Genetic and Hormonal Regulation of Chlorophyll Degradation during Maturation of Seeds with Green Embryos
Abstract
:1. Introduction
2. Photosynthesis in Seeds with Green Embryos
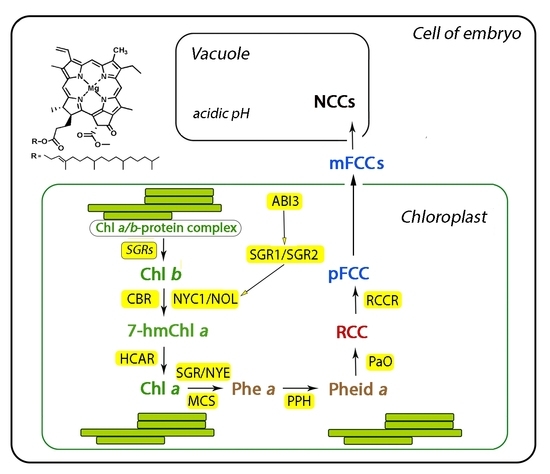
3. Catabolism of Chlorophylls in Plants
4. The Role of STAY-GREEN Genes in Degradation of Seed Chlorophylls
5. Role of Abscisic Acid (ABA) in Degradation of Chlorophylls and Seed Maturation
6. Residual Chlorophylls in Mature Seeds: The Problem of ”Green Seeds”
7. Conclusions
Acknowledgments
Conflicts of Interest
Abbreviations
| ABA | abscisic acid |
| ABI | abscisic acid insensitive |
| CCE | chlorophyll catabolic enzymes |
| NCC | non-fluorescent chlorophyll catabolite |
| NOL | NYC1-like |
| NYE | NON-YELLOWING |
| NYC | NON-YELLOW COLORING |
| mFCC | modified fluorescent chlorophyll catabolite |
| PaO | pheophorbide a oxygenase |
| pFCC | primary fluorescent chlorophyll catabolite |
| PPH | pheophytin pheophorbide hydrolase |
| RCC | red chlorophyll catabolite |
| SGR | STAY GREEN |
References
- Ruuska, S.A.; Schwender, J.; Ohlrogge, J.B. The capacity of green oilseeds to utilize photosynthesis to drive biosynthetic processes. Plant Physiol. 2004, 136, 2700–2709. [Google Scholar] [CrossRef] [PubMed]
- Galili, G.; Avin-Wittenberg, T.; Angelovici, R.; Fernie, A.R. The role of photosynthesis and amino acid metabolism in the energy status during seed development. Front. Plant Sci. 2014, 5, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Smolikova, G.N.; Medvedev, S.S. Photosynthesis in the seeds of chloroembryophytes. Russ. J. Plant Physiol. 2016, 63, 1–12. [Google Scholar] [CrossRef]
- Kranner, I.; Minibayeva, F.V.; Beckett, R.P.; Seal, C.E. What is stress? Concepts, definitions and applications in seed science. New Phytol. 2010, 188, 655–673. [Google Scholar] [CrossRef] [PubMed]
- Weber, H.; Borisjuk, L.; Wobus, U. Molecular physiology of legume seed development. Annu. Rev. Plant Biol. 2005, 56, 253–279. [Google Scholar] [CrossRef] [PubMed]
- Puthur, J.T.; Shackira, A.M.; Saradhi, P.P.; Bartels, D. Chloroembryos: A unique photosynthesis system. J. Plant Physiol. 2013, 170, 1131–1138. [Google Scholar] [CrossRef] [PubMed]
- Allen, D.K.; Ohlrogge, J.B.; Shachar-Hill, Y. The role of light in soybean seed filling metabolism. Plant J. 2009, 58, 220–234. [Google Scholar] [CrossRef] [PubMed]
- Allorent, G.; Courtois, F.; Chevalier, F.; Lerbs-Mache, S. Plastid gene expression during chloroplast differentiation and dedifferentiation into non-photosynthetic plastids during seed formation. Plant Mol. Biol. 2013, 82, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Hua, S.; Chen, Z.H.; Zhang, Y.; Yu, H.; Lin, B.; Zhang, D. Chlorophyll and carbohydrate metabolism in developing silique and seed are prerequisite to seed oil content of Brassica napus L. Bot. Stud. 2014, 55. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.A.; Xie, Y.; Sun, M.Z.; Si, J.S.; Hu, L. Comparison of the photosynthetic characteristics in the pericarp and flag leaves during wheat (Triticum aestivum L.) caryopsis development. Photosynthetica 2016, 54, 40–46. [Google Scholar] [CrossRef]
- Puthur, J.T.; Saradhi, P.P. Developing embryos of Sesbania sesban have unique potential to photosynthesize under high osmotic environment. J. Plant Physiol. 2004, 161, 1107–1118. [Google Scholar] [CrossRef] [PubMed]
- Yakovlev, M.S.; Zhukova, G.Y. Chlorophyll in embryos of angiosperm seeds, a review. Bot. Not. Ser 1980, 133, 323–336. [Google Scholar]
- Fernández-Marín, B.; Míguez, F.; Méndez-Fernández, L.; Agut, A.; Becerril, J.M.; García-Plazaola, J.I.; Kranner, I.; Colville, L. Seed carotenoid and tocochromanol composition of wild fabaceae species is shaped by phylogeny and ecological factors. Front. Plant Sci. 2017, 8, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Smolikova, G.N.; Laman, N.A.; Boriskevich, O.V. Role of chlorophylls and carotenoids in seed tolerance to abiotic stressors. Russ. J. Plant Physiol. 2011, 58, 965–973. [Google Scholar] [CrossRef]
- Chung, D.W.; Pružinská, A.; Hörtensteiner, S.; Ort, D.R. The role of pheophorbide a oxygenase expression and activity in the canola green seed problem. Plant Physiol. 2006, 142, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, R.N.; Ligterink, W.; de França-Neto, J.B.; Hilhorst, H.W.M.; da Silva, E.A.A. Gene expression profiling of the green seed problem in Soybean. BMC Plant Biology 2016, 16. [Google Scholar] [CrossRef] [PubMed]
- Jalink, H.; van der Schoor, R.; Frandas, A.; van Pijien, J.G.; Bino, R.J. Chlorophyll Fluorescence of Brassica oleracea seeds as a non-destructive marker for seed maturity and seed performance. Seed Sci. Res. 1998, 8, 437–443. [Google Scholar] [CrossRef]
- Hay, F.R.; Timple, S.; van Duijn, B. Can chlorophyll fluorescence be used to determine the optimal time to harvest rice seeds for long-term genebank storage ? Seed Sci. Res. 2015, 25, 321–334. [Google Scholar] [CrossRef]
- Johnson-Flanagan, A.M.; Singh, J.; Thiagarajah, M.R. The impact of sublethal freezing during maturation on pigment content in seeds of Brassica napus. J. Plant Physiol. 1990, 136, 385–390. [Google Scholar] [CrossRef]
- Green, B.R.; Singh, S.; Babic, I.; Bladen, C.; Johnson-Flanagan, A.M. Relashionship of chlorophyll, seed moisture and ABA levels in the maturing Brassica napus seed and effect of a mild freezing stress. Physiol. Plant. 1998, 104, 125–133. [Google Scholar] [CrossRef]
- Mailer, R.J.; Orchard, B.; Vonarx, M.M.; Wratten, N. The influence of cultivar and environment on the chlorophyll concentration of Australian canola seed. Aust. J. Exp. Agric. 2003, 43, 169–176. [Google Scholar] [CrossRef]
- Smolikova, G.N.; Kreslavski, V.D.; Shiroglazova, O.V.; Sharova, E.I.; Bilova, T.E.; Frolov, A.A.; Medvedev, S.S. Рhotochemical activity changes accompanying the embryogenesis of pea (Pisum. sativum L.) with yellow and green cotyledons. Funct. Plant Biol. 2017. [Google Scholar] [CrossRef]
- Kusaba, M.; Ito, H.; Morita, R.; Iida, S.; Sato, Y.; Fujimoto, M.; Kawasaki, S.; Tanaka, R.; Hirochika, H.; Nishimura, M.; Tanaka, A. Rice NON-YELLOW COLORING1 is involved in light-harvesting complex II and grana degradation during leaf senescence. Plant Cell 2007, 19, 1362–1375. [Google Scholar] [CrossRef] [PubMed]
- Horie, Y.; Ito, H.; Kusaba, M.; Tanaka, R.; Tanaka, A. Participation of chlorophyll b reductase in the initial step of the degradation of light-harvesting chlorophyll a/b-protein complexes in Arabidopsis. J. Biol. Chem. 2009, 284, 17449–17456. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Morita, R.; Katsuma, S.; Nishimura, M.; Tanaka, A.; Kusaba, M. Two short-chain dehydrogenase/reductases, NON-YELLOW COLORING 1 and NYC1-LIKE, are required for chlorophyll b and light-harvesting complex II degradation during senescence in rice. Plant J. 2009, 57, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Meguro, M.; Ito, H.; Takabayashi, A.; Tanaka, R.; Tanaka, A. Identification of the 7-hydroxymethyl chlorophyll a reductase of the chlorophyll cycle in Arabidopsis. Plant Cell 2011, 23, 3442–3453. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Kunieda, T.; Murai, F.; Morioka, S.; Shioi, Y. Mg-dechelation activity in radish cotyledons with artificial and native substrates, Mg-chlorophyllin a and chlorophyllide a. Plant Physiol. Biochem. 2005, 43, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, Y.; Ito, H.; Tanaka, A. Arabidopsis STAY-GREEN, Mendel’s green cotyledon gene, encodes magnesium-dechelatase. Plant Cell 2016, 28, 2147–2160. [Google Scholar] [CrossRef] [PubMed]
- Schelbert, S.; Aubry, S.; Burla, B.; Agne, B.; Kessler, F.; Krupinska, K.; Hörtensteiner, S. Pheophytin pheophorbide hydrolase (pheophytinase) is involved in chlorophyll breakdown during leaf senescence in Arabidopsis. Plant Cell 2009, 21, 767–785. [Google Scholar] [CrossRef] [PubMed]
- Pružinská, A.; Tanner, G.; Anders, I.; Roca, M.; Hörtensteiner, S. Chlorophyll breakdown: Pheophorbide a oxygenase is a Rieske-type iron–sulfur protein, encoded by the accelerated cell death 1 gene. Proc. Natl. Acad. Sci. USA 2003, 100, 15259–15264. [Google Scholar] [CrossRef]
- Pruzinska, A.; Anders, I.; Aubry, S.; Schenk, N.; Tapernoux-Luthi, E.; Muller, T.; Krautler, B.; Hortensteiner, S. In vivo participation of red chlorophyll catabolite reductase in chlorophyll breakdown. Plant Cell 2007, 19, 369–387. [Google Scholar] [CrossRef] [PubMed]
- Thomas, H.; Schellenberg, M.; Vicentini, F.; Matile, P. Gregor Mendel’s green and yellow pea seeds. Botanica. Acta. 1996, 109, 3–4. [Google Scholar] [CrossRef]
- Mendel, G. Experiments in Plant Hybridization (1865); Verhandlungen des naturforschenden Vereins Brünn: Abhandlungen, Germany, 1865; pp. 3–47. Available online: http://www.esp.org/foundations/genetics/classical/gm-65.pdf (accessed on 14 August 2017).
- Armstead, I.; Donnison, I.; Aubry, S.; Harper, J.; Hörtensteiner, S.; James, C.; Mani, J.; Moffet, M.; Ougham, H.; Roberts, L.; et al. Cross-species identification of Mendel’s locus. Science 2007, 315, 73. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Morita, R.; Nishimura, M.; Yamaguchi, H.; Kusaba, M. Mendel’s green cotyledon gene encodes a positive regulator of the chlorophyll-degrading pathway. Proc. Natl. Acad. Sci. USA 2007, 104, 14169–14174. [Google Scholar] [CrossRef] [PubMed]
- Christ, B.; Hörtensteiner, S. Mechanism and significance of chlorophyll breakdown. J. Plant Growth Regul. 2014, 33, 4–20. [Google Scholar] [CrossRef] [Green Version]
- Sakuraba, Y.; Park, S.-Y.; Paek, N.-C. The divergent roles of STAYGREEN (SGR) homologs in chlorophyll degradation. Mol. Cells 2015. [Google Scholar] [CrossRef] [PubMed]
- Aubry, S.; Mani, J.; Hörtensteiner, S. Stay-green protein, defective in Mendel’s green cotyledon mutant, acts independent and upstream of pheophorbide a oxygenase in the chlorophyll catabolic pathway. Plant Mol. Biol. 2008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, H.; Li, M.; Liang, N.; Yan, H.; Wei, Y.; Xu, X.; Liu, J.; Xu, Z.; Chen, F.; Wu, G. Molecular cloning and function analysis of the stay green gene in rice. Plant J. 2007, 52, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Barry, C.S.; McQuinn, R.P.; Chung, M.-Y.; Besuden, A.; Giovannoni, J.J. Amino acid substitutions in homologs of the STAY-GREEN protein are responsible for the green-flesh and chlorophyll retainer mutations of tomato and pepper. Plant Physiol. 2008, 147, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Barry, C.S. The stay-green revolution: Recent progress in deciphering the mechanisms of chlorophyll degradation in higher plants. Plant Sci. 2009, 176, 325–333. [Google Scholar] [CrossRef]
- Thomas, H.; Ougham, H. The stay-green trait. J. Exp. Bot. 2014, 65, 3889–3900. [Google Scholar] [CrossRef] [PubMed]
- Sakuraba, Y.; Park, S.-Y.; Kim, Y.-S.; Wanga, S.-H.; Yoo, S.-C.; Hörtensteinere, S.; Paeka, N.-C. Arabidopsis STAY-GREEN2 is a negative regulator of chlorophyll degradation during leaf senescence. Mol. Plant. 2014, 7, 1288–1302. [Google Scholar] [CrossRef] [PubMed]
- Ghandchi, F.P.; Caetano-Anolles, G.; Clough, S.J.; Ort, D.R. Investigating the control of chlorophyll degradation by genomic correlation mining. PLoS ONE. 2016, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Voss-Fels, K.; Cui, Y.; Jan, H.U.; Samans, B.; Obermeier, C.; Qian, W.; Snowdon, R.J. Deletion of a stay-green gene associates with adaptive selection in Brassica napus. Mol. Plant. 2016, 9, 1559–1569. [Google Scholar] [CrossRef] [PubMed]
- Borisjuk, L.; Rolletschek, H. The oxygen status of the developing seed. New Phytol. 2009, 182, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Allorent, G.; Osorio, S.; Ly Vu, J.; Falconet, D.; Jouhet, J.; Kuntz, M.; Fernie, A.R.; Lerbs-Mache, S.; Macherel, D.; Courtois, F.; et al. Adjustments of embryonic photosynthetic activity modulate seed fitness in Arabidopsis thaliana. New Phytol. 2014, 205, 707–719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohzuma, K.; Sato, Y.; Ito, H.; Okuzaki, A.; Watanabe, M.; Kobayashi, H.; Nakano, M.; Yamatani, H.; Masuda, Y.; Nagashima, Y.; et al. The non-Mendelian green cotyledon gene in soybean encodes a small subunit of photosystem II. Plant Physiol. 2017, 173, 2138–2147. [Google Scholar] [CrossRef] [PubMed]
- Hills, M.J. Control of storage-product synthesis in seeds. Curr. Opin. Plant Biol. 2004, 7, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Baud, S.; Lepiniec, L. Physiological and developmental regulation of seed oil production. Prog. Lipid Res. 2010, 4, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.L.; Liu, Z.H.; Hu, Z.H.; Huang, R.Z. BnWRI1 coordinates fatty acid biosynthesis and photosynthesis pathways during oil accumulation in rapeseed. J. Integr. Plant Biol. 2014, 56, 582–593. [Google Scholar] [CrossRef] [PubMed]
- Asokanthan, P.S.; Johnson, R.W.; Griffith, M.; Krol, M. The photosynthetic potential of canola embryos. Physiol. Plant. 1997, 101, 353–360. [Google Scholar] [CrossRef]
- Schwender, J.; Goffman, F.; Ohlrogge, J.B.; Shachar-Hill, Y. Rubisco without the Calvin cycle improves the carbon efficiency of developing green seeds. Nature 2004, 432, 779–782. [Google Scholar] [CrossRef] [PubMed]
- Borisjuk, L.; Rolletschek, H.; Walenta, S.; Panitz, R.; Wobus, U.; Weber, H. Energy status and its control on embryogenesis of legumes: ATP distribution within Vicia faba embryos is developmentally regulated and correlated with photosynthetic capacity. Plant J. 2003, 36, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Tschiersch, H.; Borisjuk, L.; Rutten, T.; Rolletschek, H. Gradients of seed photosynthesis and its role for oxygen balancing. Biosystem. 2011, 103, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, S.G.; Briarty, L.G. Cotyledon cell development in Arabidopsis thaliana during reserve deposition. Canadian J. Botany 1992, 70, 151–164. [Google Scholar] [CrossRef]
- Hörtensteiner, S.; Kräutler, B. Chlorophyll breakdown in oilseed rape. Photosynth. Res. 2000, 64, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, M.G.; Dalai, A.K.; Bakhshi, N.N. Utilization of green seed canola oil for biodiesel production. J. Chem. Technol. Biotechnol. 2006, 81, 1886–1893. [Google Scholar] [CrossRef]
- Diosady, L.L. Chlorophyll removal from edible oils. Int. J. App. Sci. Eng. 2005, 3, 81–88. [Google Scholar] [CrossRef]
- Chen, J.; Ren, G.; Kuai, B. The mystery of Mendel’s stay-green: Magnesium stays chelated in chlorophylls. Mol. Plant 2016, 9, 1556–1558. [Google Scholar] [CrossRef] [PubMed]
- Brzezowski, P.; Grimm, B. Chlorophyll metabolism. In eLS; John Wiley & Sons, Ltd.: Berlin, Germany, April 2013. [Google Scholar]
- Onyilagha, J.C.; Elliott, B.H.; Buckner, E.; Okiror, S.O.; Raney, P.J. Seed chlorophyll influences vigor in oilseed rape (Brassica napus L.). J. Agric. Sci. 2011, 3, 73–79. [Google Scholar] [CrossRef]
- Hörtensteiner, S. Chlorophyll degradation during senescence. Annu. Rev. Plant Biol. 2006, 57, 55–77. [Google Scholar] [CrossRef] [PubMed]
- Hörtensteiner, S. Update on the biochemistry of chlorophyll breakdown. Plant Mol. Biol. 2013, 82, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Borovsky, Y.; Paran, I. Chlorophyll breakdown during pepper fruit ripening in the chlorophyll retainer mutation is impaired at the homolog of the senescence-inducible STAY-GREEN gene. Theor. Appl. Genet. 2008, 117, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Hörtensteiner, S. Stay-green regulates chlorophyll and chlorophyll-binding protein degradation during senescence. Trends Plant Sci. 2009, 14, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Kusaba, M.; Tanaka, A.; Tanaka, R. Stay-green plants: What do they tell us about the molecular mechanism of leaf senescence. Photosynth. Res. 2013, 117, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Yu, J.W.; Park, J.S.; Li, J.; Yoo, S.C.; Lee, N.Y.; Lee, S.K.; Jeong, S.W.; Seo, H.S.; Koh, H.J.; et al. The senescence-induced stay green protein regulates chlorophyll degradation. Plant Cell 2007, 19, 1649–1664. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Miao, H.; Du, X.; Gu, J.; Xiao, K. GmSGR1, a stay-green gene in soybean (Glycine max L.), plays an important role in regulating early leaf-yellowing phenotype and plant productivity under nitrogen deprivation. Acta. Physiol. Plant. 2016, 38. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, B.; He, L.; Maa, F.F.; Wang, X.C.; Lia, H.Y.; Han, Y.H. Constitutive down-regulation of SiSGR gene is related to green millet in Setaria italica. Russ. J. Plant. Physiol. 2017, 64, 608–615. [Google Scholar] [CrossRef]
- Nakano, M.; Yamada, T.; Masuda, Y.; Sato, Y.; Kobayashi, H.; Ueda, H.; Morita, R.; Nishimura, M.; Kitamura, K.; Kusaba, M. A green-cotyledon/stay-green mutant exemplifies the ancient whole-genome duplications in soybean. Plant Cell Physiol. 2014, 55, 1763–1771. [Google Scholar] [CrossRef] [PubMed]
- Thomas, H.; Howarth, C.J. Five ways to stay green. J. Exp. Botany 2000, 51, 329–337. [Google Scholar] [CrossRef]
- Ren, G.; An, K.; Liao, Y.; Zhou, X.; Cao, Y.; Zhao, H.; Ge, X.; Kuai, B. Identification of a novel chloroplast protein AtNYE1 regulating chlorophyll degradation during leaf senescence in Arabidopsis. Plant Physiol. 2007, 144, 1429–1441. [Google Scholar] [CrossRef] [PubMed]
- Pádua, G.P.; França Neto, J.B.; Carvalho, M.L.M.; Krzyzanowski, F.C.; Guimaraes, R.M. Incidence of green soybean seeds as a function of environmental stresses during seed maturation. Rev. Bras. Sementes. 2009, 31, 150–159. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, M.; Wu, T.; Wu, C.; Jiang, B.; Guo, C.; Han, T. Physiological and molecular studies of staygreen caused by pod removal and seed injury in soybean. Crop J. 2016, 4, 435–443. [Google Scholar] [CrossRef]
- Nambara, E.; Keith, K.; McCourt, P.; Naito, S. Isolation of an internal deletion mutant of the Arabidopsis thaliana ABI3 gene. Plant Cell Physiol. 1994, 35, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Parcy, F.; Valon, C.; Kohara, A.; Miséra, S.; Giraudat, J. The ABSCISIC ACID-INSENSITIVE3, FUSCA3, and LEAFY COTYLEDON1 loci act in concert to control multiple aspects of Arabidopsis seed development. Plant Cell 1997, 9, 1265–1277. [Google Scholar] [CrossRef] [PubMed]
- Nambara, E.; Okamoto, M.; Tatematsu, K.; Yano, R.; Seo, M.; Kamiya, Y. Abscisic acid and the control of seed dormancy and germination. Seed Sci. Res. 2010, 20, 675–689. [Google Scholar] [CrossRef]
- Bewley, J.D.; Bradford, K.; Hilhorst, H.M.; Nonogaki, H. Development and Maturation. In Seeds: Physiology of Development, Germination and Dormancy, 3rd ed.; Springer: New York, NY, USA, 2013; pp. 27–83. [Google Scholar]
- Smolikova, G.N.; Medvedev, S.S. Seed Carotenoids: Synthesis, Diversity, and Function. Russ. J. Plant Physiol. 2015, 62, 1–13. [Google Scholar] [CrossRef]
- Nambara, E.; Marion-Poll, A. ABA action and interactions in seeds. Trends Plant Sci. 2003, 8, 213–217. [Google Scholar] [CrossRef]
- Boursiac, Y.; Leran, S.; Corratge-Faillie, C.; Gojon, A.; Krouk, G.; Lacombe, B. ABA transport and transporters. Trends Plant Sci. 2012, 18, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Johnson-Flanagan, A.M.; Huiwen, Z.; Geng, X.-M.; Brown, D.C.W.; Nykiforuk, C.L.; Singh, J. Frost, abscisic acid, and desiccation hasten embryo development in Brassica napus. Physiol Plant. 1992, 99, 700–706. [Google Scholar] [CrossRef]
- Delmas, F.; Sankaranarayanan, S.; Deb, S.; Widdup, E.; Bournonville, C.; Bollier, N.; Northey, J.G.B.; McCourt, P.; Samuel, M.A. ABI3 controls embryo degreening through Mendel’s I locus. Proc. Natl. Acad. Sci. USA 2013, 16, E3888–E3894. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, R.R.; Ariizumi, T.; Steber, C. Molecular aspects of seed dormancy. Annu. Rev. Plant Biol. 2008, 59, 387–415. [Google Scholar] [CrossRef] [PubMed]
- Ooms, J.J.J.; Leon-Kloosterziel, K.M.; Bartels, D.; Koornneef, M.; Karssen, C.M. Acquisition of desiccation tolerance and longevity in seeds of Arabidopsis thaliana. A comparative study using abscisic acid-insensitive abi3 mutants. Plant Physiol. 1993, 102, 1185–1191. [Google Scholar] [CrossRef] [PubMed]
- Clerkx, E.J.; Vries, H.B.; Ruys, G.J.; Groot, S.P.; Koornneef, M. Characterization of green seed, an enhancer of abi3-1 in Arabidopsis that affects seed longevity. Plant Physiol. 2003, 132, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Roscoe, T.T.; Guilleminot, J.; Bessoule, J.-J.; Berger, F.; Devic, M. Complementation of seed maturation phenotypes by ectopic expression of ABSCISIC ACID INSENSITIVE3, FUSCA3 and LEAFY COTYLEDON2 in Arabidopsis. Plant Cell Physiol. 2015, 56, 1215–1228. [Google Scholar] [CrossRef] [PubMed]
- Sakuraba, Y.; Han, S.H.; Lee, S.H.; Hortensteiner, S.; Paek, N.C. Arabidopsis NAC016 promotes chlorophyll breakdown by directly upregulating STAY GREEN1 transcription. Plant Cell Rep. 2016, 35, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Ohtsuka, T.; Tanaka, A. Conversion of chlorophyll b to chlorophyll a via 7-hydroxymethyl chlorophyll. J. Biol. Chem. 1996, 271, 1475–1479. [Google Scholar] [CrossRef] [PubMed]
- Jia, T.; Ito, H.; Hu, X.; Tanaka, A. Accumulation of the NON-YELLOW COLORING 1 protein of the chlorophyll cycle requires chlorophyll b in Arabidopsis thaliana. Plant J. 2015, 81, 586–596. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, S.; Ito, H.; Tanaka, R.; Tanaka, A. Chlorophyll b reductase plays an essential role in maturation and storability of Arabidopsis seeds. Plant Physiol. 2012, 160, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Xiang, D.; Venglat, P.; Tibiche, C.; Yang, H.; Risseeuw, E.; Cao, Y.; Babic, V.; Cloutier, M.; Keller, W.; Wang, E.; et al. Genome-wide analysis reveals gene expression and metabolic network dynamics during embryo development in Arabidopsis. Plant Physiol. 2011, 156, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Worley, E.; Udvardi, M. A NAP-AAO3 regulatory module promotes chlorophyll degradation via ABA biosynthesis in Arabidopsis leaves. Plant Cell 2014, 26, 4862–4874. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.W.; Asokanthan, P.S.; Griffith, M. Water and sucrose regulate canola embryo development. Physiol. Plant. 1997, 101, 361–366. [Google Scholar] [CrossRef]
- Johnson-Flanagan, A.M.; Maret, L.L.D.; Pomeroy, M.K. Humidification of green canola seed leads to pigment degradation in the absence of germination. Crop Sci. 1994, 34, 1618–1623. [Google Scholar] [CrossRef]
- Whitmarsh, C.J.; Ortiz-Lopez, A. The de-greening of canola. USDA Agric. Res. Mag. 2000, 48, 9. [Google Scholar]
- Johnson-Flanagan, A.M.; Go, N.; Sun, F.; Singh, J.; Robert, L.; Konschuh, M.N. Antisense RNA to Decrease The Green Seed Problem in Canola. In Proceedings of the 10th International Rapeseed Congress, Canberra, Australia, 26–29 September 1999. [Google Scholar]
- Matilla, A.; Gallardo, M.; Puga-Hermida, M.I. Structural, physiological and molecular aspects of heterogeneity in seeds. Seed Sci. Res. 2005, 15, 63–76. [Google Scholar] [CrossRef]
- Li, N.; Li, Y. Maternal control of seed size in plants. J. Exp. Bot. 2015, 66, 1087–1097. [Google Scholar] [CrossRef] [PubMed]
- Suhartonto, M.R. Chlorophyll in Tomato Seeds: Marker for Seed Performance? Ph.D. Thesis, Wageningen University, Suhartanto, The Netherlands, June 2002. Available online: http://edepot.wur.nl/192229 (accessed on 19 August 2017).


| Types of Mutants | Phenotypic Manifestation of Mutations |
|---|---|
| A (functional stay-green) | Chlorophylls are not degraded, leaf senescence onset is strongly delayed, duration of photosynthetically active stage is prolonged |
| B (functional stay-green) | Chlorophylls are not degraded, leaf senescence onset is slowed down, duration of photosynthetically active stage is prolonged |
| C (cosmetic stay-green) | Chlorophylls are not degraded, but photosynthetic activity and leaf senescence itself remain unaffected |
| D (pseudo stay-green) | Leaves are involved in programmed cell death during or before senescence onset and chlorophyll degradation |
| E (super green hyper-green) | Leaf senescence rates and photosynthetic activity are unaffected, but chlorophylls are strongly up-regulated |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smolikova, G.; Dolgikh, E.; Vikhnina, M.; Frolov, A.; Medvedev, S. Genetic and Hormonal Regulation of Chlorophyll Degradation during Maturation of Seeds with Green Embryos. Int. J. Mol. Sci. 2017, 18, 1993. https://doi.org/10.3390/ijms18091993
Smolikova G, Dolgikh E, Vikhnina M, Frolov A, Medvedev S. Genetic and Hormonal Regulation of Chlorophyll Degradation during Maturation of Seeds with Green Embryos. International Journal of Molecular Sciences. 2017; 18(9):1993. https://doi.org/10.3390/ijms18091993
Chicago/Turabian StyleSmolikova, Galina, Elena Dolgikh, Maria Vikhnina, Andrej Frolov, and Sergei Medvedev. 2017. "Genetic and Hormonal Regulation of Chlorophyll Degradation during Maturation of Seeds with Green Embryos" International Journal of Molecular Sciences 18, no. 9: 1993. https://doi.org/10.3390/ijms18091993
APA StyleSmolikova, G., Dolgikh, E., Vikhnina, M., Frolov, A., & Medvedev, S. (2017). Genetic and Hormonal Regulation of Chlorophyll Degradation during Maturation of Seeds with Green Embryos. International Journal of Molecular Sciences, 18(9), 1993. https://doi.org/10.3390/ijms18091993