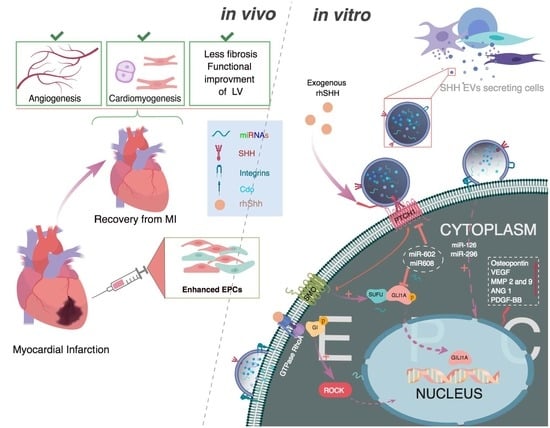
Sonic Hedgehog Signaling Pathway in Endothelial Progenitor Cell Biology for Vascular Medicine
Abstract
:1. Introduction
1.1. SHH Signaling Pathways in Vascular Development
1.2. SHH Signal Transmission by Extracellular Vesicles
1.3. Impaired SHH Signaling in Cardiovascular Diseases
1.4. Therapeutic Application of SHH Signaling in Cardiovascular Diseases
1.5. Ischemic Heart Diseases
1.6. Peripheral Arterial Diseases
1.7. Post DM Complications
2. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Asahara, T.; Murohara, T.; Sullivan, A.; Silver, M.; Zee, R.v.d.; Li, T.; Witzenbichler, B.; Schatteman, G.; Isner, J.M. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997, 275, 964–966. [Google Scholar] [CrossRef] [PubMed]
- Asahara, T.; Kawamoto, A.; Masuda, H. Concise review: Circulating endothelial progenitor cells for vascular medicine. Stem Cell 2011, 29, 1650–1655. [Google Scholar] [CrossRef] [PubMed]
- Asahara, T.; Masuda, H.; Takahashi, T.; Kalka, C.; Pastore, C.; Silver, M.; Kearne, M.; Magner, M.; Isner, J.M. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ. Res. 1999, 85, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Ingram, D.A.; Mead, L.E.; Tanaka, H.; Meade, V.; Fenoglio, A.; Mortell, K.; Pollok, K.; Ferkowicz, M.J.; Gilley, D.; Yoder, M.C. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood 2004, 104, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Aicher, A.; Rentsch, M.; Sasaki, K.; Ellwart, J.W.; Fandrich, F.; Siebert, R.; Cooke, J.P.; Dimmeler, S.; Heeschen, C. Nonbone marrow-derived circulating progenitor cells contribute to postnatal neovascularization following tissue ischemia. Circ. Res. 2007, 100, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Chinnaiya, K.; Tickle, C.; Towers, M. Sonic hedgehog-expressing cells in the developing limb measure time by an intrinsic cell cycle clock. Nat. Commun. 2014, 5, 4230. [Google Scholar] [CrossRef] [PubMed]
- Tickle, C.; Towers, M. Sonic hedgehog signaling in limb development. Front. Cell Dev. Biol. 2017, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Yao, P.J.; Petralia, R.S.; Mattson, M.P. Sonic hedgehog signaling and hippocampal neuroplasticity. Trends Neurosci. 2016, 39, 840–850. [Google Scholar] [CrossRef] [PubMed]
- Yao, P.J.; Petralia, R.S.; Ott, C.; Wang, Y.X.; Lippincott-Schwartz, J.; Mattson, M.P. Dendrosomatic sonic hedgehog signaling in hippocampal neurons regulates axon elongation. J. Neurosci. 2015, 35, 16126–16141. [Google Scholar] [CrossRef] [PubMed]
- Ihrie, R.A.; Shah, J.K.; Harwell, C.C.; Levine, J.H.; Guinto, C.D.; Lezameta, M.; Kriegstein, A.R.; Alvarez-Buylla, A. Persistent sonic hedgehog signaling in adult brain determines neural stem cell positional identity. Neuron 2011, 71, 250–262. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Moskowitz, M.A.; Sims, J.R. Sonic hedgehog inversely regulates the expression of angiopoietin-1 and angiopoietin-2 in fibroblasts. Int. J. Mol. Med. 2007, 19, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Jeong, M.-H.; Leem, Y.-E.; Kim, H.-J.; Kang, K.; Cho, H.; Kang, J.-S. A shh coreceptor cdo is required for efficient cardiomyogenesis of pluripotent stem cells. J. Mol. Cell. Cardiol. 2016, 93, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, S.; Dohle, E.; Kirkpatrick, C.J. Sonic hedgehog-mediated synergistic effects guiding angiogenesis and osteogenesis. Vitam. Horm. 2012, 88, 491–506. [Google Scholar] [PubMed]
- Yao, Q.; Renault, M.-A.; Chapouly, C.; Vandierdonck, S.; Belloc, I.; Jaspard-Vinassa, B.; Daniel-Lamazière, J.-M.; Laffargue, M.; Merched, A.; Desgranges, C.; et al. Sonic hedgehog mediates a novel pathway of pdgf-bb–dependent vessel maturation. Blood 2014, 123. blood-2013. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.R.; Liu, W.L.; Zhou, J.F.; Sun, H.Y.; Xu, H.Z.; Luo, L.; Zhang, H.; Zhou, Y.F. Sonic hedgehog protein promotes bone marrow-derived endothelial progenitor cell proliferation, migration and vegf production via pi 3-kinase/akt signaling pathways. Acta Pharmacol. Sin. 2006, 27, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Pola, R.; Ling, L.E.; Silver, M.; Corbley, M.J.; Kearney, M.; Blake Pepinsky, R.; Shapiro, R.; Taylor, F.R.; Baker, D.P.; Asahara, T.; et al. The morphogen sonic hedgehog is an indirect angiogenic agent upregulating two families of angiogenic growth factors. Nat. Med. 2001, 7, 706–711. [Google Scholar] [CrossRef] [PubMed]
- Mackie, A.R.; Klyachko, E.; Thorne, T.; Schultz, K.M.; Millay, M.; Ito, A.; Kamide, C.E.; Liu, T.; Gupta, R.; Sahoo, S.; et al. Sonic hedgehog–modified human cd34+ cells preserve cardiac function after acute myocardial infarction. Circ. Res. 2012, 122, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Straface, G.; Aprahamian, T.; Flex, A.; Gaetani, E.; Biscetti, F.; Smith, R.C.; Pecorini, G.; Pola, E.; Angelini, F.; Stigliano, E.; et al. Sonic hedgehog regulates angiogenesis and myogenesis during post-natal skeletal muscle regeneration. J. Cell. Mol. Med. 2009, 13, 2424–2435. [Google Scholar] [CrossRef] [PubMed]
- Kanaya, K.; Li, M.; Okazaki, T.; Nakamura, T.; Horii-Komatsu, M.; Alev, C.; Akimaru, H.; Kawamoto, A.; Akashi, H.; Tanaka, H.; et al. Sonic hedgehog signaling regulates vascular differentiation and function in human cd34 positive cells: Vasculogenic cd34+ cells with sonic hedgehog. Stem Cell Res. 2015, 14, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Pola, R.; Ling, L.E.; Aprahamian, T.R.; Barban, E.; Bosch-Marce, M.; Curry, C.; Corbley, M.; Kearney, M.; Isner, J.M.; Losordo, D.W. Postnatal recapitulation of embryonic hedgehog pathway in response to skeletal muscle ischemia. Circulation 2003, 108, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Briscoe, J.; Thérond, P.P. The mechanisms of hedgehog signalling and its roles in development and disease. Nat. Rev. Mol. Cell Biol. 2013, 14, 416. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Shen, L.; Law, K.; Zhang, Z.; Liu, X.; Hua, H.; Li, S.; Huang, H.; Yue, S.; Hui, C.-C.; et al. Suppressor of fused chaperones gli proteins to generate transcriptional responses to sonic hedgehog signaling. Mol. Cell. Biol. 2017, 37, e00421-16. [Google Scholar] [CrossRef] [PubMed]
- Rimkus, T.K.; Carpenter, R.L.; Qasem, S.; Chan, M.; Lo, H.W. Targeting the sonic hedgehog signaling pathway: Review of smoothened and gli inhibitors. Cancers 2016, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Mastrangelo, E.; Milani, M. Role and inhibition of gli1 protein in cancer. Lung Cancer 2018, 9, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Chinchilla, P.; Xiao, L.; Kazanietz, M.G.; Riobo, N.A. Hedgehog proteins activate pro-angiogenic responses in endothelial cells through non-canonical signaling pathways. Cell Cycle 2010, 9, 570–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, R.; Mackie, A.R.; Misener, S.; Liu, L.; Losordo, D.W.; Kishore, R. Endothelial smoothened-dependent hedgehog signaling is not required for sonic hedgehog induced angiogenesis or ischemic tissue repair. Lab. Investig. 2018, 98, 682–691. [Google Scholar] [CrossRef] [PubMed]
- Renault, M.A.; Roncalli, J.; Tongers, J.; Thorne, T.; Klyachko, E.; Misener, S.; Volpert, O.V.; Mehta, S.; Burg, A.; Luedemann, C.; et al. Sonic hedgehog induces angiogenesis via rho kinase-dependent signaling in endothelial cells. J. Mol. Cell Cardiol. 2010, 49, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Abdelmonem, M.; Kassem, S.H.; Gabr, H.; Shaheen, A.A.; Aboushousha, T. Avemar and echinacea extracts enhance mobilization and homing of cd34(+) stem cells in rats with acute myocardial infarction. Stem Cell Res. Ther. 2015, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Fritz, R.D.; Pertz, O. The dynamics of spatio-temporal rho gtpase signaling: Formation of signaling patterns. F1000Res. 2016, 5, 749. [Google Scholar] [CrossRef] [PubMed]
- Lawson, C.D.; Ridley, A.J. Rho gtpase signaling complexes in cell migration and invasion. J. Cell Biol. 2018, 217. jcb-201612069. [Google Scholar] [CrossRef] [PubMed]
- Bryan, B.A.; Dennstedt, E.; Mitchell, D.C.; Walshe, T.E.; Noma, K.; Loureiro, R.; Saint-Geniez, M.; Campaigniac, J.P.; Liao, J.K.; D’Amore, P.A. Rhoa/rock signaling is essential for multiple aspects of vegf-mediated angiogenesis. FASEB J. 2010, 24, 3186–3195. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.N.R.; Son, Y.S.; Lee, D.; Choi, Y.J.; Kwon, S.M.; Chang, H.K.; Kim, P.H.; Cho, J.Y. Hedgehog-interacting protein (hip) regulates apoptosis evasion and angiogenic function of late endothelial progenitor cells. Sci. Rep. 2017, 7, 12449. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles and friends. J. Cell. Boil. 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Zitvogel, L.; Amigorena, S. Exosomes: Composition, biogenesis and function. Nat. Rev. Immunol. 2002, 2, 569. [Google Scholar] [CrossRef] [PubMed]
- Mol, E.A.; Goumans, M.J.; Doevendans, P.A.; Sluijter, J.P.G.; Vader, P. Higher functionality of extracellular vesicles isolated using size-exclusion chromatography compared to ultracentrifugation. Nanomedicine 2017, 13, 2061–2065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vyas, N.; Walvekar, A.; Tate, D.; Lakshmanan, V.; Bansal, D.; Cicero, A.L.; Raposo, G.; Palakodeti, D.; Dhawan, J. Vertebrate hedgehog is secreted on two types of extracellular vesicles with different signaling properties. Sci. Rep. 2014, 4, 7357. [Google Scholar] [CrossRef] [PubMed]
- Fattahi, S.; Pilehchian Langroudi, M.; Akhavan-Niaki, H. Hedgehog signaling pathway: Epigenetic regulation and role in disease and cancer development. J. Cell Physiol. 2018, 233, 5726–5735. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, E.; Smaele, E.D.; Miele, E.; Laneve, P.; Po, A.; Pelloni, M.; Paganelli, A.; Marcotullio, L.D.; Caffarelli, E.; Screpanti, I.; et al. Concerted microrna control of hedgehog signalling in cerebellar neuronal progenitor and tumour cells. EMBO J. 2008, 172, 2616–2627. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wang, J.; Li, J.; Ma, C.; Chen, S.; Lei, W.; Yang, Y.; Liu, S.; Bihl, J.; Chen, C. Loading mir-210 in endothelial progenitor cells derived exosomes boosts their beneficial effects on hypoxia/reoxygeneation-injured human endothelial cells via protecting mitochondrial function. Cell. Physiol. Biochem. 2018, 46, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Makki, M.S.; Haqqi, T.M. Microrna-602 and microrna-608 regulate sonic hedgehog expression via target sites in the coding region in human chondrocytes. Arthritis Rheumatol. 2015, 67, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Huaitong, X.; Yuanyong, F.; Yueqin, T.; Peng, Z.; Wei, S.; Kai, S. Microvesicles releasing by oral cancer cells enhance endothelial cell angiogenesis via shh/rhoa signaling pathway. Cancer Boil. Ther. 2017, 18, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.C.; Andriantsitohaina, R. Microparticles in angiogenesis: Therapeutic potential. Circ. Res. 2011, 109, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Barakat, B.; Yu, L.; Lo, C.; Vu, D.; De Luca, E.; Cain, J.E.; Martelotto, L.G.; Dedhar, S.; Sadler, A.J.; Wang, D.; et al. Interaction of smoothened with integrin-linked kinase in primary cilia mediates hedgehog signalling. EMBO Rep. 2013, 14, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Mathiyalagan, P.; Liang, Y.; Kim, D.; Misener, S.; Thorne, T.; Kamide, C.E.; Klyachko, E.; Losordo, D.W.; Hajjar, R.J.; Sahoo, S. Angiogenic mechanisms of human cd34(+) stem cell exosomes in the repair of ischemic hindlimb. Circ. Res. 2017, 120, 1466–1476. [Google Scholar] [CrossRef] [PubMed]
- Semo, J.; Sharir, R.; Afek, A.; Avivi, C.; Barshack, I.; Maysel-Auslender, S.; Krelin, Y.; Kain, D.; Entin-Meer, M.; Keren, G.; et al. The 106b∼25 microRNA cluster is essential for neovascularization after hindlimb ischaemia in mice. Eur. Heart J. 2014, 35, 3212–3223. [Google Scholar] [CrossRef] [PubMed]
- Van Solingen, C.; de Boer, H.C.; Bijkerk, R.; Monge, M.; van Oeveren-Rietdijk, A.M.; Seghers, L.; de Vries, M.R.; van der Veer, E.P.; Quax, P.H.; Rabelink, T.J.; et al. Microrna-126 modulates endothelial sdf-1 expression and mobilization of sca-1(+)/lin(-) progenitor cells in ischaemia. Cardiovasc. Res. 2011, 92, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Deng, S.; Ma, Q.; Zhang, T.; Jia, C.; Zhuo, D.; Yang, F.; Wei, J.; Wang, L.; Dykxhoorn, D.M.; et al. Microrna-10a* and microrna-21 modulate endothelial progenitor cell senescence via suppressing high-mobility group a2. Circ. Res. 2013, 112, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.W.; Lo, H.H.; Chiu, Y.L.; Chang, S.J.; Huang, P.H.; Liao, K.H.; Tasi, C.F.; Wu, C.H.; Tsai, T.N.; Cheng, C.C.; et al. Dysregulated mir-361-5p/vegf axis in the plasma and endothelial progenitor cells of patients with coronary artery disease. PLoS ONE 2014, 9, e98070. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Li, J.; Chen, A.F. MicroRNA-34a induces endothelial progenitor cell senescence and impedes its angiogenesis via suppressing silent information regulator 1. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E110–E116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The Lancet Life, death and disability in 2016. Lancet 2017, 390, 1083. [CrossRef]
- Paschalaki, K.E.; Starke, R.D.; Hu, Y.; Mercado, N.; Margariti, A.; Gorgoulis, V.G.; Randi, A.M.; Barnes, P.J. Dysfunction of endothelial progenitor cells from smokers and chronic obstructive pulmonary disease patients due to increased DNA damage and senescence. Stem Cells 2013, 31, 2813–2826. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Lucke, C.; Rössig, L.; Fichtlscherer, S.; Vasa, M.; Britten, M.; Kämper, U.; Dimmeler, S.; Zeiher, A.M. Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: Proof of concept for the clinical importance of endogenous vascular repair. Circulation 2005, 111, 2981–2987. [Google Scholar] [CrossRef] [PubMed]
- Wils, J.; Favre, J.; Bellien, J. Modulating putative endothelial progenitor cells for the treatment of endothelial dysfunction and cardiovascular complications in diabetes. Pharmacol. Ther. 2017, 170, 98–115. [Google Scholar] [CrossRef] [PubMed]
- Han, B.W.; Layman, H.; Rode, N.A.; Conway, A.; Schaffer, D.V.; Boudreau, N.J.; Jackson, W.M.; Healy, K.E. Multivalent conjugates of sonic hedgehog accelerate diabetic wound healing. Tissue Eng. Part A 2015, 21, 2366–2378. [Google Scholar] [CrossRef] [PubMed]
- Palladino, M.; Gatto, I.; Neri, V.; Stigliano, E.; Smith, R.C.; Pola, E.; Straino, S.; Gaetani, E.; Capogrossi, M.; Leone, G.; et al. Combined therapy with sonic hedgehog gene transfer and bone marrow-derived endothelial progenitor cells enhances angiogenesis and myogenesis in the ischemic skeletal muscle. J. Vasc. Res. 2012, 49, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Kusano, K.F.; Pola, R.; Murayama, T.; Curry, C.; Kawamoto, A.; Iwakura, A.; Shintani, S.; Ii, M.; Asai, J.; Tkebuchava, T.; et al. Sonic hedgehog myocardial gene therapy: Tissue repair through transient reconstitution of embryonic signaling. Nat. Med. 2005, 11, 1197–1204. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; He, Y.H.; Hou, N.; Zhang, G.S.; Cai, Y.; Zhang, G.P.; Xiao, Q.; He, L.S.; Li, S.J.; Yi, Q.; et al. Sonic hedgehog improves ischemia-induced neovascularization by enhancing endothelial progenitor cell function in type 1 diabetes. Mol. Cell. Endocrinol. 2016, 423, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Nanta, R.; Sharma, J.; Gunewardena, S.; Singh, K.P.; Shankar, S.; Srivastava, R.K. Pi3k/akt/mtor and sonic hedgehog pathways cooperate together to inhibit human pancreatic cancer stem cell characteristics and tumor growth. Oncotarget 2015, 6, 32039–32060. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Hou, N.; Wang, Y.P.; He, L.S.; He, Y.H.; Zhang, G.P.; Yi, Q.; Liu, S.M.; Chen, M.S.; Luo, J.D. Impaired sonic hedgehog pathway contributes to cardiac dysfunction in type 1 diabetic mice with myocardial infarction. Cardiovasc. Res. 2012, 95, 507–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bijlsma, M.F.; Leenders, P.J.A.; Janssen, B.J.A.; Peppelenbosch, M.P.; Cate, H.T.; Spek, C.A. Endogenous hedgehog expression contributes to myocardial ischemia-reperfusion–induced injury. Exp. Biol. Med. 2008, 33, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Teraa, M.; Sprengers, R.W.; Schutgens, R.E.; Slaper-Cortenbach, I.C.; van der Graaf, Y.; Algra, A.; van der Tweel, I.; Doevendans, P.A.; Mali, W.P.; Moll, F.L.; et al. Effect of repetitive intra-arterial infusion of bone marrow mononuclear cells in patients with no-option limb ischemia: The randomized, double-blind, placebo-controlled rejuvenating endothelial progenitor cells via transcutaneous intra-arterial supplementation (juventas) trial. Circulation 2015, 131, 851–860. [Google Scholar] [PubMed]
- Loomans, C.J.M.; Koning, E.J.P.D.; Staal, F.J.T.; Rookmaaker, M.B.; Verseyden, C.; Boer, H.C.D.; Verhaar, M.C.; Braam, B.; Rabelink, T.J.; Zonneveld, A.-J.V. Endothelial progenitor cell dysfunction. Diabetes 2004, 53, 159–199. [Google Scholar] [CrossRef]
- Zimmet, H.; Porapakkham, P.; Sata, Y.; Haas, S.J.; Itescu, S.; Forbes, A.; Krum, H. Short- and long-term outcomes of intracoronary and endogenously mobilized bone marrow stem cells in the treatment of st-segment elevation myocardial infarction: A meta-analysis of randomized control trials. Eur. J. Heart Failur. 2014, 14, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Renault, M.-A.; Robbesyn, F.; Chapouly, C.; Yao, Q.; Vandierdonck, S.; Reynaud, A.; Belloc, I.; Traiffort, E.; Ruat, M.; Desgranges, C.; et al. Hedgehog-dependent regulation of angiogenesis and myogenesis is impaired in aged mice. Circ. Res. 2013, 33. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Yi, X.; Ren, F.; Liu, L.; Wu, S.; Yang, J. Activation of shh signaling pathway promotes vasculogenesis in post-myocardial ischemic-reperfusion injury. Int. J. Clin. Exp. Pathol. 2015, 8, 12464–12472. [Google Scholar] [PubMed]
- Jin, Y.; Barnett, A.; Zhang, Y.; Yu, X.; Luo, Y. Poststroke sonic hedgehog agonist treatment improves functional recovery by enhancing neurogenesis and angiogenesis. Stroke 2017, 48, 1636–1645. [Google Scholar] [CrossRef] [PubMed]
- Bisdas, T.; Borowski, M.; Stavroulakis, K.; Torsello, G.; Collaborators, C.; Adili, F.; Balzer, K.; Billing, A.; Böckler, D.; Brixner, D.; et al. Endovascular therapy versus bypass surgery as first-line treatment strategies for critical limb ischemia. JACC Cardiovasc. Interv. 2016, 24, 2557–2565. [Google Scholar] [CrossRef] [PubMed]
- Fowkes, F.G.; Rudan, D.; Rudan, I.; Aboyans, V.; Denenberg, J.O.; McDermott, M.M.; Norman, P.E.; Sampson, U.K.; Williams, L.J.; Mensah, G.A.; et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: A systematic review and analysis. Lancet 2013, 382, 1329–1340. [Google Scholar] [CrossRef]
- Jones, W.S.; Dolor, R.J.; Hasselblad, V.; Vemulapalli, S.; Subherwal, S.; Heidenfelder, B.; Patel, M.R. Comparative effectiveness of endovascular and surgical revascularization for patients with peripheral artery disease and critical limb ischemia: Systematic review of revascularization in critical limb ischemia. Am. Heart J. 2014, 167, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Liotta, F.; Annunziato, F.; Castellani, S.; Boddi, M.; Alterini, B.; Castellini, G.; Mazzanti, B.; Cosmi, L.; Acquafresca, M.; Bartalesi, F.; et al. Therapeutic efficacy of autologous non-mobilized enriched circulating endothelial progenitors in patients with critical limb ischemia- the scelta trial. Circ. J. 2018, 82, 1688–1698. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Kinoshita, M.; Furukawa, Y.; Nagano, T.; Hashimoto, H.; Hirami, Y.; Kurimoto, Y.; Arakawa, K.; Yamazaki, K.; Okada, Y.; et al. Phase ii clinical trial of cd34+ cell therapy to explore endpoint selection and timing in patients with critical limb ischemia. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 78, 490–501. [Google Scholar] [CrossRef]
- Losordo, D.W.; Henry, T.D.; Davidson, C.; Sup Lee, J.; Costa, M.A.; Bass, T.; Mendelsohn, F.; Fortuin, F.D.; Pepine, C.J.; Traverse, J.H.; et al. Intramyocardial, autologous cd34+ cell therapy for refractory angina. Circ. Res. 2011, 109, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Fadini, G.P.; Albiero, M.; Kreutzenberg, S.V.D.; Boscaro, E.; Cappellari, R.; Marescotti, M.; Poncina, N.; Agostini, C.; Avogaro, A. Diabetes impairs stem cell and proangiogenic cell mobilization in humans. Diabetes Care 2013, 36, DC_121084. [Google Scholar] [CrossRef] [PubMed]
- Caradu, C.; Guy, A.; James, C.; Reynaud, A.; Gadeau, A.P.; Renault, M.A. Endogenous sonic hedgehog limits inflammation and angiogenesis in the ischaemic skeletal muscle of mice. Cardiovasc. Res. 2018, 114, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.C.; Huang, M.; He, Q.W.; Zhang, Y.; Opoku, E.N.; Yang, H.; Jin, H.J.; Xia, Y.P.; Hu, B. Administration of sonic hedgehog protein induces angiogenesis and has therapeutic effects after stroke in rats. Neuroscience 2017, 352, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Piccioni, A.; Gaetani, E.; Neri, V.; Gatto, I.; Palladino, M.; Silver, M.; Smith, R.C.; Giarretta, I.; Pola, E.; Hlatky, L.; et al. Sonic hedgehog therapy in a mouse model of age-associated impairment of skeletal muscle regeneration. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Rivron, N.C.; Raiss, C.C.; Liu, J.; Nandakumar, A.; Sticht, C.; Gretz, N.; Truckenmüller, R.; Rouwkema, J.; van Blitterswijk, C.A. Sonic hedgehog-activated engineered blood vessels enhance bone tissue formation. Proc. Natl. Acad. Sci. USA 2012, 109, 4413–4418. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, O.; Zdunek, S.; Felker, A.; Salehpour, M.; Alkass, K.; Bernard, S.; Sjostrom, S.L.; Szewczykowska, M.; Jackowska, T.; Dos Remedios, C.; et al. Dynamics of cell generation and turnover in the human heart. Cell 2015, 161, 1566–1575. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.R.; Ilinykh, A.; Ivey, M.J.; Kuwabara, J.T.; D’Antoni, M.L.; Debuque, R.; Chandran, A.; Wang, L.; Arora, K.; Rosenthal, N.A.; et al. Revisiting cardiac cellular composition. Circ. Res. 2016, 118, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Roncalli, J.; Renault, M.A.; Tongers, J.; Misener, S.; Thorne, T.; Kamide, C.; Jujo, K.; Tanaka, T.; Ii, M.; Klyachko, E.; et al. Sonic-hedgehog–induced functional recovery after myocardial infarction is enhanced by amd3100-mediated progenitor-cell mobilization. J. Am. Coll. Cardiol. 2011, 57, 2444–2452. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.R.; Wang, Y. Controlled delivery of sonic hedgehog morphogen and its potential for cardiac repair. PLoS ONE 2013, 8, e63075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rydén, L.; Grant, P.J.; Anker, S.D.; Berne, C.; Cosentino, F.; Danchin, N.; Deaton, C.; Escaned, J.; Hammes, H.-P.; Huikuri, H.; et al. Esc guidelines on diabetes, pre-diabetes and cardiovascular diseases developed in collaboration with the easdthe task force on diabetes, pre-diabetes and cardiovascular diseases of the european society of cardiology (esc) and developed in collaboration with the european association for the study of diabetes (easd). Eur. Heart J. 2013, 34, 3035–3087. [Google Scholar] [PubMed]
- Shah, A.D.; Langenberg, C.; Rapsomaniki, E.; Denaxas, S.; Pujades-Rodriguez, M.; Gale, C.P.; Deanfield, J.; Smeeth, L.; Timmis, A.; Hemingway, H. Type 2 diabetes and incidence of cardiovascular diseases: A cohort study in 1.9 million people. Lancet. Diabetes Endocrinol. 2015, 3, 105–113. [Google Scholar] [CrossRef]


| Name of the miRNA | Expression | Target Cells | Outcome | Target Genes | Ref. |
|---|---|---|---|---|---|
| miR-126-3p | Up | EPC and EC | In vitro, presence of miR126-3p increases the length of tube formation. In vivo, it increases the MVD in animal models with HLI. | VEGF, ANG-1, ANG-2 and MMP-9 | [44] |
| miR-106b-25 | Up | EC, EPC, SCA-1 and BMMSC | Increased tube formation capacity. Overexpression of individual members of the miR-106b-25 cluster increases viability, proliferation and migration of ECs. | VEGF, SCA-1 and FLK-1 | [45] |
| miR-126 | Down | EPC, SCA-1 and Lin- | Silencing miR-126 in animal models with HLI increases mobilization of EPC, SCA-1 and Lin- cells from bone marrow to the site of injury and enhanced angiogenesis. | SDF-1 | [46] |
| miR-10A and miR-21 | Down | EPC | miR-10A and miR-21 regulates senescence in EPCs by suppressing the expression of HMGA 2. | HMGA 2 | [47] |
| miR-361 | Down | EPC | In vitro, KO of miR-361-5p restores VEGF levels and angiogenic activities in diseased EPCs. In vivo, it promotes blood flow and recovery of ischemic limbs in mice. | VEGF | [48] |
| miR-34a | Down | EPC | Overexpression of miR-34a significantly enhanced senescence and impairment in EPC that paralleled with 40% reduction in SIRT1. KO of SIRT1 by siRNA decreased angiogenesis and increased senescence in EPCs. | SIRT1 and FOXO1 | [49] |
| Disease Model | SHH Pathway and Cell Tx. | Results | Ref. |
|---|---|---|---|
| AMI | Activation of endogenous and exogenous SHH signaling by SHH–modified human CD34+ cells and its exosomes | Treatment with SHH–modified human CD34+ cells reduced infarct size, increased border zone capillary density and improved cardiac function; EF, FS, compared with unmodified CD34 cells or cells transfected with the empty vector. | [15] |
| AMI and Chronic MI | Exogenous recombinant SHH administration and gene transfer of naked DNA encoding human SHH | MI fibrosis size and apoptotic cardiomyocytes were reduced. MVD was increased. SHH gene transfer enhanced the contribution of bone marrow–derived endothelial progenitor cells to myocardial neovascularization. | [56] |
| Myocardial Ischemia-Reperfusion–Induced Injury | Activation of endogenous HH signaling and administration of exogenous recombinant SHH | Reduced apoptosis, fibrosis and increased vascularization. Exogenous SHH administration reduced apoptosis, increased vascularization and reduced | [60] |
| Post-myocardial ischemic-reperfusion injury | Activation of endogenous HH signaling and exogenous recombinant SHH administration | Exogenous SHH administration significantly increased vasculogenesis-related factors including VEGF, FGF and ANG as well as the SHH signal proteins including PTCH-1, GLI 1, GLI 2 and SMO. | [65] |
| HLI | SHH-treated human G-CSF mobilized EPCs locally injected into the HLI muscles | Incubation of CD34+ cells with exogenous SHH molecule significantly increased vasculogenesis-related factors including VEGFA, VEGFB, HGF and Pecam 1 as well as the SHH signal proteins including PTCH-1, GLI 1, GLI 2 and SMO at dose 1μg/mL. In vivo significant increase in angiogenesis and vasculogenesis and recovery by blood perfusion following HLI. | [17] |
| HLI | SHH conditioned fibroblast media or exosomes | PDGF-B, VEGF-A, HGF and IGF. PDGF-B was significantly upregulated and contributed to MVD. Improved blood flow perfusion after HLI. | [23] |
| HLI | Combined treatment with SHH and EPC | Increased incorporation of EPC within host vessels, reduced apoptotic of EPC and initiated the generation of new myocytes. | [55] |
| Diabetic wound healing | Administration of exogenous nanoscale polymer encapsulated SHH | Accelerated diabetic-induced wound closure. | [44] |
| DM type 1 mouse was inducted AMI | SHH + EPCs Tx | EPC migration, tube forming ability and mobilization were impaired in diabetic mice compared to that of control. In vivo administration of the SHH pathway receptor agonist improved all the above. SHH molecules significantly increased capillary density and blood perfusion in the ischemic hind-limbs of diabetic mice. | [57] |
| Ischemic Stroke | Administration of exogenous SHH | SHH treatment results in enhanced functional recovery both in locomotor function and in cognitive function 1-month post-stroke. Increased the cerebral blood flow map by arterial spin labeling and immunohistochemistry á-smooth muscle actin and CD31 immunostaining. | [66] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salybekov, A.A.; Salybekova, A.K.; Pola, R.; Asahara, T. Sonic Hedgehog Signaling Pathway in Endothelial Progenitor Cell Biology for Vascular Medicine. Int. J. Mol. Sci. 2018, 19, 3040. https://doi.org/10.3390/ijms19103040
Salybekov AA, Salybekova AK, Pola R, Asahara T. Sonic Hedgehog Signaling Pathway in Endothelial Progenitor Cell Biology for Vascular Medicine. International Journal of Molecular Sciences. 2018; 19(10):3040. https://doi.org/10.3390/ijms19103040
Chicago/Turabian StyleSalybekov, Amankeldi A., Ainur K. Salybekova, Roberto Pola, and Takayuki Asahara. 2018. "Sonic Hedgehog Signaling Pathway in Endothelial Progenitor Cell Biology for Vascular Medicine" International Journal of Molecular Sciences 19, no. 10: 3040. https://doi.org/10.3390/ijms19103040
APA StyleSalybekov, A. A., Salybekova, A. K., Pola, R., & Asahara, T. (2018). Sonic Hedgehog Signaling Pathway in Endothelial Progenitor Cell Biology for Vascular Medicine. International Journal of Molecular Sciences, 19(10), 3040. https://doi.org/10.3390/ijms19103040