001) and linoleic acid (C18:2n-6, p < 0.001), while female normal foetuses showed increases in cis-vaccenic acid (C18:1n-7, p < 0.01) and total MUFA (p < 0.05). During pregnancy, male foetuses showed decreases in mead acid (C20:3n-9, p < 0.001), adrenic acid (C22:4n-6, p < 0.005), arachidonic acid (C20:4n-6, p < 0.01), docosapentaenoic acid (C22:5n-6, p < 0.05), total PUFA (p < 0.05), and the unsaturated index (UI; p < 0.01). Female foetuses showed a significant decrease in α-linolenic acid (p < 0.005).
Sex-specific differences were also observed in the composition of the polar fatty acid fraction of normal foetuses. Female foetuses had less palmitoleic, α-linolenic and mead acids than males (all p < 0.05) on GD 70 and 90. During pregnancy, several fatty acids increased significantly in both sexes, including stearic, α-linoleic, homo-γ-linolenic and erucic acids (all p < 0.0001); docosahexaenoic acid (C22:6n-3, p < 0.05); total PUFA (p < 0.05); n-6 PUFA (p < 0.05); and the C18:1/C18:0 ratio (p < 0.0001). Over the same period, several fatty acids decreased significantly in both sexes, including cis-7 hexadecenoic, heptadecanoic, cis-vaccenic, α-linolenic, adrenic and docosapentaenoic acids (all p < 0.005); and the MUFA/SFA ratio (p < 0.001). Female foetuses showed a significant decrease in heptadecanoic acid (p < 0.05), while males showed a significant decrease in mead acid (p < 0.05) and an increase in ∑n-6/∑n-3 ratio (p < 0.05).
2.6. Changes in Foetal Liver Composition during Pregnancy and Sex-Related Effects
Like the intramuscular fatty acid composition, the neutral fatty acid composition of the liver of normal foetuses varied significantly with gestational age and foetal sex (
Table 6). During pregnancy, several fatty acids increased in both sexes, including myristic, palmitic, palmitoleic, vaccenic and α-linoleic acids (all
p < 0.0001); mead acid (
p < 0.005); adrenic acid (
p < 0.001); and the C18:1/C18:0 ratio (
p < 0.005). Over the same period, several fatty acids decreased in both sexes: margaric (C17:0), heptadecanoic, stearic, and α-linolenic acids (all
p < 0.0001), as well as erucic acid (
p < 0.0005). In male foetuses, arachidonic and eicosapentaenoic acids decreased (both
p < 0.05), while in females, total SFA decreased (
p < 0.01).
On GD 70 and 90, female foetuses showed higher amounts of stearic acid and total SFA (both p < 0.05), but lower amounts of cis-7 hexadecenoic acid (p < 0.005), oleic acid (p < 0.05), and MUFA (p < 0.01), as well as lower ratios of C18:1/C18:0 (p < 0.01) and MUFA/SFA (p < 0.005). On GD 70, females showed higher content of heptadecanoic acid (p < 0.05) and lower content of cis-vaccenic acid (p < 0.01) than males. On GD 90, females showed lower content of heptadecanoic acid (p < 0.05) than males.
In contrast, the composition of the polar fatty acid fraction did not vary significantly between male and female normal foetuses (
Table 7), with the exception that α-linoleic acid content was higher in females than males on GD 70 (
p < 0.05). During pregnancy, both sexes showed increases in arachidonic acid (
p < 0.0005); margaric, stearic, linoleic, and mead acids (all
p < 0.0001); myristic acid (
p < 0.05); total PUFA and n-6 PUFA (both
p < 0.0001); and UI (
p < 0.005). During the same period, both sexes showed decreases in α-linolenic, erucic, cis-7 hexadecenoic and heptadecanoic acids (all
p < 0.0001); oleic acid (
p < 0.005); vaccenic acid (
p < 0.05); total MUFA (
p < 0.0001); and the ratios C18:1/C18:0 and MUFA/SFA (both
p < 0.0005).
3. Discussion
The results of the present study support the prominent role of the maternal metabolic profile on foetal development and homeorhesis and provide new insights on the effects of offspring sex on fetoplacental development, lipid availability and fatty acid composition at non-adipose tissues involved in metabolism regulation (muscle and liver). These data may be considered relevant for humans since despite differences in placentation, human and pig foetuses and pregnant females show similar lipid metabolism and distribution [
39].
In the current study, lower cholesterol concentration in maternal plasma correlated with deficiencies in foetal development, which it is consistently with previous studies on GD 42 [
16]. In contrast, poor availability of glucose, the main energy source for developing foetuses [
40], affected foetal development on GD 90 but not on GD 70 in our study, consistent with reports that hypoglycaemia in early development is non-critical and can be compensated [
41].
The higher growth of the brain when compared to total body observed in the foetuses affected by IUGR is consistent with the “brain-sparing effect” firstly described by Rudolph (1984) [
42]. There was also a higher growth of other organs (mainly heart, liver and spleen), which is also consistent with studies in humans documenting “heart- and liver-sparing” cardiovascular adaptations—analogous to the brain-sparing effect—in response to foetal malnutrition and hypoxia [
43,
44]. Previous studies address that changes in the growth, morphology and function of various organs are dependant on the timing and severity of nutritional restriction [
45,
46], with specifically a determinant effect triggered by lipid availability. At the same time, our results support previous work indicating sex-specific differences in the growth of the different organs [
27,
31].
Maternal undernutrition in our study clearly affected foetal lipid availability. The developing foetus and placenta require large amounts of lipids for: (a) synthesis of cholesterol, which acts as a key constituent of cell membranes and organelles and is the precursor of a range of hormones and metabolic regulators necessary for successful pregnancy [
47,
48,
49,
50]; (b) secretion of key products such as lipoproteins; and (c) storage of triglycerides [
47,
48,
49]. Although placental and foetal tissues can synthesize lipids de novo [
47,
51], the building blocks must be taken up by the placenta, i.e. maternal free fatty acids; triglycerides, which placental lipases hydrolyse into fatty acid constituents; and lipoprotein-associated cholesterol [
19,
21,
23,
51]. Triglycerides are a major source of energy for the foetus [
52,
53], but a balance is needed: low levels of triglycerides delay growth, while high levels can cause foetal macrosomia [
54]. Availability of lipids to the fetoplacental unit depends on de novo synthesis by the foetus as well as maternal transfer. Cholesterol, for example, can reach the foetal circulatory system after crossing the syncytiotrophoblast as LDL-c [
49,
55]. In pig foetuses, fatty acid availability and composition depend more on foetal synthesis from precursors transferred from the mother than on direct maternal transfer [
56,
57,
58,
59,
60,
61], since fatty acids do not easily cross the placenta in ruminants, pigs or horses [
62].
Lipid availability to the foetoplacental unit also depends on the antioxidant/oxidative status of the foetus. Our results suggest that, during pregnancy, antioxidant capacity decreases and lipid peroxidation increases in foetuses affected by maternal undernutrition, as suggested by previous studies [
24,
25,
26]. These changes may explain the lower lipid availability later in pregnancy.
Cholesterol and triglyceride availability in our study did not differ significantly between male and female foetuses, consistently with prior studies [
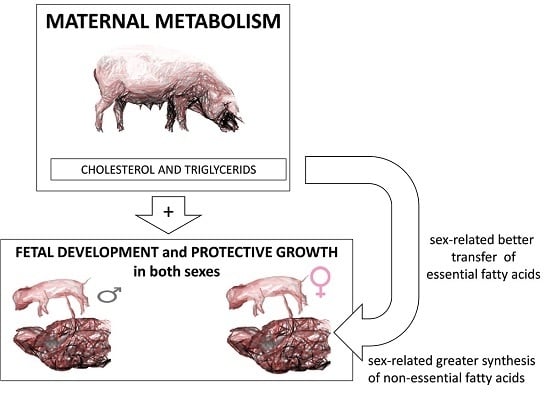
56]. Our data further show that the two sexes showed similar antioxidant capacity and lipid peroxidation. In other words, foetal development was affected to a much greater extent by gestational age—and therefore by nutritional restriction—than by sex. Nevertheless, we did observe sex-related differences in how the fatty acid composition of non-adipose tissues involved in metabolism regulation (muscle and liver) changed between GD 70 and 90 in foetuses affected by maternal undernutrition.
Our data indicate significant sex-related differences in content of essential fatty acids, which are so-called because they must be obtained from maternal transfer in the case of foetuses [
22,
63] or from diet in the case of adults [
64]. The major essential fatty acids are linolenic PUFA (an omega-3 fatty acid) and linoleic PUFA (an omega-6 fatty acid); the long-chain omega-3 PUFA eicosapentaenoic and docosahexaenoic acids and the long-chain omega-6 PUFA gamma-linolenic and arachidonic acids are also essential. Future work should examine the maternal and/or placental factors that may drive the sex-dependent differences in essential fatty acid availability that we observed.
Most of the differences in fatty acid composition between male and female foetuses that we observed in muscle and liver belonged to the neutral fraction corresponding to triglycerides, which are an essential energy source [
19]. Smaller differences were observed in the polar fraction corresponding to phospholipids, which constitute cell membranes and are essential for tissue development [
19], so sex-related differences in fatty acid composition are most related to energy partitioning than to organ development.
We found that male foetuses had a higher n-6/n-3 ratio than females, and a high n-6/n-3 ratio appears to be deleterious [
65], corresponding to the prodromal phase of insulin resistance [
66]. Optimal development depends on adequate availability of n-6 [
67], while n-3 may improve insulin function. Our results are consistent with previous reports that alterations in lipid metabolism and insulin regulation appear early in the development of male foetuses under limited nutrition [
68,
69,
70,
71]. Indeed, males in our study showed a significantly higher ratio of MUFA to SFA in muscle triglycerides than females on GD 70, although this difference was no longer significant on GD 90. On GD 70 and 90, males showed lower stearic acid content in the liver, and this acid is considered to protect metabolic health [
72,
73]. Males also showed higher ratios of MUFA to SFA and C18:1 to C18:0, indicating higher stearoyl-CoA desaturase activity. Although we observed few sex-related differences in the changes in phospholipid composition of muscle or liver during pregnancy, we did observe that females had higher content of linoleic acid in the liver than males on GD 70, and that males had higher content of mead acid in the muscle on GD 70 and 90. The greater content of mead acid in males likely reflects a worse homeorhesis state, since synthesis of mead or eicosatrienoic acid occurs in response to severe deficiency of fatty acids, mainly linoleic acid [
74,
75].