Transcriptomics Analysis on Excellent Meat Quality Traits of Skeletal Muscles of the Chinese Indigenous Min Pig Compared with the Large White Breed
Abstract
:1. Introduction
2. Results
2.1. RNA Sequencing Data Mapping and Annotation
2.2. Differentially Expressed Genes Calling and Validation by Real-Time PCR
2.3. Gene Ontology Analysis and Kyoto Encyclopedia of Genes and Genomes Pathway Analysis of DEGs
3. Discussion
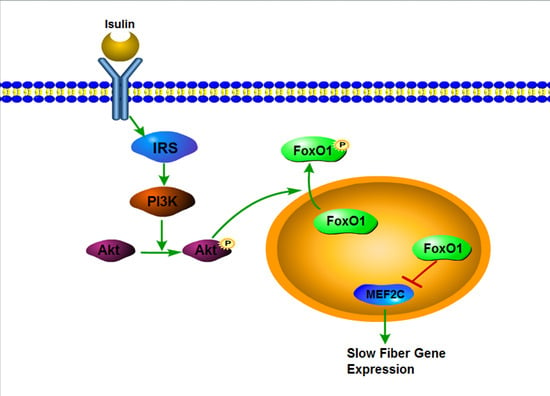
3.1. IRS1/Akt/FoxO1 Signaling Pathway
3.2. AMPK Activity and Cascade Effects
3.3. DEGs of Lipid Metabolism
3.4. Nutritional Metabolism
4. Materials and Methods
4.1. Sample Collection
4.2. RNA Preparation and Sequencing
4.3. RNA-Seq Data Analysis and DEGs Analysis
4.4. Validation of DEGs by Real-Time PCR
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yuliaxis, R.C.; Nuria, M.; Anna, E.C.; Jordi, C.; Anna, C.; Maria, B.; Jordi, E.; Noelia, I.E.; Fernández, A.I.; Miguel, P.E. Liver transcriptome profile in pigs with extreme phenotypes of intramuscular fatty acid composition. BMC Genom. 2012, 13, 547. [Google Scholar]
- Rosenvold, K.; Andersen, H.J. Factors of significance for pork quality—A review. Meat Sci. 2003, 64, 219–237. [Google Scholar] [CrossRef]
- Megens, H.J.; Crooijmans, R.P.; Cristobal, M.S.; Hui, X.; Li, N.; Groenen, M.A. Biodiversity of pig breeds from China and Europe estimated from pooled DNA samples: Differences in microsatellite variation between two areas of domestication. Genet. Sel. Evol. 2008, 40, 1–26. [Google Scholar]
- Scherf, B.D. World Watch List for Domestic Animal Diversity; Food and Agriculture Organization of the United Nations: Rome, Italy, 1993. [Google Scholar]
- D’Astous-Pagé, J.; Gariépy, C.; Blouin, R.; Cliche, S.; Méthot, S.; Sullivan, B.; Fortin, F.; Palin, M.F. Identification of single nucleotide polymorphisms in carnosine-related genes and effects of genotypes on pork meat quality attributes. Meat Sci. 2017, 134, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Lefaucheur, L. A second look into fibre typing—Relation to meat quality. Meat Sci. 2010, 84, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.; Li, S.; Deng, L.; Guan, Y.; Chen, D.; Yuan, X.; Xia, T.; He, X.; Shan, Y.; Li, C. Transcriptome Analysis Reveals Long Intergenic Noncoding RNAs Contributed to Growth and Meat Quality Differences between Yorkshire and Wannanhua Pig. Genes 2017. [Google Scholar] [CrossRef] [PubMed]
- Li, X.J.; Zhou, J.; Liu, L.Q.; Qian, K.; Wang, C.L. Identification of genes in longissimus Dorsi muscle differentially expressed between Wannanhua and Yorkshire pigs using RNA-sequencing. Anim. Genet. 2016, 47, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Shan, T.; Wu, T.; Zhu, L.N.; Ren, Y.; An, S.; Wang, Y. Comparisons of different muscle metabolic enzymes and muscle fiber types in Jinhua and Landrace pigs. J. Anim. Sci. 2011, 89, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xiong, X.; Yang, J.; Zhou, L.; Yang, B.; Ai, H.; Ma, H.; Xie, X.; Huang, Y.; Fang, S. Genome-wide association analyses for meat quality traits in Chinese Erhualian pigs and a Western Duroc × (Landrace × Yorkshire) commercial population. Genet. Sel. Evol. 2015, 47, 44. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Zhang, Z.; Yuan, Z.; Lo, L.J.; Chen, J.; Wang, Y.; Peng, J. Distinctive Genes Determine Different Intramuscular Fat and Muscle Fiber Ratios of the longissimus Dorsi Muscles in Jinhua and Landrace Pigs. PLoS ONE 2013, 8, e53181. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.S.; Seo, K.S.; Kim, K.T.; Nam, K.C. Comparison of Pork Quality Characteristics of Different Parts from Domesticated Pig Species. Korean J. Food Sci. Anim. Resour. 2011, 31, 921–927. [Google Scholar] [CrossRef]
- Choi, Y.S.; Ku, S.K.; Lee, H.J.; Sung, J.M.; Jeon, K.H.; Kim, H.W.; Kim, T.K.; Kim, Y.B. Study on Processing Quality of Different Parts of Pork and Beef. Korean J. Food Cook. Sci. 2016, 32, 157–167. [Google Scholar] [CrossRef]
- Kim, N.K.; Joh, J.H.; Park, H.R.; Kim, O.H.; Park, B.Y.; Lee, C.S. Differential expression profiling of the proteomes and their mRNAs in porcine white and red skeletal muscles. Proteomics 2004, 4, 3422–3428. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liu, Y.L.; Fu, C.Y.; Wang, J.; Chen, S.Y.; Yao, J.; Lai, S.J. Expression of MyHC genes, composition of muscle fiber type and their association with intramuscular fat, tenderness in skeletal muscle of Simmental hybrids. Mol. Biol. Rep. 2014, 41, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Cheatham, B.; Vlahos, C.J.; Cheatham, L.; Wang, L.; Blenis, J.; Kahn, C.R. Phosphatidylinositol 3-kinase activation is required for insulin stimulation of pp70 S6 kinase, DNA synthesis, and glucose transporter translocation. Mol. Cell. Biol. 1994, 14, 4902–4911. [Google Scholar] [CrossRef] [PubMed]
- Kamei, Y.; Miura, S.; Suzuki, M.; Kai, Y.; Mizukami, J.; Taniguchi, T.; Mochida, K.; Hata, T.; Matsuda, J.; Aburatani, H. Skeletal muscle FOXO1 (FKHR) transgenic mice have less skeletal muscle mass, down-regulated Type I (slow twitch/red muscle) fiber genes, and impaired glycemic control. J. Biol. Chem. 2004, 279, 41114–41123. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.E.; Zheng, X.L.; Liu, Y.G.; Yuan, Y.; Zhang, H.; Yang, G.S. Lentivirus Mediated shRNA Interference Silencing FoxO1 Gene Promoted the Expression of MyHC I in Pig Myoblasts. Chin. J. Biochem. Mol. Biol. 2011, 27, 582–588. [Google Scholar]
- Tous, N.; Lizardo, R.; Gispert, M.; Fontifurnols, M.; Estevegarcia, E. Effect of reducing dietary protein and lysine on growth performance, carcass characteristics, intramuscular fat, and fatty acid profile of finishing barrows. J. Anim. Sci. 2014, 92, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Xu, X.L.; Ma, H.M.; Jiang, J. Integrative analysis of transcriptomics and proteomics of skeletal muscles of the Chinese indigenous Shaziling pig compared with the Yorkshire breed. BMC Genet. 2016, 17, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Han, W.; Jiang, S.; Zhao, C.; Wu, C. Integrative transcriptomics and proteomics analysis of longissimus Dorsi muscles of Canadian double-muscled Large White pigs. Gene 2016, 577, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Faure, J.; Lebret, B.; Ecolan, P.; Metayer-Coustard, S.; Lefaucheur, L. Adenosine monophosphate-activated protein kinase status modulates kinetics of post-mortem pH decline and meat quality in pig Longissimus muscle. In Proceedings of the 57th International Conference of Meat Science and Technology, Ghent, Belgium, 7–12 August 2011. [Google Scholar]
- Shepherd, P.R.; Navé, B.T.; Siddle, K. Insulin stimulation of glycogen synthesis and glycogen synthase activity is blocked by wortmannin and rapamycin in 3T3-L1 adipocytes: Evidence for the involvement of phosphoinositide 3-kinase and p70 ribosomal protein-S6 kinase. Biochem. J. 1995, 305, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.C.; Cheng, Z.; Copps, K.D.; White, M.F. Insulin Receptor Substrates Irs1 and Irs2 Coordinate Skeletal Muscle Growth and Metabolism via the Akt and AMPK Pathways. Mol. Cell. Biol. 2011, 31, 430–441. [Google Scholar] [CrossRef] [PubMed]
- DeLong, A.D.; Zachary, B.; Bryon, R.; Sierra, S.; Yasutomi, K.; Shinji, M.; Osamu, E.; McLoughlin, T.J. Foxo1 Inhibits Skeletal Muscle Hypertrophy: 630: May 27 3:30 PM–3:45 PM. Med. Sci. Sports Exerc. 2009, 41. [Google Scholar] [CrossRef]
- Wang, J.Y. Effect of IRS1, Akt, FOXO1 on Occurrence of Sarcopenia and its Exercise-induced Remission. J. Chengdu Sport Univ. 2012, 38, 86–91. [Google Scholar]
- Meng, X.; Chen, X.; Chen, D.; Bing, Y.; Huang, Z. FoxO1: A novel insight into its molecular mechanisms in the regulation of skeletal muscle differentiation and fiber type specification. Oncotarget 2017, 8, 10662–10674. [Google Scholar]
- Bairwa, S.C.; Parajuli, N.; Dyck, J.R. The role of AMPK in cardiomyocyte health and survival. Biochim. Biophys. Acta 2016, 1862, 2199–2210. [Google Scholar] [CrossRef] [PubMed]
- Awazawa, M.; Ueki, K.; Inabe, K.; Yamauchi, T.; Kaneko, K.; Okazaki, Y.; Bardeesy, N.; Ohnishi, S.; Nagai, R.; Kadowaki, T. Adiponectin suppresses hepatic SREBP1c expression in an AdipoR1/LKB1/AMPK dependent pathway. Biochem. Biophys. Res. Commun. 2009, 382, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Jaworski, K.; Sarkadinagy, E.; Duncan, R.E.; Ahmadian, M.; Sul, H.S. Regulation of Triglyceride Metabolism. IV. Hormonal regulation of lipolysis in adipose tissue. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 293, G1–G4. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Qiang, L.I.; Chen, X.; Ya-Xin, L.I.; Shan, L.I. Curcumin Inhibits Lipid Deposition of Macrophage through LXRα-ABCA1 Pathway in Mouse. J. Hubei Univ. Med. 2015, 34, 120–124. [Google Scholar]
- Su, S.; Li, Q.; Liu, Z.S.; Wang, Y.P.; Zhu, H.S.; Luo, H.R.; Wu, S.; Xie, Z. Comparison of Expression Levels of LXRα in the Liver and Fat Tissue of Landes Goose Foie Gras. J. Agric. Biotechnol. 2008. [Google Scholar] [CrossRef]
- Gong, X.W.; He, Y.; Yan, H.Z.; Zhang, Y.P.; Huang, J.; Xu, Y.J.; Zhang, J.W.; Lin, C.M. Effect of Shugan Jianpi Recipe on LXRα/FAS signaling pathway mediated hepatocyte fatty deposits in NAFLD rats. Chin. J. Integr. Tradit. West. Med. 2014, 34, 1481–1486. [Google Scholar]
- Tyra, M.; Ropka-Molik, K. Effect of the FABP3 and LEPR gene polymorphisms and expression levels on intramuscular fat (IMF) content and fat cover degree in pigs. Livest. Sci. 2011, 142, 114–120. [Google Scholar] [CrossRef]
- Xiao, H.B.; Wang, J.Y.; Sun, Z.L. Icariin Changes ANGPTL3 Expression and LPL Activity to Improve Meat Quality in Swine. J. Anim. Vet. Adv. 2012, 11, 1450–1454. [Google Scholar] [CrossRef]
- Zhu, R.Z.; Yin, X.H.; Wang, Y.H.; Li, S.; Wu, D.; Wang, Q. The Gene Expression of MDH and LPL in Muscle and Their Association with Content of Intramuscular Fat and Composition of Fatty Acids in Pigs. Chin. J. Anim. Vet. Sci. 2013, 44, 1182–1188. [Google Scholar]
- Corominas, J.; Ramayo-Caldas, Y.; Castelló, A.; Muñoz, M.; Ibáñez-Escriche, N.; Folch, J.M.; Ballester, M. Evaluation of the porcine ACSL4 gene as a candidate gene for meat quality traits in pigs. Anim. Genet. 2012, 43, 714–720. [Google Scholar] [CrossRef] [PubMed]
- Jieun, L.; Wolfgang, M.J. Metabolomic profiling reveals a role for CPT1c in neuronal oxidative metabolism. BMC Biochem. 2012, 13, 23. [Google Scholar]
- Kirimura, J.; Shimizu, A.; Kimizuka, A.; Ninomiya, T.; Katsuya, N. Contribution of peptides and amino acids to the taste of foods. J. Agric. Food Chem. 1969, 17, 689–695. [Google Scholar] [CrossRef]
- Macy, R.L., Jr.; Naumann, H.D.; Bailey, M.E. Water-Soluble Flavor and Odor Precursors of Meat. I. Qualitative Study of Certain Amino Acids, Carbohydrates, Non-Amino Acid Nitrogen Compounds, and Phosphoric Acid Esters of Beef, Pork, and Lamb. J. Food Sci. 1964, 29, 136–141. [Google Scholar] [CrossRef]









| Group | Individual | Clean Reads | % Mapped Reads | Q20 (%) |
|---|---|---|---|---|
| LW_LM | LW_LM1 | 30,149,804 | 87.49 | 98.01 |
| LW_LM2 | 30,149,790 | 85.72 | 98.15 | |
| LW_LM3 | 30,149,412 | 86.25 | 97.36 | |
| Min_LM | Min_LM1 | 30,149,856 | 85.95 | 97.81 |
| Min_LM2 | 30,149,510 | 86.74 | 98.03 | |
| Min_LM3 | 30,149,878 | 84.95 | 97.83 | |
| Min_BF | Min_BF1 | 30,080,960 | 82.82 | 97.53 |
| Min_BF2 | 30,122,758 | 81.22 | 97.64 | |
| Min_BF3 | 30,455,930 | 84.11 | 98.14 |
| Pathway | p-Value | q-Value |
|---|---|---|
| Insulin resistance | 1.53 × 10−10 | 4.59 × 10−8 |
| Starch and sucrose metabolism | 7.09 × 10−6 | 1.06 × 10−3 |
| Regulation of actin cytoskeleton | 4.09 × 10−5 | 3.96 × 10−3 |
| Insulin signaling pathway | 5.28 × 10−5 | 3.96 × 10−3 |
| PPAR signaling pathway | 8.21 × 10−5 | 4.82 × 10−3 |
| Regulation of lipolysis in adipocytes | 1.07 × 10−4 | 4.82 × 10−3 |
| Adipocytokine signaling pathway | 1.12 × 10−4 | 4.82 × 10−3 |
| Glucagon signaling pathway | 1.46 × 10−4 | 4.97 × 10−3 |
| Bladder cancer | 1.49 × 10−4 | 4.97 × 10−3 |
| PI3K-Akt signaling pathway | 2.20 × 10−4 | 6.60 × 10−3 |
| Pathways in cancer | 2.81 × 10−4 | 7.51 × 10−3 |
| Complement and coagulation cascades | 3.01 × 10−4 | 7.51 × 10−3 |
| Platelet activation | 3.63 × 10−4 | 7.63 × 10−3 |
| MAPK signaling pathway | 3.67 × 10−4 | 7.63 × 10−3 |
| MicroRNAs in cancer | 3.82 × 10−4 | 7.63 × 10−3 |
| Circadian rhythm | 6.99 × 10−4 | 1.27 × 10−2 |
| Amoebiasis | 7.21 × 10−4 | 1.27 × 10−2 |
| AMPK signaling pathway | 9.67 × 10−4 | 1.46 × 10−2 |
| Small cell lung cancer | 9.72 × 10−4 | 1.46 × 10−2 |
| FoxO signaling pathway | 9.74 × 10−4 | 1.46 × 10−2 |
| Proteoglycans in cancer | 1.62 × 10−3 | 2.32 × 10−2 |
| Focal adhesion | 2.19 × 10−3 | 2.89 × 10−2 |
| Riboflavin metabolism | 2.22 × 10−3 | 2.89 × 10−2 |
| Rap1 signaling pathway | 2.36 × 10−3 | 2.95 × 10−2 |
| ECM-receptor interaction | 2.96 × 10−3 | 3.44 × 10−2 |
| Cocaine addiction | 2.98 × 10−3 | 3.44 × 10−2 |
| Hypertrophic cardiomyopathy (HCM) | 3.50 × 10−3 | 3.88 × 10−2 |
| Carbohydrate digestion and absorption | 4.85 × 10−3 | 5.20 × 10−2 |
| Pertussis | 6.19 × 10−3 | 6.40 × 10−2 |
| Protein digestion and absorption | 6.48 × 10−3 | 6.48 × 10−2 |
| Pathway | p-Value | q-Value |
|---|---|---|
| Focal adhesion | 1.74 × 10−6 | 3.05 × 10−4 |
| Pertussis | 9.30 × 10−5 | 5.45 × 10−3 |
| Protein digestion and absorption | 9.58 × 10−5 | 5.45 × 10−3 |
| Amoebiasis | 1.44 × 10−4 | 5.45 × 10−3 |
| ECM-receptor interaction | 1.55 × 10−4 | 5.45 × 10−3 |
| PI3K-Akt signaling pathway | 6.42 × 10−4 | 1.68 × 10−2 |
| Prion diseases | 7.24 × 10−4 | 1.68 × 10−2 |
| AGE-RAGE signaling pathway in diabetic complications | 7.65 × 10−4 | 1.68 × 10−2 |
| Complement and coagulation cascades | 1.10 × 10−3 | 2.14 × 10−2 |
| Platelet activation | 1.45 × 10−3 | 2.55 × 10−2 |
| Staphylococcus aureus infection | 2.54 × 10−3 | 4.06 × 10−2 |
| Regulation of actin cytoskeleton | 3.36 × 10−3 | 4.92 × 10−2 |
| Carbohydrate digestion and absorption | 5.37 × 10−3 | 7.00 × 10−2 |
| Vascular smooth muscle contraction | 5.57 × 10−3 | 7.00 × 10−2 |
| Salmonella infection | 9.99 × 10−3 | 1.17 × 10−1 |
| Oxytocin signaling pathway | 1.21 × 10−2 | 1.27 × 10−1 |
| Phenylalanine metabolism | 1.23 × 10−2 | 1.27 × 10−1 |
| MicroRNAs in cancer | 1.37 × 10−2 | 1.34 × 10−1 |
| Butanoate metabolism | 1.56 × 10−2 | 1.45 × 10−1 |
| Thyroid hormone signaling pathway | 2.02 × 10−2 | 1.78 × 10−1 |
| Pathogenic Escherichia coli infection | 2.20 × 10−2 | 1.84 × 10−1 |
| EGFR tyrosine kinase inhibitor resistance | 2.42 × 10−2 | 1.86 × 10−1 |
| Axon guidance | 2.43 × 10−2 | 1.86 × 10−1 |
| Leukocyte transendothelial migration | 2.77 × 10−2 | 2.03 × 10−1 |
| Glycine, serine and threonine metabolism | 3.22 × 10−2 | 2.27 × 10−1 |
| Tyrosine metabolism | 3.46 × 10−2 | 2.34 × 10−1 |
| Insulin secretion | 3.70 × 10−2 | 2.41 × 10−1 |
| Fat digestion and absorption | 4.76 × 10−2 | 2.91 × 10−1 |
| Gene | Primer Sequences (5′-3′) | Product Size (bp) | Tm (°C) |
|---|---|---|---|
| CPT1A | F: ACAAGCCATAGTCTTAACGAAA; R: GCCAGTCCAGGATAACAAA | 198 | 60 |
| CPT1B | F: ACTGTCTGGGCAAACCAAAC; R: CTTCTTGATGAGGCCTTTGC | 176 | 60 |
| RasGRP3 | F: TAAATCGCAGCCTACCTCCCCT; R:TTGGCAGCTATACTTTCAAAGTCCT | 198 | 60 |
| IRS1 | F: TGCCTGACCAGCAAGACCATC; R: ATCCACCTGCATCCAAAACTC | 168 | 60 |
| UCP3 | F: GACGTGGTGAAGGTTCGATT; R: CGAGTTCATGTACCGGGTCT | 330 | 60 |
| CRYAB | F: GACCCTCTCACCATTACTTCA; R: CAGCAGGCTTCTCTTCACG | 121 | 60 |
| PYGM | F: CCCAGTATGCCAGGGAGAT; R: CTGAGGGATTGCGAACAGA | 125 | 60 |
| HSPB1 | F: CCTGTCACTTTCGAGGCG; R: AGGTGGGGATGGCTGGT | 168 | 60 |
| PPARA | F: CCGAGACCGCAGATCTCAAG; R: GACGAAAGGCGGGTTATTGC | 128 | 60 |
| PPARGC1A | F: GATGTGTCGCCTTCTTGTTC; R: CATCCTTTGGGGTCTTTGAG | 93 | 60 |
| GPX3 | F: GCTTCCCCTGCAACCAATT; R:GGACATACCTGAGAGTGGACAGAA | 75 | 60 |
| AMD1 | F: TCCACAAGTCAAGTCCTCTAATG; R: CCATGGAGAGGAACGAATCAA | 108 | 60 |
| ZIC1 | F: CGACCGACGCTTTGCTAATA; R: GTAGGACTTGTCGCACATCTT | 97 | 60 |
| GAPDH | F: CTACTCGGGCCTCTTCTGTG; R: GATTCTCCCGATCAGTCAGC | 112 | 60 |
| PLP1 | F: CTTCCTTTATGGGGCCCTCC; R: ACACACCCGCTCCAAAGAAT | 181 | 60 |
| NEFM | F: GAGCAGAACAAGGAGGCCAT; R: TTGGTGCCTCGAACTGACTC | 104 | 60 |
| ACSM3 | F: AATGGCTCCACCAATCCAGG; R: ACGTTGGTCTTGGCAGTAGC | 102 | 60 |
| ISLR2 | F: CGTGCACTGAGCTCTTCAGG; R: CGGGGTTCAACTCCTTTTCC | 115 | 60 |
| ZNF503 | F: CCAAACATGCTCGCAGATCG; R: ATGTCGCTTAGCTTGAGGGG | 150 | 60 |
| HOXC6 | F: GCCTTTCTCCTGGTGTACTGT; R: TCCTGCCCTGCTCAGAACTAA | 193 | 60 |
| SIM1 | F: GGCTCTCACCGGCAGTATTT; R: TGAGCCATTACAGCCCAAGG | 114 | 60 |
| ZIC1 | F: GCCTCCATTCCCTATCCTGC; R: TGAGCGTTTGTGCTTGTTCG | 145 | 60 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Yang, X.; Jing, X.; He, X.; Wang, L.; Liu, Y.; Liu, D. Transcriptomics Analysis on Excellent Meat Quality Traits of Skeletal Muscles of the Chinese Indigenous Min Pig Compared with the Large White Breed. Int. J. Mol. Sci. 2018, 19, 21. https://doi.org/10.3390/ijms19010021
Liu Y, Yang X, Jing X, He X, Wang L, Liu Y, Liu D. Transcriptomics Analysis on Excellent Meat Quality Traits of Skeletal Muscles of the Chinese Indigenous Min Pig Compared with the Large White Breed. International Journal of Molecular Sciences. 2018; 19(1):21. https://doi.org/10.3390/ijms19010021
Chicago/Turabian StyleLiu, Yingzi, Xiuqin Yang, Xiaoyan Jing, Xinmiao He, Liang Wang, Yang Liu, and Di Liu. 2018. "Transcriptomics Analysis on Excellent Meat Quality Traits of Skeletal Muscles of the Chinese Indigenous Min Pig Compared with the Large White Breed" International Journal of Molecular Sciences 19, no. 1: 21. https://doi.org/10.3390/ijms19010021
APA StyleLiu, Y., Yang, X., Jing, X., He, X., Wang, L., Liu, Y., & Liu, D. (2018). Transcriptomics Analysis on Excellent Meat Quality Traits of Skeletal Muscles of the Chinese Indigenous Min Pig Compared with the Large White Breed. International Journal of Molecular Sciences, 19(1), 21. https://doi.org/10.3390/ijms19010021