Motor, Somatosensory, Viscerosensory and Metabolic Impairments in a Heterozygous Female Rat Model of Rett Syndrome
Abstract
:1. Introduction
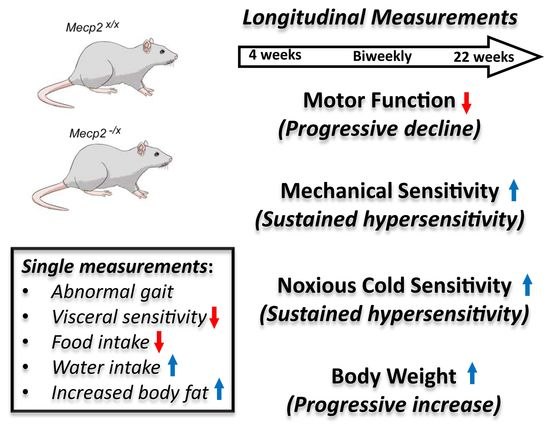
2. Results
2.1. Motor Function
2.2. Sensory Function
2.3. Metabolic Indicators
2.4. Inter-Relationships among MECP2 Mosaicism and Behavioral and Metabolic Dysfunctions
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Timelines for Measurement
4.3. Rotarod
4.4. Gait Analysis
4.5. Somatosensory Behavior: Mechanical, Thermal and Cold Sensitivity
4.6. Visceral Sensitivity
4.7. Food, Water and Activity Monitoring (Metabolic Cage)
4.8. Body Mass Composition
4.9. PBMNC Isolation, Staining and FACS
4.10. Stastical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| RTT | Rett Syndrome |
| ASD | Autism spectrum disorder |
| MECP2 | Methyl CpG-binding Protein 2 |
| WT | Wild type |
| PBMNC | Peripheral blood mononuclear cell |
| EMG | Electromyography |
| VMR | Visceromotor reflex |
References
- Amir, R.E.; Van den Veyver, I.B.; Wan, M.; Tran, C.Q.; Francke, U.; Zoghbi, H.Y. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat. Genet. 1999, 23, 185–188. [Google Scholar] [PubMed]
- Chahrour, M.; Jung, S.Y.; Shaw, C.; Zhou, X.; Wong, S.T.; Qin, J.; Zoghbi, H.Y. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science 2008, 320, 1224–1229. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.L.; Qiu, Z. MeCP2: Multifaceted roles in gene regulation and neural development. Neurosci. Bull. 2014, 30, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Chahrour, M.; Zoghbi, H.Y. The story of Rett syndrome: From clinic to neurobiology. Neuron 2007, 56, 422–437. [Google Scholar] [CrossRef] [PubMed]
- Cianfaglione, R.; Clarke, A.; Kerr, M.; Hastings, R.P.; Oliver, C.; Felce, D. A national survey of Rett syndrome: Age, clinical characteristics, current abilities, and health. Am. J. Med. Genet. Part A 2015, 167, 1493–1500. [Google Scholar] [CrossRef] [PubMed]
- Zoghbi, H.Y. MeCP2 dysfunction in humans and mice. J. Child Neurol. 2005, 20, 736–740. [Google Scholar] [CrossRef] [PubMed]
- Veeraragavan, S.; Wan, Y.W.; Connolly, D.R.; Hamilton, S.M.; Ward, C.S.; Soriano, S.; Pitcher, M.R.; McGraw, C.M.; Huang, S.G.; Green, J.R.; et al. Loss of MeCP2 in the rat models regression, impaired sociability and transcriptional deficits of Rett syndrome. Hum. Mol. Genet. 2016, 25, 3284–3302. [Google Scholar] [CrossRef] [PubMed]
- Moretti, P.; Bouwknecht, J.A.; Teague, R.; Paylor, R.; Zoghbi, H.Y. Abnormalities of social interactions and home-cage behavior in a mouse model of Rett syndrome. Hum. Mol. Genet. 2005, 14, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Gemelli, T.; Berton, O.; Nelson, E.D.; Perrotti, L.I.; Jaenisch, R.; Monteggia, L.M. Postnatal loss of methyl-CpG binding protein 2 in the forebrain is sufficient to mediate behavioral aspects of Rett syndrome in mice. Biol. Psychiatry 2006, 59, 468–476. [Google Scholar] [CrossRef] [PubMed]
- McGill, B.E.; Bundle, S.F.; Yaylaoglu, M.B.; Carson, J.P.; Thaller, C.; Zoghbi, H.Y. Enhanced anxiety and stress-induced corticosterone release are associated with increased Crh expression in a mouse model of Rett syndrome. Proc. Natl. Acad. Sci. USA 2006, 103, 18267–18272. [Google Scholar] [CrossRef] [PubMed]
- Moretti, P.; Levenson, J.M.; Battaglia, F.; Atkinson, R.; Teague, R.; Antalffy, B.; Armstrong, D.; Arancio, O.; Sweatt, J.D.; Zoghbi, H.Y. Learning and memory and synaptic plasticity are impaired in a mouse model of Rett syndrome. J. Neurosci. Off. J. Soc. Neurosci. 2006, 26, 319–327. [Google Scholar]
- Santos, M.; Silva-Fernandes, A.; Oliveira, P.; Sousa, N.; Maciel, P. Evidence for abnormal early development in a mouse model of Rett syndrome. Genes Brain Behav. 2007, 6, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Shahbazian, M.D.; Young, J.I.; Yuva-Paylor, L.A.; Spencer, C.M.; Antalffy, B.A.; Noebels, J.L.; Armstrong, D.L.; Paylor, R.; Zoghbi, H.Y. Mice with truncated MeCP2 recapitulate many Rett syndrome features and display hyperacetylation of histone H3. Neuron 2002, 35, 243–254. [Google Scholar] [CrossRef]
- Stearns, N.A.; Schaevitz, L.R.; Bowling, H.; Nag, N.; Berger, U.V.; Berger-Sweeney, J. Behavioral and anatomical abnormalities in Mecp2 mutant mice: A model for Rett syndrome. Neuroscience 2007, 146, 907–921. [Google Scholar] [CrossRef] [PubMed]
- Ricceri, L.; De Filippis, B.; Laviola, G. Mouse models of Rett syndrome: From behavioural phenotyping to preclinical evaluation of new therapeutic approaches. Behav. Pharmacol. 2008, 19, 501–517. [Google Scholar] [CrossRef] [PubMed]
- Symons, F.J.; Byiers, B.; Tervo, R.C.; Beisang, A. Parent-reported pain in Rett syndrome. Clin. J. Pain 2013, 29, 744–746. [Google Scholar] [CrossRef] [PubMed]
- Barney, C.C.; Feyma, T.; Beisang, A.; Symons, F.J. Pain experience and expression in Rett syndrome: Subjective and objective measurement approaches. J. Dev. Phys. Disabil. 2015, 27, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Peebles, K.A.; Price, T.J. Self-injurious behaviour in intellectual disability syndromes: Evidence for aberrant pain signalling as a contributing factor. J. Intell. Disabil. Res. 2012, 56, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Motil, K.J.; Schultz, R.J.; Browning, K.; Trautwein, L.; Glaze, D.G. Oropharyngeal dysfunction and gastroesophageal dysmotility are present in girls and women with Rett syndrome. J. Pediatr. Gastroenterol. Nutr. 1999, 29, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Motil, K.J.; Caeg, E.; Barrish, J.O.; Geerts, S.; Lane, J.B.; Percy, A.K.; Annese, F.; McNair, L.; Skinner, S.A.; Lee, H.S.; et al. Gastrointestinal and nutritional problems occur frequently throughout life in girls and women with Rett syndrome. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Krizsan-Agbas, D.; Winter, M.K.; Eggimann, L.S.; Meriwether, J.; Berman, N.E.; Smith, P.G.; McCarson, K.E. Gait analysis at multiple speeds reveals differential functional and structural outcomes in response to graded spinal cord injury. J. Neurotrauma 2014, 31, 846–856. [Google Scholar] [CrossRef] [PubMed]
- Bhattacherjee, A.; Mu, Y.; Winter, M.K.; Knapp, J.R.; Eggimann, L.S.; Gunewardena, S.S.; Kobayashi, K.; Kato, S.; Krizsan-Agbas, D.; Smith, P.G. Neuronal cytoskeletal gene dysregulation and mechanical hypersensitivity in a rat model of Rett syndrome. Proc. Natl. Acad. Sci. USA 2017, 114, E6952–E6961. [Google Scholar] [CrossRef] [PubMed]
- Orefice, L.L.; Zimmerman, A.L.; Chirila, A.M.; Sleboda, S.J.; Head, J.P.; Ginty, D.D. Peripheral Mechanosensory Neuron Dysfunction Underlies Tactile and Behavioral Deficits in Mouse Models of ASDs. Cell 2016, 166, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Christianson, J.A.; Gebhart, G.F. Assessment of colon sensitivity by luminal distension in mice. Nat. Protoc. 2007, 2, 2624–2631. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.; Rho, J.M.; Masino, S.A. Metabolic Dysfunction Underlying Autism Spectrum Disorder and Potential Treatment Approaches. Front. Mol. Neurosci. 2017, 10, 34. [Google Scholar] [CrossRef] [PubMed]
- El-Ansary, A.; Bjorklund, G.; Chirumbolo, S.; Alnakhli, O.M. Predictive value of selected biomarkers related to metabolism and oxidative stress in children with autism spectrum disorder. Metab. Brain Dis. 2017, 32, 1209–1221. [Google Scholar] [CrossRef] [PubMed]
- Mogil, J.S. Animal models of pain: Progress and challenges. Nat. Rev. Neurosci. 2009, 10, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Temudo, T.; Ramos, E.; Dias, K.; Barbot, C.; Vieira, J.P.; Moreira, A.; Calado, E.; Carrilho, I.; Oliveira, G.; Levy, A.; et al. Movement disorders in Rett syndrome: An analysis of 60 patients with detected MECP2 mutation and correlation with mutation type. Mov. Disord. 2008, 23, 1384–1390. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.Z.; Akbarian, S.; Tudor, M.; Jaenisch, R. Deficiency of methyl-CpG binding protein-2 in CNS neurons results in a Rett-like phenotype in mice. Nat. Genet. 2001, 27, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Guy, J.; Hendrich, B.; Holmes, M.; Martin, J.E.; Bird, A. A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nat. Genet. 2001, 27, 322–326. [Google Scholar] [CrossRef] [PubMed]
- De Filippis, B.; Musto, M.; Altabella, L.; Romano, E.; Canese, R.; Laviola, G. Deficient Purposeful Use of Forepaws in Female Mice Modelling Rett Syndrome. Neural Plast. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Naidu, S.; Bibat, G.; Kratz, L.; Kelley, R.I.; Pevsner, J.; Hoffman, E.; Cuffari, C.; Rohde, C.; Blue, M.E.; Johnston, M.V. Clinical variability in Rett syndrome. J. Child Neurol. 2003, 18, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Neul, J.L.; Lane, J.B.; Lee, H.S.; Geerts, S.; Barrish, J.O.; Annese, F.; Baggett, L.M.; Barnes, K.; Skinner, S.A.; Motil, K.J.; et al. Developmental delay in Rett syndrome: Data from the natural history study. J. Neurodev. Disord. 2014, 6, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chin Wong, L.; Hung, P.L.; Jan, T.Y.; Lee, W.T.; Taiwan Rett Syndrome Association. Variations of stereotypies in individuals with Rett syndrome: A nationwide cross-sectional study in Taiwan. Autism Res. Off. J. Int. Soc. Autism Res. 2017, 10, 1204–1214. [Google Scholar] [CrossRef] [PubMed]
- Marco, E.J.; Hinkley, L.B.; Hill, S.S.; Nagarajan, S.S. Sensory processing in autism: A review of neurophysiologic findings. Pediatr. Res. 2011, 69, 48R–54R. [Google Scholar] [CrossRef] [PubMed]
- Riquelme, I.; Hatem, S.M.; Montoya, P. Abnormal Pressure Pain, Touch Sensitivity, Proprioception, and Manual Dexterity in Children with Autism Spectrum Disorders. Neural Plast. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Baikie, G.; Ravikumara, M.; Downs, J.; Naseem, N.; Wong, K.; Percy, A.; Lane, J.; Weiss, B.; Ellaway, C.; Bathgate, K.; et al. Gastrointestinal dysmotility in Rett syndrome. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Farmer, A.D.; Aziz, Q. Gut pain & visceral hypersensitivity. Br. J. Pain 2013, 7, 39–47. [Google Scholar] [PubMed]
- Kiba, T. Relationships between the autonomic nervous system, humoral factors and immune functions in the intestine. Digestion 2006, 74, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Tougas, G. The autonomic nervous system in functional bowel disorders. Can. J. Gastroenterol. 1999, 13 (Suppl. A), 15A–17A. [Google Scholar] [CrossRef] [PubMed]
- Uesaka, T.; Young, H.M.; Pachnis, V.; Enomoto, H. Development of the intrinsic and extrinsic innervation of the gut. Dev. Biol. 2016, 417, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A. Gut feelings: The emerging biology of gut-brain communication. Nat. Rev. Neurosci. 2011, 12, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, J.M.; Ward, C.S.; Neul, J.L. Breathing challenges in Rett syndrome: Lessons learned from humans and animal models. Respir. Physiol. Neurobiol. 2013, 189, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Lioy, D.T.; Wu, W.W.; Bissonnette, J.M. Autonomic dysfunction with mutations in the gene that encodes methyl-CpG-binding protein 2: Insights into Rett syndrome. Auton. Neurosci. Basic Clin. 2011, 161, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Panighini, A.; Duranti, E.; Santini, F.; Maffei, M.; Pizzorusso, T.; Funel, N.; Taddei, S.; Bernardini, N.; Ippolito, C.; Virdis, A.; et al. Vascular dysfunction in a mouse model of Rett syndrome and effects of curcumin treatment. PLoS ONE 2013, 8, e64863. [Google Scholar] [CrossRef] [PubMed]
- Fyffe, S.L.; Neul, J.L.; Samaco, R.C.; Chao, H.T.; Ben-Shachar, S.; Moretti, P.; McGill, B.E.; Goulding, E.H.; Sullivan, E.; Tecott, L.H.; et al. Deletion of Mecp2 in Sim1-expressing neurons reveals a critical role for MeCP2 in feeding behavior, aggression, and the response to stress. Neuron 2008, 59, 947–958. [Google Scholar] [CrossRef] [PubMed]
- Samaco, R.C.; Fryer, J.D.; Ren, J.; Fyffe, S.; Chao, H.T.; Sun, Y.; Greer, J.J.; Zoghbi, H.Y.; Neul, J.L. A partial loss of function allele of methyl-CpG-binding protein 2 predicts a human neurodevelopmental syndrome. Hum. Mol. Genet. 2008, 17, 1718–1727. [Google Scholar] [CrossRef] [PubMed]
- Ward, C.S.; Arvide, E.M.; Huang, T.W.; Yoo, J.; Noebels, J.L.; Neul, J.L. MeCP2 is critical within HoxB1-derived tissues of mice for normal lifespan. J. Neurosci. 2011, 31, 10359–10370. [Google Scholar] [CrossRef] [PubMed]
- Zappella, M.; Meloni, I.; Longo, I.; Hayek, G.; Renieri, A. Preserved speech variants of the Rett syndrome: Molecular and clinical analysis. Am. J. Med. Genet. 2001, 104, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Segatto, M.; Trapani, L.; Di Tunno, I.; Sticozzi, C.; Valacchi, G.; Hayek, J.; Pallottini, V. Cholesterol metabolism is altered in Rett syndrome: A study on plasma and primary cultured fibroblasts derived from patients. PLoS ONE 2014, 9, e104834. [Google Scholar] [CrossRef] [PubMed]
- Buchovecky, C.M.; Turley, S.D.; Brown, H.M.; Kyle, S.M.; McDonald, J.G.; Liu, B.; Pieper, A.A.; Huang, W.; Katz, D.M.; Russell, D.W.; et al. A suppressor screen in Mecp2 mutant mice implicates cholesterol metabolism in Rett syndrome. Nat. Genet. 2013, 45, 1013–1020. [Google Scholar] [CrossRef] [PubMed]
- Iannitti, T.; Graham, A.; Dolan, S. Increased central and peripheral inflammation and inflammatory hyperalgesia in Zucker rat model of leptin receptor deficiency and genetic obesity. Exp. Physiol. 2012, 97, 1236–1245. [Google Scholar] [CrossRef] [PubMed]
- Okifuji, A.; Hare, B.D. The association between chronic pain and obesity. J. Pain Res. 2015, 8, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Gigli, C.C.; Scaramuzza, L.; Gandaglia, A.; Bellini, E.; Gabaglio, M.; Parolaro, D.; Kilstrup-Nielsen, C.; Landsberger, N.; Bedogni, F. MeCP2 related studies benefit from the use of CD1 as genetic background. PLoS ONE 2016, 11, e0153473. [Google Scholar]
- Vogel Ciernia, A.; Pride, M.C.; Durbin-Johnson, B.; Noronha, A.; Chang, A.; Yasui, D.H.; Crawley, J.N.; LaSalle, J.M. Early motor phenotype detection in a female mouse model of Rett syndrome is improved by cross-fostering. Hum. Mol. Genet. 2017, 26, 1839–1854. [Google Scholar] [CrossRef] [PubMed]




| Test | Source of Variation | DF | F | P |
|---|---|---|---|---|
| Body Weight | Genotype | 1 | 12.487 | 0.002 |
| Weeks | 9 | 310.219 | <0.001 | |
| Genotype × Weeks | 9 | 6.613 | <0.001 | |
| Rotarod | Genotype | 1 | 83.617 | <0.001 |
| Weeks | 9 | 16.738 | <0.001 | |
| Genotype × Weeks | 9 | 8.143 | <0.001 | |
| Von Frey | Genotype | 1 | 21.737 | <0.001 |
| Weeks | 9 | 3.649 | <0.001 | |
| Genotype × Weeks | 9 | 0.281 | 0.979 | |
| Cold Plate | Genotype | 1 | 36.002 | <0.001 |
| Weeks | 9 | 2.266 | 0.021 | |
| Genotype × Weeks | 9 | 0.780 | 0.635 | |
| VMR | Genotype | 1 | 6.514 | 0.021 |
| Pressure | 3 | 33.405 | <0.001 | |
| Genotype x Pressure | 3 | 4.600 | 0.007 |
| Light | |||||
| Parameters | WT Mean | WT SEM | Het Mean | Het SEM | t-Test p-Value |
| Average Energy Expenditure | 1.85 | 0.04 | 1.67 | 0.09 | 0.199 |
| Total Energy Expenditure | 25.96 | 0.56 | 23.43 | 1.27 | 0.199 |
| Average VO2 | 6.41 | 0.14 | 5.88 | 0.31 | 0.26 |
| Average VCO2 | 5.09 | 0.16 | 4.28 | 0.28 | 0.073 |
| Average RQ | 0.79 | 0.02 | 0.72 | 0.02 | 0.03 |
| Dark | |||||
| Parameters | WT Mean | WT SEM | Het Mean | Het SEM | t-Test p-Value |
| Average Energy Expenditure | 2.29 | 0.03 | 2.01 | 0.12 | 0.138 |
| Total Energy Expenditure | 22.85 | 0.31 | 20.09 | 1.2 | 0.138 |
| Average VO2 | 7.86 | 0.13 | 7.01 | 0.41 | 0.172 |
| Average VCO2 | 6.42 | 0.11 | 5.3 | 0.38 | 0.064 |
| Average RQ | 0.82 | 0.02 | 0.75 | 0.02 | 0.054 |
| Parameters Compared | R2 | Adjusted R2 | F-Statistic vs. Constant Model | p-Value |
|---|---|---|---|---|
| Weight gain rate and day time food intake | 0.0152 | −0.108 | 0.124 | 0.734 |
| Weight gain rate and night time food intake | 0.0522 | −0.0663 | 0.441 | 0.525 |
| Weight gain rate and day time water intake | 0.0405 | −0.0794 | 0.338 | 0.577 |
| Weight gain rate and night time water intake | 5.73 × 10−6 | −0.125 | 4.58 × 10−5 | 0.995 |
| von Frey and Brake | 0.195 | −0.00572 | 0.972 | 0.38 |
| von Frey and Propel | 0.307 | 0.134 | 1.77 | 0.254 |
| von Frey and Swing | 0.0428 | −0.196 | 0.179 | 0.694 |
| Sensitivity (% withdrawal) to a 4 mg filament and brake | 0.564 | 0.456 | 5.18 | 0.0851 |
| Sensitivity (% withdrawal) to a 4 mg filament and Propel | 0.514 | 0.393 | 4.23 | 0.109 |
| Sensitivity (% withdrawal) to a 4 mg filament and Swing | 0.0482 | −0.19 | 0.202 | 0.676 |
| Rotarod and Brake | 0.204 | 0.00471 | 1.02 | 0.369 |
| Rotarod and Propel | 0.0255 | −0.218 | 0.105 | 0.762 |
| Rotarod and Swing | 0.706 | 0.633 | 9.62 | 0.0362 |
| Test | Frequency of Measure | Age |
|---|---|---|
| Rotarod | Biweekly | Weeks 4–22 |
| DigiGait | Once | Week 18 |
| Somatosensory (mechanical, thermal, cold) | Biweekly | Weeks 4–22 |
| Visceral sensitivity | Once | Week 18 |
| Metabolic (food, water, activity) | Once | Week 18 |
| Body mass composition | Once | Week 18 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhattacherjee, A.; Winter, M.K.; Eggimann, L.S.; Mu, Y.; Gunewardena, S.; Liao, Z.; Christianson, J.A.; Smith, P.G. Motor, Somatosensory, Viscerosensory and Metabolic Impairments in a Heterozygous Female Rat Model of Rett Syndrome. Int. J. Mol. Sci. 2018, 19, 97. https://doi.org/10.3390/ijms19010097
Bhattacherjee A, Winter MK, Eggimann LS, Mu Y, Gunewardena S, Liao Z, Christianson JA, Smith PG. Motor, Somatosensory, Viscerosensory and Metabolic Impairments in a Heterozygous Female Rat Model of Rett Syndrome. International Journal of Molecular Sciences. 2018; 19(1):97. https://doi.org/10.3390/ijms19010097
Chicago/Turabian StyleBhattacherjee, Aritra, Michelle K. Winter, Linda S. Eggimann, Ying Mu, Sumedha Gunewardena, Zhaohui Liao, Julie A. Christianson, and Peter G. Smith. 2018. "Motor, Somatosensory, Viscerosensory and Metabolic Impairments in a Heterozygous Female Rat Model of Rett Syndrome" International Journal of Molecular Sciences 19, no. 1: 97. https://doi.org/10.3390/ijms19010097
APA StyleBhattacherjee, A., Winter, M. K., Eggimann, L. S., Mu, Y., Gunewardena, S., Liao, Z., Christianson, J. A., & Smith, P. G. (2018). Motor, Somatosensory, Viscerosensory and Metabolic Impairments in a Heterozygous Female Rat Model of Rett Syndrome. International Journal of Molecular Sciences, 19(1), 97. https://doi.org/10.3390/ijms19010097