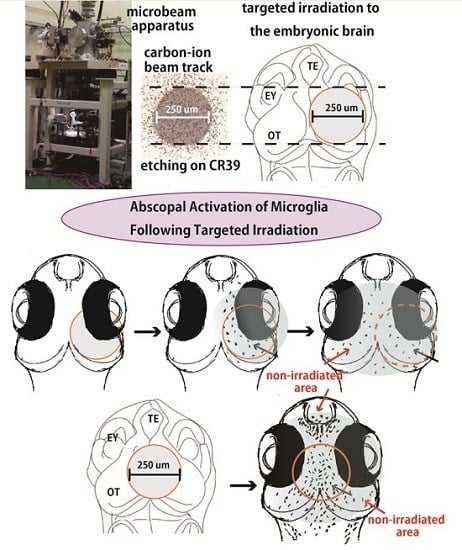
Abscopal Activation of Microglia in Embryonic Fish Brain Following Targeted Irradiation with Heavy-Ion Microbeam
Abstract
:1. Introduction
2. Results
2.1. Sequential Process of Microglial Activation Following γ-Ray Irradiation with 10 Gy
2.2. Collimated Carbon Ion Microbeam-Induced Neural Apoptosis only in the Targeted Area of OT in Medaka Embryos
2.3. Microglia in the Nonirradiated Area of OT Were Activated to Express ApoE after Irradiation
3. Discussion
4. Materials and Methods
4.1. Ethics
4.2. Fish and Embryos
4.3. Irradiation
4.4. Acridine Orange Staining Assay
4.5. Histology
4.6. Immunohistochemistry
4.7. Whole-Mount In Situ Hybridization (WISH)
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ransohoff, R.M.; Cardona, A.E. The myeloid cells of the central nervous system parenchyma. Nature 2010, 468, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Eyo, U.; Dailey, M.E. Effects of oxygen-glucose deprivation on microglial mobility and viability in developing mouse hippocampal tissues. Glia 2012, 60, 1747–1760. [Google Scholar] [CrossRef] [PubMed]
- Prinz, M.; Priller, J. Microglia and brain macrophages in the molecular age: From origin to neuropsychiatric disease. Nat. Rev. Neurosci. 2014, 15, 300–312. [Google Scholar] [CrossRef] [PubMed]
- Lyons, D.A.; Talbot, W.S. Glial cell development and function in zebrafish. Cold Spring Harb. Perspect. Biol. 2014, 7, 020586. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, T.; Oda, S.; Hibi, Y.; Satoh, S.; Nagata, K.; Hirakawa, K.; Kutsuna, N.; Sagara, H.; Mitani, H. Embryonic medaka model of microglia in the developing cns allowing in vivo analysis of their spatiotemporal recruitment in response to irradiation. PLoS ONE 2015, 10, e0127325. [Google Scholar] [CrossRef] [PubMed]
- Morrens, J.; Van Den Broeck, W.; Kempermann, G. Glial cells in adult neurogenesis. Glia 2012, 60, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Cui, J.L.; Li, L.; Hitchcock, P.F.; Li, Y.H. The role of microglia in the neurogenesis of zebrafish retina. Biochem. Biophys. Res. Commun. 2012, 421, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Sieger, D.; Peri, F. Animal models for studying microglia: The first, the popular, and the new. Glia 2013, 61, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Peri, F.; Nusslein-Volhard, C. Live imaging of neuronal degradation by microglia reveals a role for v0-ATPase a1 in phagosomal fusion in vivo. Cell 2008, 133, 916–927. [Google Scholar] [CrossRef] [PubMed]
- Ellett, F.; Pase, L.; Hayman, J.W.; Andrianopoulos, A.; Lieschke, G.J. mpeg1 promoter transgenes direct macrophage-lineage expression in zebrafish. Blood 2011, 117, e49–e56. [Google Scholar] [CrossRef] [PubMed]
- Davalos, D.; Grutzendler, J.; Yang, G.; Kim, J.V.; Zuo, Y.; Jung, S.; Littman, D.R.; Dustin, M.L.; Gan, W.B. ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci. 2005, 8, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Nimmerjahn, A.; Kirchhoff, F.; Helmchen, F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 2005, 308, 1314–1318. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Du, X.F.; Liu, C.S.; Wen, Z.L.; Du, J.L. Reciprocal regulation between resting microglial dynamics and neuronal activity in vivo. Dev. Cell 2012, 23, 1189–1202. [Google Scholar] [CrossRef] [PubMed]
- Hanisch, U.K.; Kettenmann, H. Microglia: Active sensor and versatile effector cells in the normal and pathologic brain. Nat. Neurosci. 2007, 10, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Marz, M.; Schmidt, R.; Rastegar, S.; Strahle, U. Regenerative response following stab injury in the adult zebrafish telencephalon. Dev. Dyn. 2011, 240, 2221–2231. [Google Scholar] [CrossRef] [PubMed]
- Baumgart, E.V.; Barbosa, J.S.; Bally-Cuif, L.; Gotz, M.; Ninkovic, J. Stab wound injury of the zebrafish telencephalon: A model for comparative analysis of reactive gliosis. Glia 2012, 60, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Oosterhof, N.; Holtman, I.R.; Kuil, L.E.; van der Linde, H.C.; Boddeke, E.W.; Eggen, B.J.; van Ham, T.J. Identification of a conserved and acute neurodegeneration-specific microglial transcriptome in the zebrafish. Glia 2017, 65, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Sieger, D.; Moritz, C.; Ziegenhals, T.; Prykhozhij, S.; Peri, F. Long-range Ca2+ waves transmit brain-damage signals to microglia. Dev. Cell 2012, 22, 1138–1148. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, L.; Astell, K.R.; Velikova, G.; Sieger, D. A zebrafish live imaging model reveals differential responses of microglia toward glioblastoma cells in vivo. Zebrafish 2016, 13, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Astell, K.R.; Sieger, D. Investigating microglia-brain tumor cell interactions in vivo in the larval zebrafish brain. Methods Cell Biol. 2017, 138, 593–626. [Google Scholar] [PubMed]
- Haynes, S.E.; Hollopeter, G.; Yang, G.; Kurpius, D.; Dailey, M.E.; Gan, W.B.; Julius, D. The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat. Neurosci. 2006, 9, 1512–1519. [Google Scholar] [CrossRef] [PubMed]
- Elliott, M.R.; Chekeni, F.B.; Trampont, P.C.; Lazarowski, E.R.; Kadl, A.; Walk, S.F.; Park, D.; Woodson, R.I.; Ostankovich, M.; Sharma, P.; et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 2009, 461, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Davis, E.J.; Foster, T.D.; Thomas, W.E. Cellular forms and functions of brain microglia. Brain Res. Bull. 1994, 34, 73–78. [Google Scholar] [CrossRef]
- Svahn, A.J.; Graeber, M.B.; Ellett, F.; Lieschke, G.J.; Rinkwitz, S.; Bennett, M.R.; Becker, T.S. Development of ramified microglia from early macrophages in the zebrafish optic tectum. Dev. Neurobiol. 2013, 73, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Du, X.; Pei, G.; Du, J.; Zhao, J. β-Arrestin1 regulates the morphology and dynamics of microglia in zebrafish in vivo. Eur. J. Neurosci. 2016, 43, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Li, Y.; Mao, J.; Pei, G. Neurogenesis-promoting natural product α-Asarone modulates morphological dynamics of activated microglia. Front. Cell. Neurosci. 2016, 10, 280. [Google Scholar] [CrossRef] [PubMed]
- Mazaheri, F.; Breus, O.; Durdu, S.; Haas, P.; Wittbrodt, J.; Gilmour, D.; Peri, F. Distinct roles for BAI1 and TIM-4 in the engulfment of dying neurons by microglia. Nat. Commun. 2014, 5, 4046. [Google Scholar] [CrossRef] [PubMed]
- Van Ham, T.J.; Brady, C.A.; Kalicharan, R.D.; Oosterhof, N.; Kuipers, J.; Veenstra-Algra, A.; Sjollema, K.A.; Peterson, R.T.; Kampinga, H.H.; Giepmans, B.N. Intravital correlated microscopy reveals differential macrophage and microglial dynamics during resolution of neuroinflammation. Dis. Model. Mech. 2014, 7, 857–869. [Google Scholar] [CrossRef] [PubMed]
- Morsch, M.; Radford, R.; Lee, A.; Don, E.K.; Badrock, A.P.; Hall, T.E.; Cole, N.J.; Chung, R. In vivo characterization of microglial engulfment of dying neurons in the zebrafish spinal cord. Front. Cell. Neurosci. 2015, 9, 321. [Google Scholar] [CrossRef] [PubMed]
- Welzel, G.; Fleckenstein, K.; Mai, S.K.; Hermann, B.; Kraus-Tiefenbacher, U.; Wenz, F. Acute neurocognitive impairment during cranial radiation therapy in patients with intracranial tumors. Strahlenther. Onkol. 2008, 184, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Greene-Schloesser, D.; Moore, E.; Robbins, M.E. Molecular pathways: Radiation-induced cognitive impairment. Clin. Cancer Res. 2013, 19, 2294–2300. [Google Scholar] [CrossRef] [PubMed]
- Makale, M.T.; McDonald, C.R.; Hattangadi-Gluth, J.A.; Kesari, S. Mechanisms of radiotherapy-associated cognitive disability in patients with brain tumours. Nat. Rev. Neurol. 2017, 13, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.L.; Tian, D.S.; Li, Z.W.; Qu, W.S.; Zhan, Y.; Xie, M.J.; Yu, Z.Y.; Wang, W.; Wu, G. Tamoxifen alleviates irradiation-induced brain injury by attenuating microglial inflammatory response in vitro and in vivo. Brain Res. 2010, 1316, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Dong, J.H.; Huang, G.D.; Qu, X.F.; Wu, G.; Dong, X.R. NF-κB signaling modulates radiationinduced microglial activation. Oncol. Rep. 2014, 31, 2555–2560. [Google Scholar] [PubMed]
- Zhang, J.; Tong, F.; Cai, Q.; Chen, L.J.; Dong, J.H.; Wu, G.; Dong, X.R. Shenqi Fuzheng Injection attenuates irradiation-induced brain injury in mice via inhibition of the NF-κB signaling pathway and microglial activation. Acta Pharmacol. Sin. 2015, 36, 1288–1299. [Google Scholar] [CrossRef] [PubMed]
- Formenti, S.C.; Demaria, S. Systemic effects of local radiotherapy. Lancet Oncol. 2009, 10, 718–726. [Google Scholar] [CrossRef]
- Postow, M.A.; Callahan, M.K.; Barker, C.A.; Yamada, Y.; Yuan, J.; Kitano, S.; Mu, Z.; Rasalan, T.; Adamow, M.; Ritter, E.; et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N. Engl. J. Med. 2012, 366, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Durante, M.; Reppingen, N.; Held, K.D. Immunologically augmented cancer treatment using modern radiotherapy. Trends Mol. Med. 2013, 19, 565–582. [Google Scholar] [CrossRef] [PubMed]
- Formenti, S.C.; Demaria, S. Combining radiotherapy and cancer immunotherapy: A paradigm shift. J. Natl. Cancer Inst. 2013, 105, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Bernier, J. Immuno-oncology: Allying forces of radio- and immuno-therapy to enhance cancer cell killing. Crit. Rev. Oncol. Hematol. 2016, 108, 97–108. [Google Scholar] [CrossRef] [PubMed]
- McGinnis, G.J.; Friedman, D.; Young, K.H.; Torres, E.R.; Thomas, C.R., Jr.; Gough, M.J.; Raber, J. Neuroinflammatory and cognitive consequences of combined radiation and immunotherapy in a novel preclinical model. Oncotarget 2017, 8, 9155–9173. [Google Scholar] [CrossRef] [PubMed]
- Wittbrodt, J.; Shima, A.; Schartl, M. Medaka-a model organism from the far East. Nat. Rev. Genet. 2002, 3, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Nawa, D.; Tateno, H.; Yasuda, T.; Oda, S.; Mitani, H.; Nishimaki, T.; Katsumura, T.; Oota, H.; Hanihara, T.; et al. Generation of monoclonal antibodies against the Galβ1–4Gal epitope: A key tool in studies of species-specific glycans expressed in fish, amphibians and birds. Glycobiology 2013, 23, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Nagata, K.; Hashimoto, C.; Watanabe-Asaka, T.; Itoh, K.; Yasuda, T.; Ohta, K.; Oonishi, H.; Igarashi, K.; Suzuki, M.; Funayama, T.; et al. In vivo 3D analysis of systemic effects after local heavy-ion beam irradiation in an animal model. Sci. Rep. 2016, 6, 28691. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, Y. Medakafish as a model system for vertebrate developmental genetics. Bioessays 2000, 22, 487–495. [Google Scholar] [CrossRef]
- Shima, A.; Mitani, H. Medaka as a research organism: Past, present and future. Mech. Dev. 2004, 121, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, T.; Oda, S.; Ishikawa, Y.; Watanabe-Asaka, T.; Hidaka, M.; Yasuda, H.; Anzai, K.; Mitani, H. Live imaging of radiation-induced apoptosis by yolk injection of Acridine orange in the developing optic tectum of medaka. J. Radiat. Res. 2009, 50, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, T.; Oda, S.; Yasuda, H.; Hibi, Y.; Anzai, K.; Mitani, H. Neurocytotoxic effects of iron-ions on the developing brain measured in vivo using medaka (Oryzias latipes), a vertebrate model. Int. J. Radiat. Biol. 2011, 87, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, T.; Oda, S.; Li, Z.; Kimori, Y.; Kamei, Y.; Ishikawa, T.; Todo, T.; Mitani, H. γ-ray irradiation promotes premature meiosis of spontaneously differentiating testis-ova in the testis of p53-deficient medaka (Oryzias latipes). Cell Death Dis. 2012, 3, e395. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Liu, R.; Zhang, R.; Hu, Y. A method for real-time measurement of respiratory rhythms in medaka (Oryzias latipes) using computer vision for water quality monitoring. Ecotoxicol. Environ. Saf. 2014, 100, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Morley, S.C. The actin-bundling protein l-plastin supports T-cell motility and activation. Immunol. Rev. 2013, 256, 48–62. [Google Scholar] [CrossRef] [PubMed]
- Leblanc, A.C.; Poduslo, J.F. Regulation of apolipoprotein-E gene-expression after injury of the rat sciatic-nerve. J. Neurosci. Res. 1990, 25, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Herbomel, P.; Thisse, B.; Thisse, C. Zebrafish early macrophages colonize cephalic mesenchyme and developing brain, retina, and epidermis through, a M-CSF receptor-dependent invasive process. Dev. Biol. 2001, 238, 274–288. [Google Scholar] [CrossRef] [PubMed]
- Funayama, T.; Wada, S.; Yokota, Y.; Fukamoto, K.; Sakashita, T.; Taguchi, M.; Kakizaki, T.; Hamada, N.; Suzuki, M.; Furusawa, Y.; et al. Heavy-ion microbeam system at JAEA-Takasaki for microbeam biology. J. Radiat. Res. 2008, 49, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, T.; Fukamoto, K.; Sakashita, T.; Suzuki, E.; Kakizaki, T.; Hamada, N.; Funayama, T.; Suzuki, H.; Ishioka, N.; Wada, S.; et al. Targeted heavy-ion microbeam irradiation of the embryo but not yolk in the diapause-terminated egg of the silkworm, bombyx mori, induces the somatic mutation. J. Radiat. Res. 2009, 50, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Burrell, K.; Hill, R.P.; Zadeh, G. High-resolution in vivo analysis of normal brain response to cranial irradiation. PLoS ONE 2012, 7, e38366. [Google Scholar] [CrossRef] [PubMed]
- Buga, A.M.; Di Napoli, M.; Popa-Wagner, A. Preclinical models of stroke in aged animals with or without comorbidities: Role of neuroinflammation. Biogerontology 2013, 14, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Sandu, R.E.; Buga, A.M.; Balseanu, A.T.; Moldovan, M.; Popa-Wagner, A. Twenty-four hours hypothermia has temporary efficacy in reducing brain infarction and inflammation in aged rats. Neurobiol. Aging 2016, 38, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Junker, H.; Suofu, Y.; Venz, S.; Sascau, M.; Herndon, J.G.; Kessler, C.; Walther, R.; Popa-Wagner, A. Proteomic identification of an upregulated isoform of annexin A3 in the rat brain following reversible cerebral ischemia. Glia 2007, 55, 1630–1637. [Google Scholar] [CrossRef] [PubMed]
- Joseph, C.; Buga, A.M.; Vintilescu, R.; Balseanu, A.T.; Moldovan, M.; Junker, H.; Walker, L.; Lotze, M.; Popa-Wagner, A. Prolonged gaseous hypothermia prevents the upregulation of phagocytosis-specific protein Annexin 1 and causes low-amplitude EEG activity in the aged rat brain after cerebral ischemia. J. Cerebr. Blood Flow Metab. 2012, 32, 1632–1642. [Google Scholar] [CrossRef] [PubMed]
- Amor, S.; Puentes, F.; Baker, D.; van der Valk, P. Inflammation in neurodegenerative diseases. Immunology 2010, 129, 154–169. [Google Scholar] [CrossRef] [PubMed]
- Acharya, M.M.; Green, K.N.; Allen, B.D.; Najafi, A.R.; Syage, A.; Minasyan, H.; Le, M.T.; Kawashita, T.; Giedzinski, E.; Parihar, V.K.; et al. Elimination of microglia improves cognitive function following cranial irradiation. Sci. Rep. 2016, 6, 31545. [Google Scholar] [CrossRef] [PubMed]
- Hyodo-Taguchi, Y.; Egami, N. Establishment of inbred strains of the medaka Oryzias latipes and the usefulness of the strains for biomedical-research. Zool. Sci. 1985, 2, 305–316. [Google Scholar]
- Iwamatsu, T. Stages of normal development in the medaka Oryzias latipes. Mech. Dev. 2004, 121, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, Y.; Yamamoto, N.; Yoshimoto, M.; Yasuda, T.; Maruyama, K.; Kage, T.; Takeda, H.; Ito, H. Developmental origin of diencephalic sensory relay nuclei in teleosts. Brain Behav. Evol. 2007, 69, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Abrams, J.M.; White, K.; Fessler, L.I.; Steller, H. Programmed cell death during Drosophila embryogenesis. Development 1993, 117, 29–43. [Google Scholar] [PubMed]
- Furutani-Seiki, M.; Jiang, Y.J.; Brand, M.; Heisenberg, C.P.; Houart, C.; Beuchle, D.; van Eeden, F.J.; Granato, M.; Haffter, P.; Hammerschmidt, M.; et al. Neural degeneration mutants in the zebrafish, Danio rerio. Development 1996, 123, 229–239. [Google Scholar] [CrossRef]
- Yasuda, T.; Yoshimoto, M.; Maeda, K.; Matsumoto, A.; Maruyama, K.; Ishikawa, Y. Rapid and simple method for quantitative evaluation of neurocytotoxic effects of radiation on developing Medaka brain. J. Radiat. Res. 2008, 49, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, T.; Aoki, K.; Matsumoto, A.; Maruyama, K.; Hyodo-Taguchi, Y.; Fushiki, S.; Ishikawa, Y. Radiation-induced brain cell death can be observed in living Medaka embryos. J. Radiat. Res. 2006, 47, 295–303. [Google Scholar] [CrossRef] [PubMed]





© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yasuda, T.; Kamahori, M.; Nagata, K.; Watanabe-Asaka, T.; Suzuki, M.; Funayama, T.; Mitani, H.; Oda, S. Abscopal Activation of Microglia in Embryonic Fish Brain Following Targeted Irradiation with Heavy-Ion Microbeam. Int. J. Mol. Sci. 2017, 18, 1428. https://doi.org/10.3390/ijms18071428
Yasuda T, Kamahori M, Nagata K, Watanabe-Asaka T, Suzuki M, Funayama T, Mitani H, Oda S. Abscopal Activation of Microglia in Embryonic Fish Brain Following Targeted Irradiation with Heavy-Ion Microbeam. International Journal of Molecular Sciences. 2017; 18(7):1428. https://doi.org/10.3390/ijms18071428
Chicago/Turabian StyleYasuda, Takako, Miyuki Kamahori, Kento Nagata, Tomomi Watanabe-Asaka, Michiyo Suzuki, Tomoo Funayama, Hiroshi Mitani, and Shoji Oda. 2017. "Abscopal Activation of Microglia in Embryonic Fish Brain Following Targeted Irradiation with Heavy-Ion Microbeam" International Journal of Molecular Sciences 18, no. 7: 1428. https://doi.org/10.3390/ijms18071428
APA StyleYasuda, T., Kamahori, M., Nagata, K., Watanabe-Asaka, T., Suzuki, M., Funayama, T., Mitani, H., & Oda, S. (2017). Abscopal Activation of Microglia in Embryonic Fish Brain Following Targeted Irradiation with Heavy-Ion Microbeam. International Journal of Molecular Sciences, 18(7), 1428. https://doi.org/10.3390/ijms18071428