Antiangiogenic Effect of Flavonoids and Chalcones: An Update
Abstract
:1. Introduction
2. Methods
3. Angiogenesis and Current Antiangiogenic Therapy
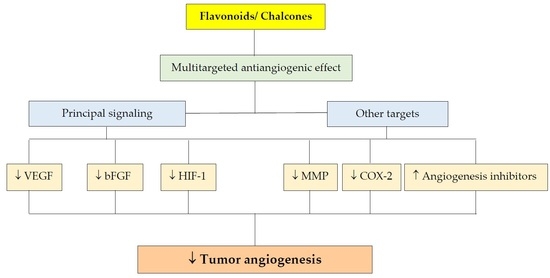
4. Effect of Polyphenols on Angiogenesis
4.1. Flavonoids
4.1.1. Effect on Signaling Pathways
VEGF Signaling Pathway
bFGF Signaling Pathway
HIF-1 Signaling Pathway
Effect of Flavonoids on Matrix Metalloproteinases
Other Targets
4.2. Chalcones
4.2.1. Chalcone-Induced Antiangiogenic Effects Proved by Common Angiogenesis Assays
Antiangiogenic Effects of Naturally Occurring Chalcones
Antiangiogenic Effects of Chalcone Analogues
4.2.2. VEGF-Related Antiangiogenic Effects of Chalcones
Naturally Occurring Chalcones in Regulation of the VEGF-Mediated Angiogenic Pathway
Synthetic Chalcone Derivatives Involvement in VEGF- and HIF-Controlled Angiogenesis
4.2.3. Suppression of Extracellular Signal-Regulated Kinase (ERK)
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dzialo, M.; Mierziak, J.; Korzun, U.; Preisner, M.; Szopa, J.; Kulma, A. The Potential of Plant Phenolics in Prevention and Therapy of Skin Disorders. Int. J. Mol. Sci. 2016, 17, 160. [Google Scholar] [CrossRef] [PubMed]
- Brglez Mojzer, E.; Knez Hrncic, M.; Skerget, M.; Knez, Z.; Bren, U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules 2016, 21. [Google Scholar] [CrossRef] [PubMed]
- Lattanzio, V.; Lattanzio, V.M.T.; Cardinali, A. Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects. In Phytochemistry: Advances in Research; Imperato, F., Ed.; Research Signpost: Kerala, India, 2006; pp. 23–26. [Google Scholar]
- Bhuiyan, M.N.; Mitsuhashi, S.; Sigetomi, K.; Ubukata, M. Quercetin inhibits advanced glycation end product formation via chelating metal ions, trapping methylglyoxal, and trapping reactive oxygen species. Biosci. Biotechnol. Biochem. 2017, 81, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Losada-Barreiro, S.; Bravo-Diaz, C. Free radicals and polyphenols: The redox chemistry of neurodegenerative diseases. Eur. J. Med. Chem. 2017, 133, 379–402. [Google Scholar] [CrossRef] [PubMed]
- Makhafola, T.J.; Elgorashi, E.E.; McGaw, L.J.; Verschaeve, L.; Eloff, J.N. The correlation between antimutagenic activity and total phenolic content of extracts of 31 plant species with high antioxidant activity. BMC Complement. Altern. Med. 2016, 16, 490. [Google Scholar] [CrossRef] [PubMed]
- Rahman, I.; Chung, S. Dietary polyphenols, deacetylases and chromatin remodeling in inflammation. World Rev. Nutr. Diet. 2010, 101, 84–94. [Google Scholar] [PubMed]
- Alissa, E.M.; Ferns, G.A. Dietary fruits and vegetables and cardiovascular diseases risk. Crit. Rev. Food Sci. Nutr. 2017, 57, 1950–1962. [Google Scholar] [CrossRef] [PubMed]
- Torres, N.; Guevara-Cruz, M.; Velazquez-Villegas, L.A.; Tovar, A.R. Nutrition and Atherosclerosis. Arch. Med. Res. 2015, 46, 408–426. [Google Scholar] [CrossRef] [PubMed]
- Ben Lagha, A.; Haas, B.; Grenier, D. Tea polyphenols inhibit the growth and virulence properties of Fusobacterium nucleatum. Sci. Rep. 2017, 7, 44815. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Oh, M.; Seok, J.H.; Kim, S.; Lee, D.B.; Bae, G.; Bae, H.I.; Bae, S.Y.; Hong, Y.M.; Kwon, S.O.; et al. Antiviral Effects of Black Raspberry (Rubus coreanus) Seed and Its Gallic Acid against Influenza Virus Infection. Viruses 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; D’Souza, D.H. Naturally occurring flavonoids against human norovirus surrogates. Food Environ. Virol. 2013, 5, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Hashemzaei, M.; Delarami Far, A.; Yari, A.; Heravi, R.E.; Tabrizian, K.; Taghdisi, S.M.; Sadegh, S.E.; Tsarouhas, K.; Kouretas, D.; Tzanakakis, G.; et al. Anticancer and apoptosisinducing effects of quercetin in vitro and in vivo. Oncol. Rep. 2017, 38, 819–828. [Google Scholar] [PubMed]
- Kubatka, P.; Kapinova, A.; Kello, M.; Kruzliak, P.; Kajo, K.; Vybohova, D.; Mahmood, S.; Murin, R.; Viera, T.; Mojzis, J.; et al. Fruit peel polyphenols demonstrate substantial anti-tumour effects in the model of breast cancer. Eur. J. Nutr. 2016, 55, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Secme, M.; Eroglu, C.; Dodurga, Y.; Bagci, G. Investigation of anticancer mechanism of oleuropein via cell cycle and apoptotic pathways in SH-SY5Y neuroblastoma cells. Gene 2016, 585, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Mocanu, M.M.; Nagy, P.; Szollosi, J. Chemoprevention of Breast Cancer by Dietary Polyphenols. Molecules 2015, 20, 22578–22620. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, A.R.; Chow, H.S.; Martinez, J.A. Effects of resveratrol on drug- and carcinogen-metabolizing enzymes, implications for cancer prevention. Pharmacol. Res. Perspect. 2017, 5, e00294. [Google Scholar] [CrossRef] [PubMed]
- Kello, M.; Kulikova, L.; Vaskova, J.; Nagyova, A.; Mojzis, J. Fruit Peel Polyphenolic Extract-Induced Apoptosis in Human Breast Cancer Cells Is Associated with ROS Production and Modulation of p38MAPK/Erk1/2 and the Akt Signaling Pathway. Nutr. Cancer 2017, 69, 920–931. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.K.; Gupta, S.C.; Nabavizadeh, A.; Aggarwal, B.B. Regulation of cell signaling pathways by dietary agents for cancer prevention and treatment. Semin. Cancer Biol. 2017, 46, 158–181. [Google Scholar] [CrossRef] [PubMed]
- Dandawate, P.R.; Subramaniam, D.; Jensen, R.A.; Anant, S. Targeting cancer stem cells and signaling pathways by phytochemicals: Novel approach for breast cancer therapy. Semin. Cancer Biol. 2016, 40–41, 192–208. [Google Scholar] [CrossRef] [PubMed]
- Curti, V.; Di Lorenzo, A.; Dacrema, M.; Xiao, J.; Nabavi, S.M.; Daglia, M. In vitro polyphenol effects on apoptosis: An update of literature data. Semin. Cancer Biol. 2017, 46, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Niaz, K.; Maqbool, F.; Ismail Hassan, F.; Abdollahi, M.; Nagulapalli Venkata, K.C.; Nabavi, S.M.; Bishayee, A. Molecular Targets Underlying the Anticancer Effects of Quercetin: An Update. Nutrients 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Coccia, A.; Mosca, L.; Puca, R.; Mangino, G.; Rossi, A.; Lendaro, E. Extra-virgin olive oil phenols block cell cycle progression and modulate chemotherapeutic toxicity in bladder cancer cells. Oncol. Rep. 2016, 36, 3095–3104. [Google Scholar] [CrossRef] [PubMed]
- Zielinska-Przyjemska, M.; Kaczmarek, M.; Krajka-Kuzniak, V.; Luczak, M.; Baer-Dubowska, W. The effect of resveratrol, its naturally occurring derivatives and tannic acid on the induction of cell cycle arrest and apoptosis in rat C6 and human T98G glioma cell lines. Toxicol. In Vitro 2017, 43, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Cerezo, A.B.; Winterbone, M.S.; Moyle, C.W.; Needs, P.W.; Kroon, P.A. Molecular structure-function relationship of dietary polyphenols for inhibiting VEGF-induced VEGFR-2 activity. Mol. Nutr. Food Res. 2015, 59, 2119–2131. [Google Scholar] [CrossRef] [PubMed]
- Park, J.J.; Hwang, S.J.; Park, J.H.; Lee, H.J. Chlorogenic acid inhibits hypoxia-induced angiogenesis via down-regulation of the HIF-1α/AKT pathway. Cell. Oncol. 2015, 38, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.K.; Warrier, S.; Kumar, A.P.; Sethi, G.; Arfuso, F. Potential Role of Natural Compounds as Anti-Angiogenic Agents in Cancer. Curr. Vasc. Pharmacol. 2017, 15, 503–519. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, J.; Nandy, S.K.; Chowdhury, A.; Chakraborti, T.; Chakraborti, S. Inhibition of MMP-9 by green tea catechins and prediction of their interaction by molecular docking analysis. Biomed. Pharmacother. 2016, 84, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Lai, C.Q.; Nie, L.; Ordovas, J.; Band, M.; Moser, L.; Meydani, M. The modulation of endothelial cell gene expression by green tea polyphenol-EGCG. Mol. Nutr. Food Res. 2008, 52, 1182–1192. [Google Scholar] [CrossRef] [PubMed]
- Oak, M.H.; El Bedoui, J.; Schini-Kerth, V.B. Antiangiogenic properties of natural polyphenols from red wine and green tea. J. Nutr. Biochem. 2005, 16, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bouck, N.; Stellmach, V.; Hsu, S.C. How tumors become angiogenic. Adv. Cancer Res. 1996, 69, 135–174. [Google Scholar] [PubMed]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Donnem, T.; Hu, J.; Ferguson, M.; Adighibe, O.; Snell, C.; Harris, A.L.; Gatter, K.C.; Pezzella, F. Vessel co-option in primary human tumors and metastases: An obstacle to effective anti-angiogenic treatment? Cancer Med. 2013, 2, 427–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maniotis, A.J.; Folberg, R.; Hess, A.; Seftor, E.A.; Gardner, L.M.; Pe’er, J.; Trent, J.M.; Meltzer, P.S.; Hendrix, M.J. Vascular channel formation by human melanoma cells in vivo and in vitro: Vasculogenic mimicry. Am. J. Pathol. 1999, 155, 739–752. [Google Scholar] [CrossRef]
- Tang, S.; Xiang, T.; Huang, S.; Zhou, J.; Wang, Z.; Xie, R.; Long, H.; Zhu, B. Ovarian cancer stem-like cells differentiate into endothelial cells and participate in tumor angiogenesis through autocrine CCL5 signaling. Cancer Lett. 2016, 376, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Auguste, P.; Lemiere, S.; Larrieu-Lahargue, F.; Bikfalvi, A. Molecular mechanisms of tumor vascularization. Crit. Rev. Oncol. Hematol. 2005, 54, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Tumor angiogenesis: Therapeutic implications. N. Engl. J. Med. 1971, 285, 1182–1186. [Google Scholar] [PubMed]
- Jain, R.K. Antiangiogenesis strategies revisited: From starving tumors to alleviating hypoxia. Cancer Cell 2014, 26, 605–622. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.H.; Gootenberg, J.; Keegan, P.; Pazdur, R. FDA drug approval summary: Bevacizumab plus FOLFOX4 as second-line treatment of colorectal cancer. Oncologist 2007, 12, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Burstein, H.J. Bevacizumab for advanced breast cancer: All tied up with a RIBBON? J. Clin. Oncol. 2011, 29, 1232–1235. [Google Scholar] [CrossRef] [PubMed]
- Jeong, W.; Doroshow, J.H.; Kummar, S. United States Food and Drug Administration approved oral kinase inhibitors for the treatment of malignancies. Curr. Probl. Cancer 2013, 37, 110–144. [Google Scholar] [CrossRef] [PubMed]
- Kerbel, R.S. Inhibition of tumor angiogenesis as a strategy to circumvent acquired resistance to anti-cancer therapeutic agents. Bioessays 1991, 13, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Bergers, G.; Hanahan, D. Modes of resistance to anti-angiogenic therapy. Nat. Rev. Cancer 2008, 8, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, H.F. Tumor Stroma, Tumor Blood Vessels, and Antiangiogenesis Therapy. Cancer J. 2015, 21, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.N.; Tan, M.H.; Yang, J.P.; Cao, Y. Revisiting tumor angiogenesis: Vessel co-option, vessel remodeling, and cancer cell-derived vasculature formation. Chin. J. Cancer 2016, 35, 10. [Google Scholar] [CrossRef] [PubMed]
- Cartea, M.E.; Francisco, M.; Soengas, P.; Velasco, P. Phenolic compounds in Brassica vegetables. Molecules 2010, 16, 251–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes, F.G.; Nedel, F.; Alves, A.M.; Nor, J.E.; Tarquinio, S.B. Tumor angiogenesis and lymphangiogenesis: Tumor/endothelial crosstalk and cellular/microenvironmental signaling mechanisms. Life Sci. 2013, 92, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Verheul, H.M.; Pinedo, H.M. The role of vascular endothelial growth factor (VEGF) in tumor angiogenesis and early clinical development of VEGF-receptor kinase inhibitors. Clin. Breast Cancer 2000, 1 (Suppl. S1), S80–S84. [Google Scholar] [CrossRef] [PubMed]
- McMahon, G. VEGF receptor signaling in tumor angiogenesis. Oncologist 2000, 5 (Suppl. S1), 3–10. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Gerber, H.P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, K.; Aggarwal, B.B.; Singh, R.B.; Buttar, H.S.; Wilson, D.; De Meester, F. Food Antioxidants and Their Anti-Inflammatory Properties: A Potential Role in Cardiovascular Diseases and Cancer Prevention. Diseases 2016, 4, 28. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska, H.; Kalinowska, M.; Lewandowski, W.; Stepkowski, T.M.; Brzoska, K. The role of natural polyphenols in cell signaling and cytoprotection against cancer development. J. Nutr. Biochem. 2016, 32, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Pratheeshkumar, P.; Budhraja, A.; Son, Y.O.; Wang, X.; Zhang, Z.; Ding, S.; Wang, L.; Hitron, A.; Lee, J.C.; Xu, M.; et al. Quercetin inhibits angiogenesis mediated human prostate tumor growth by targeting VEGFR-2 regulated AKT/mTOR/P70S6K signaling pathways. PLoS ONE 2012, 7, e47516. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Qin, C.; Fan, X.; Li, Y.; Gu, B. Inhibitory effects of quercetin on angiogenesis in larval zebrafish and human umbilical vein endothelial cells. Eur. J. Pharmacol. 2014, 723, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Bai, Y.; Zhao, M.; Huang, L.; Li, S.; Li, X.; Chen, Y. Quercetin inhibits vascular endothelial growth factor-induced choroidal and retinal angiogenesis in vitro. Ophthalmic Res. 2015, 53, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Maurya, A.K.; Vinayak, M. Quercetin Attenuates Cell Survival, Inflammation, and Angiogenesis via Modulation of AKT Signaling in Murine T-Cell Lymphoma. Nutr. Cancer 2017, 69, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Somerset, S.M.; Johannot, L. Dietary flavonoid sources in Australian adults. Nutr. Cancer 2008, 60, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Rankin, G.O.; Juliano, N.; Jiang, B.H.; Chen, Y.C. Kaempferol inhibits VEGF expression and in vitro angiogenesis through a novel ERK-NFkappaB-cMyc-p21 pathway. Food Chem. 2012, 130, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Rankin, G.O.; Liu, L.; Daddysman, M.K.; Jiang, B.H.; Chen, Y.C. Kaempferol inhibits angiogenesis and VEGF expression through both HIF dependent and independent pathways in human ovarian cancer cells. Nutr. Cancer 2009, 61, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Pratheeshkumar, P.; Son, Y.O.; Budhraja, A.; Wang, X.; Ding, S.; Wang, L.; Hitron, A.; Lee, J.C.; Kim, D.; Divya, S.P.; et al. Luteolin inhibits human prostate tumor growth by suppressing vascular endothelial growth factor receptor 2-mediated angiogenesis. PLoS ONE 2012, 7, e52279. [Google Scholar] [CrossRef] [PubMed]
- Ambasta, R.K.; Jha, S.K.; Kumar, D.; Sharma, R.; Jha, N.K.; Kumar, P. Comparative study of anti-angiogenic activities of luteolin, lectin and lupeol biomolecules. J. Transl. Med. 2015, 13, 307. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Lu, W.; Ye, T.; Lu, M.; Wang, J.; Huo, J.; Qian, S.; Wang, X.; Cao, P. The molecular mechanism of luteolin-induced apoptosis is potentially related to inhibition of angiogenesis in human pancreatic carcinoma cells. Oncol. Rep. 2012, 28, 1353–1361. [Google Scholar] [CrossRef] [PubMed]
- Ravishankar, D.; Watson, K.A.; Boateng, S.Y.; Green, R.J.; Greco, F.; Osborn, H.M. Exploring quercetin and luteolin derivatives as antiangiogenic agents. Eur. J. Med. Chem. 2015, 97, 259–274. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Cai, W.; Pei, C.G.; Shao, Y. Rhamnazin, a novel inhibitor of VEGFR2 signaling with potent antiangiogenic activity and antitumor efficacy. Biochem. Biophys. Res. Commun. 2015, 458, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Lecumberri, E.; Dupertuis, Y.M.; Miralbell, R.; Pichard, C. Green tea polyphenol epigallocatechin-3-gallate (EGCG) as adjuvant in cancer therapy. Clin. Nutr. 2013, 32, 894–903. [Google Scholar] [CrossRef] [PubMed]
- Niedzwiecki, A.; Roomi, M.W.; Kalinovsky, T.; Rath, M. Anticancer Efficacy of Polyphenols and Their Combinations. Nutrients 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Moyle, C.W.; Cerezo, A.B.; Winterbone, M.S.; Hollands, W.J.; Alexeev, Y.; Needs, P.W.; Kroon, P.A. Potent inhibition of VEGFR-2 activation by tight binding of green tea epigallocatechin gallate and apple procyanidins to VEGF: Relevance to angiogenesis. Mol. Nutr. Food Res. 2015, 59, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Shirakami, Y.; Sakai, H.; Yasuda, Y.; Kubota, M.; Adachi, S.; Tsurumi, H.; Hara, Y.; Moriwaki, H. (−)-Epigallocatechin gallate inhibits growth and activation of the VEGF/VEGFR axis in human colorectal cancer cells. Chem. Biol. Interact. 2010, 185, 247–252. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhang, E.; Shi, J.; Li, X.; Zhou, K.; Zhang, Q.; Le, A.D.; Tang, X. (−)-Epigallocatechin-3-gallate inhibits human papillomavirus (HPV)-16 oncoprotein-induced angiogenesis in non-small cell lung cancer cells by targeting HIF-1α. Cancer Chemother. Pharmacol. 2013, 71, 713–725. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Feng, Y.; Liu, J.; Feng, X.; Zhou, K.; Tang, X. Epigallocatechin-3-gallate inhibits IGF-I-stimulated lung cancer angiogenesis through downregulation of HIF-1α and VEGF expression. J. Nutrigenet. Nutrigenom. 2013, 6, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.L.; Liu, F.; Zhang, W.Z.; Liu, X.; Lin, B.H.; Tang, X.D. Epigallocatechin-3-gallate inhibits nicotine-induced migration and invasion by the suppression of angiogenesis and epithelial-mesenchymal transition in non-small cell lung cancer cells. Oncol. Rep. 2015, 33, 2972–2980. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.H.; Chen, H.Y.; Zhan, W.H.; Wang, C.Y.; Cai, S.R.; Wang, Z.; Zhang, C.H.; He, Y.L. (−)-Epigallocatechin-3-gallate inhibits VEGF expression induced by IL-6 via Stat3 in gastric cancer. World J. Gastroenterol. 2011, 17, 2315–2325. [Google Scholar] [CrossRef] [PubMed]
- Doleckova, I.; Rarova, L.; Gruz, J.; Vondrusova, M.; Strnad, M.; Krystof, V. Antiproliferative and antiangiogenic effects of flavone eupatorin, an active constituent of chloroform extract of Orthosiphon stamineus leaves. Fitoterapia 2012, 83, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Rankin, G.O.; Tu, Y.; Chen, Y.C. Theaflavin-3,3′-digallate decreases human ovarian carcinoma OVCAR-3 cell-induced angiogenesis via Akt and Notch-1 pathways, not via MAPK pathways. Int. J. Oncol. 2016, 48, 281–292. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Wu, Y.; Lin, L.; Wang, J.; Wu, Y.; Chen, Y.; Yi, Z.; Liu, M.; Pang, X. Hispidulin, a small flavonoid molecule, suppresses the angiogenesis and growth of human pancreatic cancer by targeting vascular endothelial growth factor receptor 2-mediated PI3K/Akt/mTOR signaling pathway. Cancer Sci. 2011, 102, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Chen, A.Y.; Rojanasakul, Y.; Ye, X.; Rankin, G.O.; Chen, Y.C. Dietary compounds galangin and myricetin suppress ovarian cancer cell angiogenesis. J. Funct. Foods 2015, 15, 464–475. [Google Scholar] [CrossRef] [PubMed]
- Keravis, T.; Favot, L.; Abusnina, A.A.; Anton, A.; Justiniano, H.; Soleti, R.; Alabed Alibrahim, E.; Simard, G.; Andriantsitohaina, R.; Lugnier, C. Delphinidin Inhibits Tumor Growth by Acting on VEGF Signalling in Endothelial Cells. PLoS ONE 2015, 10, e0145291. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.H.; Alex, D.; Lam, I.K.; Tsui, S.K.; Yang, Z.F.; Lee, S.M. Nobiletin, a polymethoxylated flavonoid from citrus, shows anti-angiogenic activity in a zebrafish in vivo model and HUVEC in vitro model. J. Cell. Biochem. 2011, 112, 3313–3321. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.M.; Chen, Y.H.; Ong, J.R.; Ma, H.P.; Shyu, K.G.; Bai, K.J. Functional role of wogonin in anti-angiogenesis. Am. J. Chin. Med. 2012, 40, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.M.; Shyu, K.G.; Wang, B.W.; Chang, H.; Chen, Y.H.; Chiu, J.H. Chrysin suppresses IL-6-induced angiogenesis via down-regulation of JAK1/STAT3 and VEGF: An in vitro and in ovo approach. J. Agric. Food Chem. 2010, 58, 7082–7087. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Song, X.; Huang, Y.; Wei, L.; Li, Z.; You, Q.; Guo, Q.; Lu, N. Wogonin inhibits H2O2-induced angiogenesis via suppressing PI3K/Akt/NF-κB signaling pathway. Vascul. Pharmacol. 2014, 60, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Lieu, C.; Heymach, J.; Overman, M.; Tran, H.; Kopetz, S. Beyond VEGF: Inhibition of the fibroblast growth factor pathway and antiangiogenesis. Clin. Cancer Res. 2011, 17, 6130–6139. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.; Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Giacomini, A.; Chiodelli, P.; Matarazzo, S.; Rusnati, M.; Presta, M.; Ronca, R. Blocking the FGF/FGFR system as a “two-compartment” antiangiogenic/antitumor approach in cancer therapy. Pharmacol. Res. 2016, 107, 172–185. [Google Scholar] [CrossRef] [PubMed]
- Kunimasa, K.; Ikekita, M.; Sato, M.; Ohta, T.; Yamori, Y.; Ikeda, M.; Kuranuki, S.; Oikawa, T. Nobiletin, a citrus polymethoxyflavonoid, suppresses multiple angiogenesis-related endothelial cell functions and angiogenesis in vivo. Cancer Sci. 2010, 101, 2462–2469. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.; Han, Y.; Gao, H.; Xin, S.; Chen, S.; Wang, N.; Qin, W.; Zhong, H.; Lin, S.; Yao, X.; et al. Kaempferol Identified by Zebrafish Assay and Fine Fractionations Strategy from Dysosma versipellis Inhibits Angiogenesis through VEGF and FGF Pathways. Sci. Rep. 2015, 5, 14468. [Google Scholar] [CrossRef] [PubMed]
- Rabiau, N.; Kossai, M.; Braud, M.; Chalabi, N.; Satih, S.; Bignon, Y.J.; Bernard-Gallon, D.J. Genistein and daidzein act on a panel of genes implicated in cell cycle and angiogenesis by polymerase chain reaction arrays in human prostate cancer cell lines. Cancer Epidemiol. 2010, 34, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, S.R.; Karmakar, S.; Banik, N.L.; Ray, S.K. Synergistic efficacy of sorafenib and genistein in growth inhibition by down regulating angiogenic and survival factors and increasing apoptosis through upregulation of p53 and p21 in malignant neuroblastoma cells having N-Myc amplification or non-amplification. Investig. New Drugs 2010, 28, 812–824. [Google Scholar]
- Varinska, L.; Gal, P.; Mojzisova, G.; Mirossay, L.; Mojzis, J. Soy and breast cancer: Focus on angiogenesis. Int. J. Mol. Sci. 2015, 16, 11728–11749. [Google Scholar] [CrossRef] [PubMed]
- Brahimi-Horn, M.C.; Pouyssegur, J. The hypoxia-inducible factor and tumor progression along the angiogenic pathway. Int. Rev. Cytol. 2005, 242, 157–213. [Google Scholar] [PubMed]
- Yang, Y.; Sun, M.; Wang, L.; Jiao, B. HIFs, angiogenesis, and cancer. J. Cell. Biochem. 2013, 114, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Hickey, M.M.; Simon, M.C. Regulation of angiogenesis by hypoxia and hypoxia-inducible factors. Curr. Top. Dev. Biol. 2006, 76, 217–257. [Google Scholar] [PubMed]
- Song, X.; Yao, J.; Wang, F.; Zhou, M.; Zhou, Y.; Wang, H.; Wei, L.; Zhao, L.; Li, Z.; Lu, N.; et al. Wogonin inhibits tumor angiogenesis via degradation of HIF-1α protein. Toxicol. Appl. Pharmacol. 2013, 271, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Chen, Y.; Wang, X.P.; An, T.; Tao, L.; Zhou, Y.X.; Huang, Y.J.; Chen, B.A.; Li, Z.Y.; You, Q.D.; et al. Wogonin inhibits multiple myeloma-stimulated angiogenesis via c-Myc/VHL/HIF-1α signaling axis. Oncotarget 2016, 7, 5715–5727. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, A.Y.; Huang, H.; Ye, X.; Rollyson, W.D.; Perry, H.E.; Brown, K.C.; Rojanasakul, Y.; Rankin, G.O.; Dasgupta, P.; et al. The flavonoid nobiletin inhibits tumor growth and angiogenesis of ovarian cancers via the Akt pathway. Int. J. Oncol. 2015, 46, 2629–2638. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.W.; Makey, K.L.; Tucker, K.B.; Chinchar, E.; Mao, X.; Pei, I.; Thomas, E.Y.; Miele, L. EGCG, a major green tea catechin suppresses breast tumor angiogenesis and growth via inhibiting the activation of HIF-1α and NFκB, and VEGF expression. Vascular Cell 2013, 5, 9. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.Q.; Xu, M.; Zhong, W.T.; Cui, Z.Y.; Liu, F.M.; Zhou, K.Y.; Li, X.Y. EGCG decreases the expression of HIF-1α and VEGF and cell growth in MCF-7 breast cancer cells. J. BUON 2014, 19, 435–439. [Google Scholar] [PubMed]
- Rashidi, B.; Malekzadeh, M.; Goodarzi, M.; Masoudifar, A.; Mirzaei, H. Green tea and its anti-angiogenesis effects. Biomed. Pharmacother. 2017, 89, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Genis, L.; Galvez, B.G.; Gonzalo, P.; Arroyo, A.G. MT1-MMP: Universal or particular player in angiogenesis? Cancer Metastasis Rev. 2006, 25, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Pilatova, M.; Stupakova, V.; Varinska, L.; Sarissky, M.; Mirossay, L.; Mirossay, A.; Gal, P.; Kraus, V.; Dianiskova, K.; Mojzis, J. Effect of selected flavones on cancer and endothelial cells. Gen. Physiol. Biophys. 2010, 29, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Scoditti, E.; Calabriso, N.; Massaro, M.; Pellegrino, M.; Storelli, C.; Martines, G.; De Caterina, R.; Carluccio, M.A. Mediterranean diet polyphenols reduce inflammatory angiogenesis through MMP-9 and COX-2 inhibition in human vascular endothelial cells: A potentially protective mechanism in atherosclerotic vascular disease and cancer. Arch. Biochem. Biophys. 2012, 527, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.W.; Hsu, S.C.; Chueh, F.S.; Chen, Y.Y.; Yang, J.S.; Lin, J.P.; Lien, J.C.; Tsai, C.H.; Chung, J.G. Quercetin inhibits migration and invasion of SAS human oral cancer cells through inhibition of NF-κB and matrix metalloproteinase-2/-9 signaling pathways. Anticancer Res. 2013, 33, 1941–1950. [Google Scholar] [PubMed]
- Li, C.; Zhao, Y.; Yang, D.; Yu, Y.; Guo, H.; Zhao, Z.; Zhang, B.; Yin, X. Inhibitory effects of kaempferol on the invasion of human breast carcinoma cells by downregulating the expression and activity of matrix metalloproteinase-9. Biochem. Cell Biol. 2015, 93, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.W.; Chen, P.N.; Chen, M.K.; Yang, W.E.; Tang, C.H.; Yang, S.F.; Hsieh, Y.S. Kaempferol reduces matrix metalloproteinase-2 expression by down-regulating ERK1/2 and the activator protein-1 signaling pathways in oral cancer cells. PLoS ONE 2013, 8, e80883. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Lu, N.; Ling, Y.; Chen, Y.; Hui, H.; Lu, Z.; Song, X.; Li, Z.; You, Q.; Guo, Q. Inhibitory effects of wogonin on the invasion of human breast carcinoma cells by downregulating the expression and activity of matrix metalloproteinase-9. Toxicology 2011, 282, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.L.; Hsieh, M.J.; Yang, J.S.; Lin, C.W.; Lue, K.H.; Lu, K.H.; Yang, S.F. Nobiletin inhibits human osteosarcoma cells metastasis by blocking ERK and JNK-mediated MMPs expression. Oncotarget 2016, 7, 35208–35223. [Google Scholar] [CrossRef] [PubMed]
- Farabegoli, F.; Govoni, M.; Spisni, E.; Papi, A. EGFR inhibition by (−)-epigallocatechin-3-gallate and IIF treatments reduces breast cancer cell invasion. Biosci. Rep. 2017, 37. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.K.; Lee, K.W.; Byun, S.; Lee, E.J.; Kim, J.E.; Bode, A.M.; Dong, Z.; Lee, H.J. Myricetin inhibits UVB-induced angiogenesis by regulating PI-3 kinase in vivo. Carcinogenesis 2010, 31, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Lamy, S.; Akla, N.; Ouanouki, A.; Lord-Dufour, S.; Beliveau, R. Diet-derived polyphenols inhibit angiogenesis by modulating the interleukin-6/STAT3 pathway. Exp. Cell Res. 2012, 318, 1586–1596. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.H.; Cheng, C.H.; Lin, C.Y.; Huang, Y.T.; Lee, L.T.; Kandaswami, C.C.; Lin, Y.C.; Lee, K.P.; Hung, C.C.; Hwang, J.J.; et al. Dietary Flavonoids Luteolin and Quercetin Suppressed Cancer Stem Cell Properties and Metastatic Potential of Isolated Prostate Cancer Cells. Anticancer Res. 2016, 36, 6367–6380. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, B.; Volpert, O.V.; Crawford, S.E.; Febbraio, M.; Silverstein, R.L.; Bouck, N. Signals leading to apoptosis-dependent inhibition of neovascularization by thrombospondin-1. Nat. Med. 2000, 6, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Jiang, X.; Song, L.; Wang, H.; Mei, Z.; Xu, Z.; Xing, N. Quercetin inhibits angiogenesis through thrombospondin-1 upregulation to antagonize human prostate cancer PC-3 cell growth in vitro and in vivo. Oncol. Rep. 2016, 35, 1602–1610. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Colome, A.M.; Lee-Rivera, I.; Benavides-Hidalgo, R.; Lopez, E. Paxillin: A crossroad in pathological cell migration. J. Hematol. Oncol. 2017, 10, 50. [Google Scholar] [CrossRef] [PubMed]
- Sp, N.; Kang, D.Y.; Joung, Y.H.; Park, J.H.; Kim, W.S.; Lee, H.K.; Song, K.D.; Park, Y.M.; Yang, Y.M. Nobiletin Inhibits Angiogenesis by Regulating Src/FAK/STAT3-Mediated Signaling through PXN in ER(+) Breast Cancer Cells. Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.D.; Chen, L.; Zhang, M.; Qi, H.J.; Chen, L.; Chen, H.F.; Zhong, M.K.; Shi, X.J.; Li, Q.Y. Downregulation of ERRα inhibits angiogenesis in human umbilical vein endothelial cells through regulating VEGF production and PI3K/Akt/STAT3 signaling pathway. Eur. J. Pharmacol. 2015, 769, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, Y.; Zhang, L.; Chen, L.; Du, Y.; Ye, T.; Shi, X. Naringenin exerts anti-angiogenic effects in human endothelial cells: Involvement of ERRα/VEGF/KDR signaling pathway. Fitoterapia 2016, 111, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Xu, F.; Lu, T.; Duan, Z.; Zhang, Z. Interleukin-6 signaling pathway in targeted therapy for cancer. Cancer Treat. Rev. 2012, 38, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Huang, J.; Zhong, H.; Shen, N.; Faggioni, R.; Fung, M.; Yao, Y. Targeting interleukin-6 in inflammatory autoimmune diseases and cancers. Pharmacol. Ther. 2014, 141, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liao, Y.; Fan, J.; Ye, T.; Sun, X.; Dong, S. Apigenin inhibits the expression of IL-6, IL-8, and ICAM-1 in DEHP-stimulated human umbilical vein endothelial cells and in vivo. Inflammation 2012, 35, 1466–1476. [Google Scholar] [CrossRef] [PubMed]
- Dhar, D.N. The Chemistry of Chalcones and Related Compounds; Wiley: New York, NY, USA, 1981; p. 285. [Google Scholar]
- Yadav, V.R.; Prasad, S.; Sung, B.; Aggarwal, B.B. The role of chalcones in suppression of NF-κB-mediated inflammation and cancer. Int. Immunopharmacol. 2011, 11, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Batovska, D.I.; Todorova, I.T. Trends in utilization of the pharmacological potential of chalcones. Curr. Clin. Pharmacol. 2010, 5, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Dimmock, J.R.; Elias, D.W.; Beazely, M.A.; Kandepu, N.M. Bioactivities of chalcones. Curr. Med. Chem. 1999, 6, 1125–1149. [Google Scholar] [PubMed]
- Go, M.L.; Wu, X.; Liu, X.L. Chalcones: An update on cytotoxic and chemoprotective properties. Curr. Med. Chem. 2005, 12, 481–499. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, R.M.P.; Muniz-Ramirez, A.; Sauceda, J.V. Review: The potential of chalcones as a source of drugs. Afr. J. Pharmac. Pharm. 2015, 9, 237–257. [Google Scholar]
- Mojzis, J.; Varinska, L.; Mojzisova, G.; Kostova, I.; Mirossay, L. Antiangiogenic effects of flavonoids and chalcones. Pharmacol. Res. 2008, 57, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Katsori, A.M.; Hadjipavlou-Litina, D. Chalcones in cancer: Understanding their role in terms of QSAR. Curr. Med. Chem. 2009, 16, 1062–1081. [Google Scholar] [CrossRef] [PubMed]
- Winter, E.; Locatelli, C.; Di Pietro, A.; Creczynski-Pasa, T.B. Recent trends of chalcones potentialities as antiproliferative and antiresistance agents. Anticancer Agents Med. Chem. 2015, 15, 592–604. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, D.K.; Bharti, S.K.; Asati, V. Anti-cancer chalcones: Structural and molecular target perspectives. Eur. J. Med. Chem. 2015, 98, 69–114. [Google Scholar] [CrossRef] [PubMed]
- Oikawa, T.; Hirotani, K.; Nakamura, O.; Shudo, K.; Hiragun, A.; Iwaguchi, T. A highly potent antiangiogenic activity of retinoids. Cancer Lett. 1989, 48, 157–162. [Google Scholar] [CrossRef]
- Kong, Y.; Wang, K.; Edler, M.C.; Hamel, E.; Mooberry, S.L.; Paige, M.A.; Brown, M.L. A boronic acid chalcone analog of combretastatin A-4 as a potent anti-proliferation agent. Bioorg. Med. Chem. 2010, 18, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.; Abramjuk, C.; Vasquez, L.; Gamboa, N.; Dominguez, J.; Nitzsche, B.; Hopfner, M.; Georgieva, R.; Baumler, H.; Stephan, C.; et al. New 4-maleamic acid and 4-maleamide peptidyl chalcones as potential multitarget drugs for human prostate cancer. Pharm. Res. 2011, 28, 907–919. [Google Scholar] [CrossRef] [PubMed]
- Kolundzija, B.; Markovic, V.; Stanojkovic, T.; Joksovic, L.; Matic, I.; Todorovic, N.; Nikolic, M.; Joksovic, M.D. Novel anthraquinone-based chalcone analogues containing an imine fragment: Synthesis, cytotoxicity and anti-angiogenic activity. Bioorg. Med. Chem. Lett. 2014, 24, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.F.; Mohamed, M.S.; Fathi, M.M.; Shouman, S.A.; Abdelhamid, I.A. Chalcones incorporated pyrazole ring inhibit proliferation, cell cycle progression, angiogenesis and induce apoptosis of MCF7 cell line. Anticancer Agents Med. Chem. 2014, 14, 1282–1292. [Google Scholar] [CrossRef] [PubMed]
- Abu, N.; Akhtar, M.N.; Yeap, S.K.; Lim, K.L.; Ho, W.Y.; Zulfadli, A.J.; Omar, A.R.; Sulaiman, M.R.; Abdullah, M.P.; Alitheen, N.B. Flavokawain A induces apoptosis in MCF-7 and MDA-MB231 and inhibits the metastatic process in vitro. PLoS ONE 2014, 9, e105244. [Google Scholar] [CrossRef] [PubMed]
- Abu, N.; Akhtar, M.N.; Yeap, S.K.; Lim, K.L.; Ho, W.Y.; Abdullah, M.P.; Ho, C.L.; Omar, A.R.; Ismail, J.; Alitheen, N.B. Flavokawain B induced cytotoxicity in two breast cancer cell lines, MCF-7 and MDA-MB231 and inhibited the metastatic potential of MDA-MB231 via the regulation of several tyrosine kinases in vitro. BMC Complement. Altern. Med. 2016, 16, 86. [Google Scholar] [CrossRef] [PubMed]
- Rossette, M.C.; Moraes, D.C.; Sacramento, E.K.; Romano-Silva, M.A.; Carvalho, J.L.; Gomes, D.A.; Caldas, H.; Friedman, E.; Bastos-Rodrigues, L.; De Marco, L. The In Vitro and In Vivo Antiangiogenic Effects of Flavokawain B. Phytother. Res. 2017, 31, 1607–1613. [Google Scholar] [CrossRef] [PubMed]
- Albini, A.; Dell’Eva, R.; Vene, R.; Ferrari, N.; Buhler, D.R.; Noonan, D.M.; Fassina, G. Mechanisms of the antiangiogenic activity by the hop flavonoid xanthohumol: NF-κB and Akt as targets. FASEB J. 2006, 20, 527–529. [Google Scholar] [CrossRef] [PubMed]
- Dell’Eva, R.; Ambrosini, C.; Vannini, N.; Piaggio, G.; Albini, A.; Ferrari, N. AKT/NF-κB inhibitor xanthohumol targets cell growth and angiogenesis in hematologic malignancies. Cancer 2007, 110, 2007–2011. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, R.; Calhau, C.; Silva, A.O.; Pinheiro-Silva, S.; Guerreiro, S.; Gartner, F.; Azevedo, I.; Soares, R. Xanthohumol inhibits inflammatory factor production and angiogenesis in breast cancer xenografts. J. Cell. Biochem. 2008, 104, 1699–1707. [Google Scholar] [CrossRef] [PubMed]
- Negrao, R.; Costa, R.; Duarte, D.; Gomes, T.T.; Coelho, P.; Guimaraes, J.T.; Guardao, L.; Azevedo, I.; Soares, R. Xanthohumol-supplemented beer modulates angiogenesis and inflammation in a skin wound healing model. Involvement of local adipocytes. J. Cell. Biochem. 2012, 113, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Negrao, R.; Costa, R.; Duarte, D.; Taveira Gomes, T.; Mendanha, M.; Moura, L.; Vasques, L.; Azevedo, I.; Soares, R. Angiogenesis and inflammation signaling are targets of beer polyphenols on vascular cells. J. Cell. Biochem. 2010, 111, 1270–1279. [Google Scholar] [CrossRef] [PubMed]
- Gallo, C.; Dallaglio, K.; Bassani, B.; Rossi, T.; Rossello, A.; Noonan, D.M.; D’Uva, G.; Bruno, A.; Albini, A. Hop derived flavonoid xanthohumol inhibits endothelial cell functions via AMPK activation. Oncotarget 2016, 7, 59917–59931. [Google Scholar] [CrossRef] [PubMed]
- Nuti, E.; Bassani, B.; Camodeca, C.; Rosalia, L.; Cantelmo, A.; Gallo, C.; Baci, D.; Bruno, A.; Orlandini, E.; Nencetti, S.; et al. Synthesis and antiangiogenic activity study of new hop chalcone Xanthohumol analogues. Eur. J. Med. Chem. 2017, 138, 890–899. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.A.; Lee, M.S.; Ryu, H.W.; Kwak, T.K.; Kim, H.; Kang, M.; Jung, O.; Kim, H.J.; Park, K.H.; Lee, J.W. Differential inhibition of transmembrane 4 L six family member 5 (TM4SF5)-mediated tumorigenesis by TSAHC and sorafenib. Cancer Biol. Ther. 2011, 11, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Pilatova, M.; Varinska, L.; Perjesi, P.; Sarissky, M.; Mirossay, L.; Solar, P.; Ostro, A.; Mojzis, J. In vitro antiproliferative and antiangiogenic effects of synthetic chalcone analogues. Toxicol. In Vitro 2010, 24, 1347–1355. [Google Scholar] [CrossRef] [PubMed]
- Varinska, L.; van Wijhe, M.; Belleri, M.; Mitola, S.; Perjesi, P.; Presta, M.; Koolwijk, P.; Ivanova, L.; Mojzis, J. Anti-angiogenic activity of the flavonoid precursor 4-hydroxychalcone. Eur. J. Pharmacol. 2012, 691, 125–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivanova, L.; Varinska, L.; Pilatova, M.; Gal, P.; Solar, P.; Perjesi, P.; Smetana, K., Jr.; Ostro, A.; Mojzis, J. Cyclic chalcone analogue KRP6 as a potent modulator of cell proliferation: An in vitro study in HUVECs. Mol. Biol. Rep. 2013, 40, 4571–4580. [Google Scholar] [CrossRef] [PubMed]
- Moon, D.O.; Choi, Y.H.; Moon, S.K.; Kim, W.J.; Kim, G.Y. Butein suppresses the expression of nuclear factor-κB-mediated matrix metalloproteinase-9 and vascular endothelial growth factor in prostate cancer cells. Toxicol. In Vitro 2010, 24, 1927–1934. [Google Scholar] [CrossRef] [PubMed]
- Padmavathi, G.; Rathnakaram, S.R.; Monisha, J.; Bordoloi, D.; Roy, N.K.; Kunnumakkara, A.B. Potential of butein, a tetrahydroxychalcone to obliterate cancer. Phytomedicine 2015, 22, 1163–1171. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.H.; Chang, C.H.; Chen, S.S.; Wang, H.H.; Yen, J.Y.; Hsiao, C.J.; Wu, N.L.; Chen, Y.L.; Huang, T.F.; Wang, P.C.; et al. Butein Inhibits Angiogenesis of Human Endothelial Progenitor Cells via the Translation Dependent Signaling Pathway. Evid. Based Complement. Alternat. Med. 2013, 2013, 943187. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Baba, K. Antitumor and antimetastatic activities of Angelica keiskei roots, part 1: Isolation of an active substance, xanthoangelol. Int. J. Cancer 2003, 106, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.W.; Choi, J.S.; Choi, Y.J.; Bae, J.Y.; Li, J.; Kim, D.S.; Kim, J.L.; Shin, S.Y.; Lee, Y.J.; Kwun, I.S.; et al. Licorice isoliquiritigenin dampens angiogenic activity via inhibition of MAPK-responsive signaling pathways leading to induction of matrix metalloproteinases. J. Nutr. Biochem. 2010, 21, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.J.; Chen, G.; Zhang, W.; Hu, X.; Huang, C.F.; Wang, Y.F.; Jia, J.; Zhao, Y.F. Mammalian target of rapamycin pathway promotes tumor-induced angiogenesis in adenoid cystic carcinoma: Its suppression by isoliquiritigenin through dual activation of c-Jun NH2-terminal kinase and inhibition of extracellular signal-regulated kinase. J. Pharmacol. Exp. Ther. 2010, 334, 500–512. [Google Scholar] [CrossRef] [PubMed]
- Jhanji, V.; Liu, H.; Law, K.; Lee, V.Y.; Huang, S.F.; Pang, C.P.; Yam, G.H. Isoliquiritigenin from licorice root suppressed neovascularisation in experimental ocular angiogenesis models. Br. J. Ophthalmol. 2011, 95, 1309–1315. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, N.; Han, S.; Wang, D.; Mo, S.; Yu, L.; Huang, H.; Tsui, K.; Shen, J.; Chen, J. Dietary compound isoliquiritigenin inhibits breast cancer neoangiogenesis via VEGF/VEGFR-2 signaling pathway. PLoS ONE 2013, 8, e68566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, L.; Yuan, Y.; Zhao, L.; He, Q.; Li, Y.; Chen, X.; Liu, X.; Liu, K. Tracking antiangiogenic components from Glycyrrhiza uralensis Fisch. based on zebrafish assays using high-speed countercurrent chromatography. J. Sep. Sci. 2012, 35, 1167–1172. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Peng, F.; Huang, X.; Xie, X.; Chen, B.; Shen, J.; Gao, F.; You, J.; Xie, X.; Chen, J. Neoisoliquiritigenin inhibits tumor progression by targeting GRP78-β-catenin signaling in breast cancer. Curr. Cancer Drug Targets 2017. [Google Scholar] [CrossRef]
- Lai, S.L.; Cheah, S.C.; Wong, P.F.; Noor, S.M.; Mustafa, M.R. In vitro and in vivo anti-angiogenic activities of Panduratin A. PLoS ONE 2012, 7, e38103. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Salomon, C.; Tapia, J.; Illanes, S.E.; Mitchell, M.D.; Rice, G.E. Ovarian cancer cell invasiveness is associated with discordant exosomal sequestration of Let-7 miRNA and miR-200. J. Transl. Med. 2014, 12, 4. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.L.; Wong, P.F.; Lim, T.K.; Lin, Q.; Mustafa, M.R. iTRAQ-based proteomic identification of proteins involved in anti-angiogenic effects of Panduratin A on HUVECs. Phytomedicine 2015, 22, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Xi, S.; Zhang, Q.; Xie, H.; Liu, L.; Liu, C.; Gao, X.; Zhang, J.; Wu, L.; Qian, L.; Deng, X. Effects of hydroxy safflor yellow A on blood vessel and mRNA expression with VEGF and bFGF of transplantation tumor with gastric adenocarcinoma cell line BGC-823 in nude mice. Zhongguo Zhong Yao Za Zhi 2009, 34, 605–610. [Google Scholar] [PubMed]
- Xi, S.Y.; Zhang, Q.; Liu, C.Y.; Xie, H.; Yue, L.F.; Gao, X.M. Effects of hydroxy safflower yellow-A on tumor capillary angiogenesis in transplanted human gastric adenocarcinoma BGC-823 tumors in nude mice. J. Tradit. Chin. Med. 2012, 32, 243–248. [Google Scholar] [CrossRef]
- Xi, S.; Zhang, Q.; Liu, C.; Xie, H.; Yue, L.; Zhao, Y.; Zang, B.; Gao, X. Effects of HSYA on expression of bFGF protein and MMP-9 in BGC-823 transplantation tumor of nude mice. Zhongguo Zhong Yao Za Zhi 2010, 35, 2877–2881. [Google Scholar] [PubMed]
- Si, N.; Wang, J.; Xu, Y.; Liu, L.; Wang, X.; Sun, H.; Lin, Z.; Wang, X.; Liu, L.; Zhang, Q.; et al. Inductive effect of hydroxyl safflower yellow-A on apoptosis in abnormal HUVEC via the mitochondrial pathway. J. Tradit. Chin. Med. Sci. 2015, 2, 25–31. [Google Scholar] [CrossRef]
- Yang, F.; Li, J.; Zhu, J.; Wang, D.; Chen, S.; Bai, X. Hydroxysafflor yellow A inhibits angiogenesis of hepatocellular carcinoma via blocking ERK/MAPK and NF-κB signaling pathway in H22 tumor-bearing mice. Eur. J. Pharmacol. 2015, 754, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, J.; Wang, X.; Liu, L.; Hu, J.; Yu, X.; Xu, Y.; Niu, X.; Lin, Z.; Zhang, Y.; et al. Molecular mechanism of inhibition of the abnormal proliferation of human umbilical vein endothelial cells by hydroxysafflor-yellow A. Pharm. Biol. 2016, 54, 1800–1807. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.J.; Yoon, G.; Park, J.S.; Kim, M.H.; Baek, M.K.; Kim, N.H.; Shin, B.A.; Ahn, B.W.; Cheon, S.H.; Jung, Y.D. Induction of apoptosis by the licochalcone E in endothelial cells via modulation of NF-κB and Bcl-2 Family. Biol. Pharm. Bull. 2007, 30, 2290–2293. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.J.; Park, S.Y.; Kwon, G.T.; Lee, K.W.; Kang, Y.H.; Choi, M.S.; Yun, J.W.; Jeon, J.H.; Jun, J.G.; Park, J.H. Licochalcone E present in licorice suppresses lung metastasis in the 4T1 mammary orthotopic cancer model. Cancer Prev. Res. 2013, 6, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.J.; Chae, J.I.; Yoon, G.; Kim, K.H.; Cho, J.H.; Cho, S.S.; Cho, Y.S.; Shim, J.H. Licochalcone A, a natural chalconoid isolated from Glycyrrhiza inflata root, induces apoptosis via Sp1 and Sp1 regulatory proteins in oral squamous cell carcinoma. Int. J. Oncol. 2014, 45, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Shin, E.K.; Kim, D.H.; Lee, H.H.; Park, J.H.; Kim, J.K. Antiangiogenic effect of licochalcone A. Biochem. Pharmacol. 2010, 80, 1152–1159. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Yuan, X.; Yu, L.; Gao, C.; Sun, X.; Wang, D.; Zheng, Q. Licochalcone A-induced human gastric cancer BGC-823 cells apoptosis by regulating ROS-mediated MAPKs and PI3K/AKT signaling pathways. Sci. Rep. 2015, 5, 10336. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.S.; Jiang, F.S.; Zhang, K.; Zhu, X.X.; Jin, B.; Lu, J.J.; Ding, Z.S. Flavonoids from the leaves of Carya cathayensis Sarg. inhibit vascular endothelial growth factor-induced angiogenesis. Fitoterapia 2014, 92, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.S.; Tian, S.S.; Lu, J.J.; Ding, X.H.; Qian, C.D.; Ding, B.; Ding, Z.S.; Jin, B. Cardamonin Regulates miR-21 Expression and Suppresses Angiogenesis Induced by Vascular Endothelial Growth Factor. BioMed Res. Int. 2015, 2015, 501581. [Google Scholar] [CrossRef] [PubMed]
- Niu, P.G.; Zhang, Y.X.; Shi, D.H.; Liu, Y.; Chen, Y.Y.; Deng, J. Cardamonin Inhibits Metastasis of Lewis Lung Carcinoma Cells by Decreasing mTOR Activity. PLoS ONE 2015, 10, e0127778. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.G.; Li, T.; Wei, R.R.; Liu, W.M.; Sang, Z.P.; Song, Z.W. Characterization of Chalcones from Medicago sativa L. and Their Hypolipidemic and Antiangiogenic Activities. J. Agric. Food Chem. 2016, 64, 8138–8145. [Google Scholar] [CrossRef] [PubMed]
- Ku, B.M.; Ryu, H.W.; Lee, Y.K.; Ryu, J.; Jeong, J.Y.; Choi, J.; Cho, H.J.; Park, K.H.; Kang, S.S. 4′-Acetoamido-4-hydroxychalcone, a chalcone derivative, inhibits glioma growth and invasion through regulation of the tropomyosin 1 gene. Biochem. Biophys. Res. Commun. 2010, 402, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Kang, Y.; Kim, J.T.; Thapa, D.; Lee, E.S.; Kim, J.A. The anti-angiogenic and anti-tumor activity of synthetic phenylpropenone derivatives is mediated through the inhibition of receptor tyrosine kinases. Eur. J. Pharmacol. 2012, 677, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Lee, D.H.; Jung, J.Y.; Koh, D.; Kim, G.S.; Ahn, Y.S.; Lee, Y.H.; Lim, Y.; Shin, S.Y. A synthetic chalcone derivative, 2-hydroxy-3′,5,5′-trimethoxychalcone (DK-139), suppresses the TNFα-induced invasive capability of MDA-MB-231 human breast cancer cells by inhibiting NF-κB-mediated GROα expression. Bioorg. Med. Chem. Lett. 2016, 26, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, G.; Lu, X.; Wang, S.; Han, S.; Li, Y.; Ping, G.; Jiang, X.; Li, H.; Yang, J.; et al. Novel chalcone derivatives as hypoxia-inducible factor (HIF)-1 inhibitor: Synthesis, anti-invasive and anti-angiogenic properties. Eur. J. Med. Chem. 2015, 89, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.H.; Li, H.H.; Li, M.; Wang, S.; Jiang, X.R.; Li, Y.; Ping, G.F.; Cao, Q.; Liu, X.; Fang, W.H.; et al. SL4, a chalcone-based compound, induces apoptosis in human cancer cells by activation of the ROS/MAPK signalling pathway. Cell Prolif. 2015, 48, 718–728. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.H.; Jiang, X.R.; Chen, G.L.; Guo, W.; Zhang, J.Y.; Cui, L.J.; Li, H.H.; Li, M.; Liu, X.; Yang, J.Y.; et al. Anti-tumor activity of SL4 against breast cancer cells: Induction of G2/M arrest through modulation of the MAPK-dependent p21 signaling pathway. Sci. Rep. 2016, 6, 36486. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Ye, H.; Wan, L.; Han, X.; Wang, G.; Hu, J.; Tang, M.; Duan, X.; Fan, Y.; He, S.; et al. Millepachine, a novel chalcone, induces G2/M arrest by inhibiting CDK1 activity and causing apoptosis via ROS-mitochondrial apoptotic pathway in human hepatocarcinoma cells in vitro and in vivo. Carcinogenesis 2013, 34, 1636–1643. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, Z.; Wen, J.; Ma, F.; Wang, F.; Yu, K.; Tang, M.; Wu, W.; Dong, Y.; Cheng, X.; et al. SKLB-M8 induces apoptosis through the AKT/mTOR signaling pathway in melanoma models and inhibits angiogenesis with decrease of ERK1/2 phosphorylation. J. Pharmacol. Sci. 2014, 126, 198–207. [Google Scholar] [CrossRef] [PubMed]

| Target | Drug | Mechanism of Action | Clinical Stage |
|---|---|---|---|
| Growth factors | Bevacizumab | recombinant mAb against VEGF-A | Approved |
| Aflibercept | chimeric soluble receptor; binds VEGF-A, -B and PlGF | Approved | |
| Ramucirumab | human mAb; blocks VEGFR2 signaling | Approved | |
| Thalidomide, Lenalidomide | inhibitor of endothelial cells proliferation | Approved | |
| Icrucumab | human mAb; blocks VEGFR1signaling | In clinical trials | |
| Tyrosine kinases | Sunitinib | inhibits signaling of VEGFRs, PDGFRs, FLT-3, CSF1R | Approved |
| Sorafenib | inhibits signaling of VEGFRs Raf, PDGFRs, KIT | Approved | |
| Pazopanib | inhibits signaling of VEGFRs, PDGFRs, KIT | Approved | |
| Axitinib | Inhibits signaling of VEGFRs, PDGFRs, KIT | Approved | |
| Vandetanib | inhibits signaling of VEGFRs, PDGFRs, EGFR | Approved | |
| Regorafenib | inhibits signaling of VEGFRs Raf, PDGFRs, KIT | Approved | |
| Cabozantinib | inhibits signaling of VEGFRs Raf, PDGFRs, cMET, RET, KIT | Approved | |
| Erlotinib | inhibits signaling of EGFR | Approved | |
| Lenvatinib | inhibits signaling of VEGFR, PDGFR and FGFR | Approved | |
| Tivozanib | inhibits signaling of VEGFRs, PDGFRs, KIT | In clinical trials | |
| Motesanib | inhibits signaling of VEGFRs, PDGFRs, KIT | In clinical trials | |
| Cediranib | inhibits signaling of VEGFRs, PDGFRs, KIT | Discontinued | |
| Intergrins | Etaracizumab | blocks αvβ3 integrin | Discontinued |
| Volociximab | chimeric mAb; blocks α5β1 integrin | Discontinued | |
| mTOR | Everolimus, Temsirolimus | mTOR inhibitor | Approved |
| Human anti-angiogenic factors | Endostatin | recombinant human protein; endogenous inhibitor of angiogenesis | In clinical trials |
| Thrombospondin-1 mimetic | mimetic peptide; endogenous inhibitor of angiogenesis | Discontinued | |
| Angiopoietin | Trebananib | angiopoietin-1/-2-neutralizing peptibody | In clinical trials |
| MMPs | Andecaliximab | anti-MMP-9 mAb | In clinical trials |
| Flavonoid | Possible Mechanism | Reference |
|---|---|---|
| Quercetin | ↓ VEGFR2 phosphorylation; ↓ VEGFR2 mRNA expression; ↓ ERK signaling pathway; modulation of AKT/mTOR/P70S6K signaling pathways; ↓ MAPK and PI3K/AKT signaling pathways ↓ COX-2 expression; ↓ secretion of MMP-2 and MMP-9 | [53,56,100,101,102] |
| Apigenin | inhibition of Smad2/3 and Src/FAK/AKT pathways; inhibition of IL-6/STAT3 pathway; ↓ MMP-2 and MMP-9 activity; ↓ mRNA and protein expression of IL-6, IL-8 and intercellular adhesion molecule-1 | [109,119] |
| Kaempferol | ↓ VEGF secretion, modulation of ERK-NF-κB-cMyc-p21-VEGF pathway; ↓ VEGF mRNA and protein expression; ↓ AKT phosphorylation; ↓ MMP-2 and MMP-9 activity; ↓ PKC/MAPK/AP-1 | [58,59,86,103] |
| EGCG | down-regulation of HIF-1α and VEGF expression; suppression of VEGF/VEGFR2; ↓ VEGFR2 phosphorylation; ↓ ERK/AKT phosphorylation; inhibition of PI3K/AKT/mTOR signaling pathway; STAT3 activity modulation; ↓ MMP-2 and MMP-9 activity; | [67,68,69,70,72,96,98] |
| Genistein | suppression of MMP-9 transcription via inhibition AP-1 and NF-κB activity; inhibition of basal VEGF and hypoxia-stimulated VEGF expression; down-regulation of EGF and IGF; inhibition of PTK activity and MAPK activation; decrease in MMPs production and activity; inhibition of expression/excretion of proangiogenic factors—MMPs, PDGF, TF, uPA, VEGF; up-regulation of angiogenesis inhibitors TSP-1,—PAI-1, endostatin, angiostatin; | [87,88,89] |
| Nobiletin | ↓ FGF-induced phosporylation of ERK1/2 and JNK; ↓ AKT, HIF-1α, NF-κB and VEGF activity; ↓ MMP-2 and MMP-9 activity; | [85,95,105] |
| Wogonin | down-regulation of the expression of HIF-1α; ↓ VEGF secretion; ↑ ubiquitination of HIF-1α; ↓ VEGF, PDGF and bFGF secretion via c-Myc/HIF-1a signaling axis; ↓ MMP-2 and MMP-9 activity; | [93,94] |
| Luteolin | ↓ VEGFR2 phosphorylation; ↓ AKT/mTOR/ERK signaling pathways; ↓ IL-1b, IL-6, IL-8, and TNF-α; ↓ MMP-2 and MMP-9 activity; inhibition of IL-6/STAT3 pathway; | [60,109] |
| Theaflavin-3, 3′-digallate | ↓ AKT/mTOR/p70S6K/4E-BP1 and AKT/c-Myc pathways | [74] |
| Myricetin | ↓ MMP-9 and MMP-13 activity; down-regulation of HIF-1α; ↓ AKT/PI3 signaling | [108] |
| Rhamnazin | inhibition VEGFR2 kinase; ↓ VEGFR2 phosphorylation; ↓ MAPK, AKT, and STAT3 phosphorylation | [64] |
| Chrysin | down-regulation of soluble IL-6 receptor; ↓ JAK1, STAT3, and VEGF phosphorylation; | [80] |
| Chalcone | Possible Mechanism | Reference |
|---|---|---|
| Flavokawain A | ↓ HUVEC tube formation; ↓ outgrowth of vessels from rat aortic rings | [135] |
| Flavokawain B | ↓ formation of vessels in HUVECs; ↓ outgrowth of vessels from rat aortic rings; ↓ EC migration and tube formation; ↓ subintestinal vein formation with their marked or complete obliteration in zebrafish model | [136,137] |
| Xanthohumol and isoxanthohumol | ↓ VEGF secretion; ↓ EC growth, invasion and migration; ↓ tube formation; ↓ MMPs production; ↓ NF-κB and AKT pathways; ↓ vessel number in mouse matrigel plug and rat skin wound-healing assays; ↑ AMPK phosphorylation and activity resulting in ↓ nitric oxide levels in EC | [138,139,140,141,142,143] |
| Butein | ↓ VEGF and MMP-9 activities via the suppression of NF-κB activity; ↓ expression of VEGF and MMP-9 induced by TNF-α and PMA; ↓ of serum- and VEGF-induced cell proliferation, migration, and tube formation of human endothelial progenitor cells; abrogation of VEGF-induced vessels sprouting from aortic rings; ↓ microvessel formation in the matrigel implant assay in vivo; ↓ phosphorylation of AKT, mTOR, and their major downstream effectors in endothelial progenitor cells; ↓ effect on STAT3 and CXCR4 | [149,150,151] |
| Xanthoangelol | ↓ of matrigel-induced formation of capillary-like tubes; ↓ of tumor-induced neovascularization in vivo; ↓ VEGF binding to HUVECs | [121,152] |
| 4-hydroxyderricin | ↓ of matrigel-induced formation of capillary-like tubes by HUVECs | [121] |
| Isoliquiritigenin and neoisoliquiritigenin | ↓PMA-induced migration, tube formation and expression of MMPs in EC mediated through the JNK and p38 MAPK pathways; ↓ of tumor-induced angiogenesis caused by down-regulation of mTOR pathway-dependent VEGF production with concurrent activation of JNK and inhibition of ERK; ↓ microvessel outgrowth induced by conditioned medium; ↓ VEGF-induced neovascularization in ocular angiogenesis models; ↓ of new vessel formation by VEGF ↓ via promoting HIF-1a proteasome degradation; ↓ of blood circulation and vascular outgrowth in zebrafish model | [153,154,155,156,157,158] |
| Panduratin A | ↓ survival and proliferation in VEGF-induced HUVECs; selective HUVECs cytotoxicity; ↓ of endothelial cell migration, invasion, and morphogenesis or tube formation; suppression of MMP-2 secretion and activation, and F-actin stress fiber formation; ↓ of neo-vessels formation in murine matrigel plugs, and angiogenesis in zebrafish embryos; ↓ of the expressions of ARPC2, CTNND1, GRB-2, ICAM-2 and STAB-1 accompanied with the suppression of mTOR signaling induced by VEGF | [159,160,161] |
| Hydroxy safflower yellow A | ↓ of the microvessel count and density in transplanted human gastric adenocarcinoma BGC-823 in mice; ↓ of mRNA expression of VEGF, bFGF and MMP-9; promotion of apoptosis of abnormal HUVECs with concomitant ↑.mRNA expression of caspase-3 and Bax and ↓ expression of mutant p53, Bcl-2, Fas, and Fas-L; block of ERK1/2 phosphorylation and restrain the activation of NF-κB; ↓ of the expression of VEGF and kinase insert domain receptor with simultaneous ↓ of the expression of oncogene and transcription factors through the Ras-Raf-MEK-ERK1/2 pathway of the MAPK family | [162,163,164,165,166,167] |
| Licochalcone A | ↓ of the migration and tube formation of endothelial cells; ↓ of neovascular outgrowth in aortic ring assays; ↓ of multiple angiogenic growth factors release; block of VEGFR2 phosphorylation; interference with PI3K/AKT and MAPK signaling cascades | [170,171,172] |
| Licochalcone E | ↓ of the constitutive NF- κB activation; change in the Bax/Bcl-2 ratio; ↓ of the expression of vascular tumor marker CD31, VEGF-A and C, VEGFR2, and lymphatic vessel endothelial receptor-1; ↓ of in vitro tube formation | [168,169] |
| Cardamonin | mTOR suppression; ↓ of VEGF-induced ERK and AKT phosphorylation; ↓ of VEGF-induced angiogenesis via miRNAs; ↓ of mRNA expression of VEGF; ↓ of angiogenesis in a CAM model | [173,174] |
| Chalcone | Possible Mechanism | Reference |
|---|---|---|
| Xanthohumol derivatives | ↓ HUVECs proliferation, adhesion, migration, invasion and their ability to form capillary-like structures | [144] |
| (E)-2-(4′-methoxybenzylidene)-1-benzosuberone | ↓ of VEGF-induced migration of HUVECs; decreased secretion of MMP-9 and VEGF | [146] |
| 4-hydroxychalcone | ↓ endothelial cell proliferation, migration and tube formation in activated endothelial cells; modulation of both VEGF- and bFGF- induced phosphorylation of ERK-1/-2 and AKT kinase; ↓ effect on bFGF-driven neovascularization in vivo in CAM assay | [147] |
| (E)-3-(20-methoxybenzylidene)-4-chromanone | modulation of AKT phosphorylation and MAPKs such as ERK-1/-2 and p38 kinase selectively in activated endothelial cells | [148] |
| 4′-acetoamido-4-hydroxychalcone | ↓ of VEGF-induced migration, invasion, and tube formation in HUVECs; | [177] |
| SL4 chalcone derivative | HIF-1 inhibitory effects together with ↓ of VEGF-induced migration and invasion of HUVECs | [180,181,182] |
| 1,3-diphenyl-propenone | multi-target receptor-tyrosine kinases ↓ including VEGFR2; ↓ of down-stream signaling, including ERK phosphorylation and NF-κB activation; ↓ tumor-induced angiogenesis in CAM assay | [178] |
| 2-hydroxy-3′,5,5′-trimethoxychalcone | ↓ of NF-κB-mediated GROα expression | [11,179] |
| (E)-3-(3-amino-4-methoxyphenyl)-1-(5-methoxy-2,2-dimethyl-2H-chromen-8-yl)prop-2-en-1-one hydrochloride | rapid endothelial cell shape changes; ↓ of HUVEC migration, invasion, and tube formation through disrupting microtubule stability and via suppression of the expression of ERK | [184] |
| Boronic acid-chalcone analogues | ↓ of HUVEC tube formation and vessel growth in aortic ring assay | [131] |
| 4-maleamic acid and 4-maleamide peptidyl chalcone derivatives | reduction of neovascularization in chick embryos and MMP-9 activity | [132] |
| Imine derivatives of hybrid chalcone analogues | ↓ of tubulogenesis and exhibition of a strong anti-angiogenic effect | [133] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirossay, L.; Varinská, L.; Mojžiš, J. Antiangiogenic Effect of Flavonoids and Chalcones: An Update. Int. J. Mol. Sci. 2018, 19, 27. https://doi.org/10.3390/ijms19010027
Mirossay L, Varinská L, Mojžiš J. Antiangiogenic Effect of Flavonoids and Chalcones: An Update. International Journal of Molecular Sciences. 2018; 19(1):27. https://doi.org/10.3390/ijms19010027
Chicago/Turabian StyleMirossay, Ladislav, Lenka Varinská, and Ján Mojžiš. 2018. "Antiangiogenic Effect of Flavonoids and Chalcones: An Update" International Journal of Molecular Sciences 19, no. 1: 27. https://doi.org/10.3390/ijms19010027
APA StyleMirossay, L., Varinská, L., & Mojžiš, J. (2018). Antiangiogenic Effect of Flavonoids and Chalcones: An Update. International Journal of Molecular Sciences, 19(1), 27. https://doi.org/10.3390/ijms19010027