Involvement of Aquaporins in the Pathogenesis, Diagnosis and Treatment of Sjögren’s Syndrome
Abstract
:1. Introduction
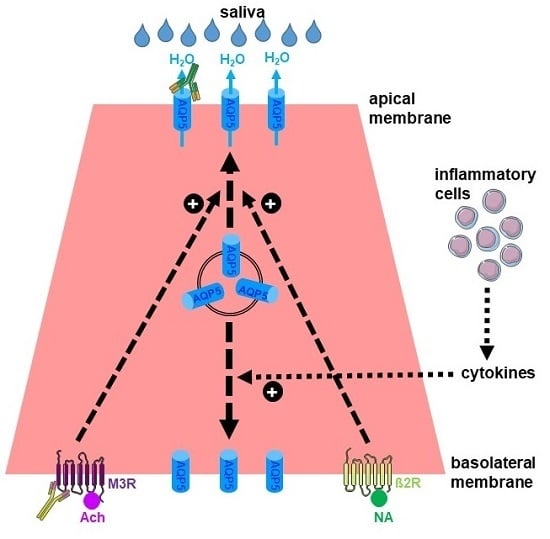
2. Expression and Function of AQPs in Salivary Glands
3. Defective Localization of AQPs in the Salivary Glands of SS
4. Autoantibodies against AQP in SS
5. Role of AQPs in the Treatment of Sjögren’s Syndrome
5.1. Drug Therapies
5.2. Gene Therapy
5.3. Ultrasound Therapies
5.4. Regenerative Therapies
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AQP | Aquaporin |
| pSS | Primary Sjögren’s syndrome |
| SG | Salivary gland |
| SS | Sjögren’s syndrome |
References
- Mariette, X.; Criswell, L.A. Primary Sjogren’s Syndrome. N. Engl. J. Med. 2018, 378, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Tzioufas, A.G.; Voulgarelis, M. Update on Sjogren’s syndrome autoimmune epithelitis: From classification to increased neoplasias. Best Pract. Res. Clin. Rheumatol. 2007, 21, 989–1010. [Google Scholar] [CrossRef] [PubMed]
- Shiboski, C.H.; Shiboski, S.C.; Seror, R.; Criswell, L.A.; Labetoulle, M.; Lietman, T.M.; Rasmussen, A.; Scofield, H.; Vitali, C.; Bowman, S.J.; et al. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjogren’s syndrome: A consensus and data-driven methodology involving three international patient cohorts. Ann. Rheum. Dis. 2017, 76, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Mavragani, C.P. Mechanisms and New Strategies for Primary Sjogren’s Syndrome. Ann. Rev. Med. 2017, 68, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Nocturne, G.; Mariette, X. Advances in understanding the pathogenesis of primary Sjogren’s syndrome. Nat. Rev. Rheumatol. 2013, 9, 544–556. [Google Scholar] [CrossRef] [PubMed]
- Goules, A.V.; Kapsogeorgou, E.K.; Tzioufas, A.G. Insight into pathogenesis of Sjogren’s syndrome: Dissection on autoimmune infiltrates and epithelial cells. Clin. Immunol. 2017, 182, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Brito-Zeron, P.; Baldini, C.; Bootsma, H.; Bowman, S.J.; Jonsson, R.; Mariette, X.; Sivils, K.; Theander, E.; Tzioufas, A.; Ramos-Casals, M. Sjogren syndrome. Nat. Rev. Dis. Primers 2016, 2, 16047. [Google Scholar] [CrossRef] [PubMed]
- Youinou, P.; Saraux, A.; Pers, J.O. B-lymphocytes govern the pathogenesis of Sjogren’s syndrome. Curr. Pharm. Biotechnol. 2012, 13, 2071–2077. [Google Scholar] [CrossRef] [PubMed]
- Mitsias, D.I.; Kapsogeorgou, E.K.; Moutsopoulos, H.M. Sjogren’s syndrome: Why autoimmune epithelitis? Oral Dis. 2006, 12, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Manoussakis, M.N.; Kapsogeorgou, E.K. The role of intrinsic epithelial activation in the pathogenesis of Sjogren’s syndrome. J. Autoimmun. 2010, 35, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Agre, P.; Preston, G.M.; Smith, B.L.; Jung, J.S.; Raina, S.; Moon, C.; Guggino, W.B.; Nielsen, S. Aquaporin CHIP: The archetypal molecular water channel. Am. J. Physiol. 1993, 265, F463–F476. [Google Scholar] [CrossRef] [PubMed]
- Agre, P.; King, L.S.; Yasui, M.; Guggino, W.B.; Ottersen, O.P.; Fujiyoshi, Y.; Engel, A.; Nielsen, S. Aquaporin water channels—From atomic structure to clinical medicine. J. Physiol. 2002, 542, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Rojek, A.; Praetorius, J.; Frokiaer, J.; Nielsen, S.; Fenton, R.A. A current view of the mammalian aquaglyceroporins. Annu. Rev. Physiol. 2008, 70, 301–327. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, K.; Morishita, Y.; Tanaka, Y. The Evolutionary Aspects of Aquaporin Family. Adv. Exp. Med. Biol. 2017, 969, 35–50. [Google Scholar] [PubMed]
- Delporte, C. Aquaporins and Gland Secretion. Adv. Exp. Med. Biol. 2017, 969, 63–79. [Google Scholar] [PubMed]
- Delporte, C.; Bryla, A.; Perret, J. Aquaporins in Salivary Glands: From Basic Research to Clinical Applications. Int. J. Mol. Sci. 2016, 17, 166. [Google Scholar] [CrossRef] [PubMed]
- Ichiyama, T.; Nakatani, E.; Tatsumi, K.; Hideshima, K.; Urano, T.; Nariai, Y.; Sekine, J. Expression of aquaporin 3 and 5 as a potential marker for distinguishing dry mouth from Sjogren’s syndrome. J. Oral Sci. 2018, 60, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Sisto, M.; Lorusso, L.; Ingravallo, G.; Nico, B.; Ribatti, D.; Ruggieri, S.; Lofrumento, D.D.; Lisi, S. Abnormal distribution of AQP4 in minor salivary glands of primary Sjogren’s syndrome patients. Autoimmunity 2017, 50, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Hosoi, K. Physiological role of aquaporin 5 in salivary glands. Pflugers Arch. 2016, 468, 519–539. [Google Scholar] [CrossRef] [PubMed]
- Mangos, J.A.; McSherry, N.R. Micropuncture study of urea excretion in parotid saliva of the rat. Am. J. Physiol. 1970, 218, 1329–1332. [Google Scholar] [CrossRef] [PubMed]
- Melvin, J.E.; Yule, D.; Shuttleworth, T.; Begenisich, T. Regulation of fluid and electrolyte secretion in salivary gland acinar cells. Annu. Rev. Physiol. 2005, 67, 445–469. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.G.; Ohana, E.; Park, H.W.; Yang, D.; Muallem, S. Molecular mechanism of pancreatic and salivary gland fluid and HCO3 secretion. Physiol. Rev. 2012, 92, 39–74. [Google Scholar] [CrossRef] [PubMed]
- Murdiastuti, K.; Purwanti, N.; Karabasil, M.R.; Li, X.; Yao, C.; Akamatsu, T.; Kanamori, N.; Hosoi, K. A naturally occurring point mutation in the rat aquaporin 5 gene, influencing its protein production by and secretion of water from salivary glands. Am. J. Physiol. 2006, 291, G1081–G1088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karabasil, M.R.; Hasegawa, T.; Azlina, A.; Purwanti, N.; Yao, C.; Akamatsu, T.; Tomioka, S.; Hosoi, K. Effects of naturally occurring G103D point mutation of AQP5 on its water permeability, trafficking and cellular localization in the submandibular gland of rats. Biol. Cell 2011, 103, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Song, Y.; Gillespie, A.; Carlson, E.J.; Epstein, C.J.; Verkman, A.S. Defective secretion of saliva in transgenic mice lacking aquaporin-5 water channels. J. Biol. Chem. 1999, 274, 20071–20074. [Google Scholar] [CrossRef] [PubMed]
- Krane, C.M.; Melvin, J.E.; Nguyen, H.V.; Richardson, L.; Towne, J.E.; Doetschman, T.; Menon, A.G. Salivary acinar cells from aquaporin 5-deficient mice have decreased membrane water permeability and altered cell volume regulation. J. Biol. Chem. 2001, 276, 23413–23420. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.S.; Yang, B.; Song, Y.; Manley, G.T.; Ma, T. Role of water channels in fluid transport studied by phenotype analysis of aquaporin knockout mice. Exp. Physiol. 2000, 85, 233S–241S. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Song, Y.; Zhao, D.; Verkman, A.S. Phenotype analysis of aquaporin-8 null mice. Am. J. Physiol. 2005, 288, C1161–C1170. [Google Scholar] [CrossRef] [PubMed]
- Delporte, C. Aquaporins in secretory glands and their role in Sjogren’s syndrome. In Aquaporins. Handbook of Experimental Pharmacology; Beitz, E., Ed.; Springer: Berlin/Heidelberg, Germany, 2009; Volume 190, pp. 185–201. [Google Scholar]
- Soyfoo, M.S.; De Vriese, C.; Debaix, H.; Martin-Martinez, M.D.; Mathieu, C.; Devuyst, O.; Steinfeld, S.D.; Delporte, C. Modified aquaporin 5 expression and distribution in submandibular glands from NOD mice displaying autoimmune exocrinopathy. Arthritis Rheum. 2007, 56, 2566–2574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinfeld, S.; Cogan, E.; King, L.S.; Agre, P.; Kiss, R.; Delporte, C. Abnormal distribution of aquaporin-5 water channel protein in salivary glands from Sjogren’s syndrome patients. Lab. Investig. 2001, 81, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, S.; Nakamura, H.; Horai, Y.; Nakajima, H.; Shiraishi, H.; Hayashi, T.; Takahashi, T.; Kawakami, A. Abnormal distribution of AQP5 in labial salivary glands is associated with poor saliva secretion in patients with Sjogren’s syndrome including neuromyelitis optica complicated patients. Mod. Rheumatol. 2016, 26, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Konttinen, Y.T.; Tensing, E.K.; Laine, M.; Porola, P.; Tornwall, J.; Hukkanen, M. Abnormal distribution of aquaporin-5 in salivary glands in the NOD mouse model for Sjogren’s syndrome. J. Rheumatol. 2005, 32, 1071–1075. [Google Scholar] [PubMed]
- Enger, T.B.; Aure, M.H.; Jensen, J.L.; Galtung, H.K. Calcium signaling and cell volume regulation are altered in Sjogren’s Syndrome. Acta Odontol. Scand. 2014, 72, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Beroukas, D.; Hiscock, J.; Jonsson, R.; Waterman, S.A.; Gordon, T.P. Subcellular distribution of aquaporin 5 in salivary glands in primary Sjogren’s syndrome. Lancet 2001, 358, 1875–1876. [Google Scholar] [CrossRef]
- Gresz, V.; Horvath, A.; Gera, I.; Nielsen, S.; Zelles, T. Immunolocalization of AQP5 in resting and stimulated normal labial glands and in Sjogren’s syndrome. Oral Dis. 2015, 21, e114–e120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teos, L.Y.; Zhang, Y.; Cotrim, A.P.; Swaim, W.; Won, J.H.; Ambrus, J.; Shen, L.; Bebris, L.; Grisius, M.; Jang, S.I.; et al. IP3R deficit underlies loss of salivary fluid secretion in Sjogren’s Syndrome. Sci. Rep. 2015, 5, 13953. [Google Scholar] [CrossRef] [PubMed]
- Soyfoo, M.S.; Bolaky, N.; Depoortere, I.; Delporte, C. Relationship between aquaporin-5 expression and saliva flow in streptozotocin-induced diabetic mice? Oral Dis. 2012, 18, 501–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soyfoo, M.S.; Konno, A.; Bolaky, N.; Oak, J.S.; Fruman, D.; Nicaise, C.; Takiguchi, M.; Delporte, C. Link between inflammation and aquaporin-5 distribution in submandibular gland in Sjogren’s syndrome? Oral Dis. 2012, 18, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Bikker, A.; van Woerkom, J.M.; Kruize, A.A.; Wenting-van Wijk, M.; de Jager, W.; Bijlsma, J.W.; Lafeber, F.P.; van Roon, J.A. Increased expression of interleukin-7 in labial salivary glands of patients with primary Sjogren’s syndrome correlates with increased inflammation. Arthritis Rheum. 2010, 62, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Sandhya, P.; Kurien, B.T.; Danda, D.; Scofield, R.H. Update on Pathogenesis of Sjogren’s Syndrome. Curr. Rheumatol. Rev. 2017, 13, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Verstappen, G.M.; Corneth, O.B.J.; Bootsma, H.; Kroese, F.G.M. Th17 cells in primary Sjogren’s syndrome: Pathogenicity and plasticity. J. Autoimmun. 2018, 87, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Argyropoulou, O.D.; Valentini, E.; Ferro, F.; Leone, M.C.; Cafaro, G.; Bartoloni, E.; Baldini, C. One year in review 2018: Sjogren’s syndrome. Clin. Exp. Rheumatol. 2018, 36, 14–26. [Google Scholar] [PubMed]
- Bodewes, I.L.A.; Versnel, M.A. Interferon activation in primary Sjogren’s syndrome: Recent insights and future perspective as novel treatment target. Expert Rev. Clin. Immunol. 2018, 14, 817–829. [Google Scholar] [CrossRef] [PubMed]
- Bodewes, I.L.A.; Al-Ali, S.; van Helden-Meeuwsen, C.G.; Maria, N.I.; Tarn, J.; Lendrem, D.W.; Schreurs, M.W.J.; Steenwijk, E.C.; van Daele, P.L.A.; Both, T.; et al. Systemic interferon type I and type II signatures in primary Sjogren’s syndrome reveal differences in biological disease activity. Rheumatology 2018, 57, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Banete, A.; Seaver, K.; Bakshi, D.; Gee, K.; Basta, S. On taking the STING out of immune activation. J. Leukoc. Biol. 2018, 103, 1189–1195. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.Q.; Yin, H.; Lee, B.H.; Carcamo, W.C.; Chiorini, J.A.; Peck, A.B. Pathogenic effect of interleukin-17A in induction of Sjogren’s syndrome-like disease using adenovirus-mediated gene transfer. Arthritis Res. Ther. 2010, 12, R220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, C.Q.; Yin, H.; Lee, B.H.; Chiorini, J.A.; Peck, A.B. IL17: Potential therapeutic target in Sjogren’s syndrome using adenovirus-mediated gene transfer. Lab. Investig. 2011, 91, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.O.; Yu, Q. Systemic administration of TLR3 agonist induces IL-7 expression and IL-7-dependent CXCR3 ligand production in the lung. J. Leukoc. Biol. 2013, 93, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Kawai, T.; Yu, Q. Pathogenic role of endogenous TNF-alpha in the development of Sjogren’s-like sialadenitis and secretory dysfunction in non-obese diabetic mice. Lab. Investig. 2017, 97, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Lopalco, G.; Cantarini, L.; Vitale, A.; Iannone, F.; Anelli, M.G.; Andreozzi, L.; Lapadula, G.; Galeazzi, M.; Rigante, D. Interleukin-1 as a common denominator from autoinflammatory to autoimmune disorders: Premises, perils, and perspectives. Mediators Inflamm. 2015, 2015, 194864. [Google Scholar] [CrossRef] [PubMed]
- Both, T.; Dalm, V.A.; van Hagen, P.M.; van Daele, P.L. Reviewing primary Sjogren’s syndrome: Beyond the dryness - From pathophysiology to diagnosis and treatment. Int. J. Med. Sci. 2017, 14, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Soyfoo, M.S.; Steinfeld, S.; Delporte, C. Usefulness of mouse models to study the pathogenesis of Sjogren’s syndrome. Oral Dis. 2007, 13, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.S.; Gauna, A.E.; Cha, S. Mouse Models of Primary Sjogren’s Syndrome. Curr. Pharm. Des. 2015, 21, 2350–2364. [Google Scholar] [CrossRef] [PubMed]
- Peck, A.B.; Nguyen, C.Q. What can Sjogren’s syndrome-like disease in mice contribute to human Sjogren’s syndrome? Clin. Immunol. 2017, 182, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Jin, J.O.; Kawai, T.; Yu, Q. Endogenous programmed death ligand-1 restrains the development and onset of Sjgren’s syndrome in non-obese diabetic mice. Sci. Rep. 2016, 6, 39105. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.K.; Siddiqui, A.A.; Modica, L.A.; Dykes, R.; Simmons, C.; Schmidt, J.; Krishnaswamy, G.A.; Berk, S.L. Interferon-alpha upregulates gene expression of aquaporin-5 in human parotid glands. J. Interferon Cytokine Res. 1999, 19, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Papinska, J.; Bagavant, H.; Gmyrek, G.B.; Sroka, M.; Tummala, S.; Fitzgerald, K.A.; Deshmukh, U.S. Activation of Stimulator of Interferon Genes (STING) and Sjogren Syndrome. J. Dent. Res. 2018, 97, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yu, Q. Anti-IL-7 receptor-alpha treatment ameliorates newly established Sjogren’s-like exocrinopathy in non-obese diabetic mice. Biochim. Biophys. Acta 2018, 1864, 2438–2447. [Google Scholar] [CrossRef] [PubMed]
- Nandula, S.R.; Amarnath, S.; Molinolo, A.; Bandyopadhyay, B.C.; Hall, B.; Goldsmith, C.M.; Zheng, C.; Larsson, J.; Sreenath, T.; Chen, W.; et al. Female mice are more susceptible to developing inflammatory disorders due to impaired transforming growth factor beta signaling in salivary glands. Arthritis Rheum. 2007, 56, 1798–1805. [Google Scholar] [CrossRef] [PubMed]
- Ring, T.; Kallenbach, M.; Praetorius, J.; Nielsen, S.; Melgaard, B. Successful treatment of a patient with primary Sjogren’s syndrome with Rituximab. Clin. Rheumatol. 2006, 25, 891–894. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.S.; Wee, Y.; Yang, C.H.; Melvin, J.E.; Baker, O.J. ALX/FPR2 Modulates Anti-Inflammatory Responses in Mouse Submandibular Gland. Sci. Rep. 2016, 6, 24244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beroukas, D.; Hiscock, J.; Gannon, B.J.; Jonsson, R.; Gordon, T.P.; Waterman, S.A. Selective down-regulation of aquaporin-1 in salivary glands in primary Sjogren’s syndrome. Lab. Investig. 2002, 82, 1547–1552. [Google Scholar] [CrossRef] [PubMed]
- Lennon, V.A.; Kryzer, T.J.; Pittock, S.J.; Verkman, A.S.; Hinson, S.R. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J. Exp. Med. 2005, 202, 473–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tzartos, J.S.; Stergiou, C.; Kilidireas, K.; Zisimopoulou, P.; Thomaidis, T.; Tzartos, S.J. Anti-aquaporin-1 autoantibodies in patients with neuromyelitis optica spectrum disorders. PLoS ONE 2013, 8, e74773. [Google Scholar] [CrossRef] [PubMed]
- Tuzun, E.; Tzartos, J.; Ekizoglu, E.; Stergiou, C.; Zisimopoulou, P.; Coban, A.; Shugaiv, E.; Turkoglu, R.; Kurtuncu, M.; Baykan, B.; et al. Aquaporin-1 antibody in neuromyelitis optical patients. Eur. Neurol. 2014, 72, 271–272. [Google Scholar] [CrossRef] [PubMed]
- Patterson, S.L.; Goglin, S.E. Neuromyelitis Optica. Rheum. Dis. Clin. N. Am. 2017, 43, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Lennon, V.A.; Wingerchuk, D.M.; Kryzer, T.J.; Pittock, S.J.; Lucchinetti, C.F.; Fujihara, K.; Nakashima, I.; Weinshenker, B.G. A serum autoantibody marker of neuromyelitis optica: Distinction from multiple sclerosis. Lancet 2004, 364, 2106–2112. [Google Scholar] [CrossRef]
- Lucchinetti, C.F.; Guo, Y.; Popescu, B.F.; Fujihara, K.; Itoyama, Y.; Misu, T. The pathology of an autoimmune astrocytopathy: Lessons learned from neuromyelitis optica. Brain Pathol. 2014, 24, 83–97. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, M.C.; Verkman, A.S. Aquaporin 4 and neuromyelitis optica. Lancet Neurol. 2012, 11, 535–544. [Google Scholar] [CrossRef] [Green Version]
- Tzartos, J.S.; Stergiou, C.; Daoussis, D.; Zisimopoulou, P.; Andonopoulos, A.P.; Zolota, V.; Tzartos, S.J. Antibodies to aquaporins are frequent in patients with primary Sjogren’s syndrome. Rheumatology 2017, 56, 2114–2122. [Google Scholar] [CrossRef] [PubMed]
- Alam, J.; Koh, J.H.; Kim, N.; Kwok, S.K.; Park, S.H.; Song, Y.W.; Park, K.; Choi, Y. Detection of autoantibodies against aquaporin-5 in the sera of patients with primary Sjogren’s syndrome. Immunol. Res. 2016, 64, 848–856. [Google Scholar] [CrossRef] [PubMed]
- Tradtrantip, L.; Zhang, H.; Saadoun, S.; Phuan, P.W.; Lam, C.; Papadopoulos, M.C.; Bennett, J.L.; Verkman, A.S. Anti-aquaporin-4 monoclonal antibody blocker therapy for neuromyelitis optica. Ann. Neurol. 2012, 71, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Proctor, G.B.; Carpenter, G.H. Regulation of salivary gland function by autonomic nerves. Auton. Neurosci. 2007, 133, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Ambudkar, I.S. Ca(2)(+) signaling and regulation of fluid secretion in salivary gland acinar cells. Cell Calcium 2014, 55, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Ambudkar, I.S. Calcium signaling defects underlying salivary gland dysfunction. Biochem. Biophys. Acta 2018, 1865, 1771–1777. [Google Scholar] [CrossRef] [PubMed]
- Derouiche, S.; Takayama, Y.; Murakami, M.; Tominaga, M. TRPV4 heats up ANO1-dependent exocrine fluid secretion. FASEB J. 2018, 32, 1841–1854. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, T.; Suzuki, T.; Koyama, H.; Tanaka, S.; Takata, K. Aquaporin-5 (AQP5), a water channel protein, in the rat salivary and lacrimal glands: Immunolocalization and effect of secretory stimulation. Cell Tissue Res. 1999, 295, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Cho, G.; Bragiel, A.M.; Wang, D.; Pieczonka, T.D.; Skowronski, M.T.; Shono, M.; Nielsen, S.; Ishikawa, Y. Activation of muscarinic receptors in rat parotid acinar cells induces AQP5 trafficking to nuclei and apical plasma membrane. Biochim. Biophys. Acta 2015, 1850, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Salum, F.G.; Medella-Junior, F.A.C.; Figueiredo, M.A.Z.; Cherubini, K. Salivary hypofunction: An update on therapeutic strategies. Gerodontology 2018. [Google Scholar] [CrossRef] [PubMed]
- Mercadante, V.; Al Hamad, A.; Lodi, G.; Porter, S.; Fedele, S. Interventions for the management of radiotherapy-induced xerostomia and hyposalivation: A systematic review and meta-analysis. Oral Oncol. 2017, 66, 64–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamad, A.A.; Lodi, G.; Porter, S.; Fedele, S.; Mercadante, V. Interventions for dry mouth and hyposalivation in Sjogren’s syndrome: A systematic review and meta-analysis. Oral Dis. 2018. [Google Scholar] [CrossRef] [PubMed]
- Takakura, K.; Takaki, S.; Takeda, I.; Hanaue, N.; Kizu, Y.; Tonogi, M.; Yamane, G.Y. Effect of cevimeline on radiation-induced salivary gland dysfunction and AQP5 in submandibular gland in mice. Bull. Tokyo Dent. Coll. 2007, 48, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, H.; Yakeishi, A.; Saga, T.; Yamaki, K. Effects of cevimeline on the immunolocalization of aquaporin-5 and the ultrastructure of salivary glands in Sjogren’s syndrome model mice. Kurume Med. J. 2009, 56, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Saga, T.; Watanabe, K.; Takahashi, N.; Tabira, Y.; Kusukawa, J.; Yamaki, K. An immunohistochemistry-based study on aquaporin (AQP)-1, 3, 4, 5 and 8 in the parotid glands, submandibular glands and sublingual glands of Sjogren’s syndrome mouse models chronically administered cevimeline. Kurume Med. J. 2013, 60, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Szymczak, M.; Ahuja, M.; Zheng, C.; Yin, H.; Swaim, W.; Chiorini, J.A.; Bridges, R.J.; Muallem, S. Restoration of CFTR Activity in Ducts Rescues Acinar Cell Function and Reduces Inflammation in Pancreatic and Salivary Glands of Mice. Gastroenterology 2017, 153, 1148–1159. [Google Scholar] [CrossRef] [PubMed]
- Samuni, Y.; Baum, B.J. Gene delivery in salivary glands: From the bench to the clinic. Biochim. Biophys. Acta 2011, 1812, 1515–1521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delporte, C.; O’Connell, B.C.; He, X.; Lancaster, H.E.; O’Connell, A.C.; Agre, P.; Baum, B.J. Increased fluid secretion after adenoviral-mediated transfer of the aquaporin-1 cDNA to irradiated rat salivary glands. Proc. Natl. Acad. Sci. USA 1997, 94, 3268–3273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teos, L.Y.; Zheng, C.Y.; Liu, X.; Swaim, W.D.; Goldsmith, C.M.; Cotrim, A.P.; Baum, B.J.; Ambudkar, I.S. Adenovirus-mediated hAQP1 expression in irradiated mouse salivary glands causes recovery of saliva secretion by enhancing acinar cell volume decrease. Gene Ther. 2016, 23, 572–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shan, Z.; Li, J.; Zheng, C.; Liu, X.; Fan, Z.; Zhang, C.; Goldsmith, C.M.; Wellner, R.B.; Baum, B.J.; Wang, S. Increased fluid secretion after adenoviral-mediated transfer of the human aquaporin-1 cDNA to irradiated miniature pig parotid glands. Mol. Ther. 2005, 11, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Yan, X.; Zheng, C.; Goldsmith, C.M.; Afione, S.; Hai, B.; Xu, J.; Zhou, J.; Zhang, C.; Chiorini, J.A.; et al. AAV2-mediated transfer of the human aquaporin-1 cDNA restores fluid secretion from irradiated miniature pig parotid glands. Gene Ther. 2011, 18, 38–42. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, A.C.; Baccaglini, L.; Fox, P.C.; O’Connell, B.C.; Kenshalo, D.; Oweisy, H.; Hoque, A.T.; Sun, D.; Herscher, L.L.; Braddon, V.R.; et al. Safety and efficacy of adenovirus-mediated transfer of the human aquaporin-1 cDNA to irradiated parotid glands of non-human primates. Cancer Gene Ther. 1999, 6, 505–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baum, B.J.; Alevizos, I.; Zheng, C.; Cotrim, A.P.; Liu, S.; McCullagh, L.; Goldsmith, C.M.; Burbelo, P.D.; Citrin, D.E.; Mitchell, J.B.; et al. Early responses to adenoviral-mediated transfer of the aquaporin-1 cDNA for radiation-induced salivary hypofunction. Proc. Natl. Acad. Sci. USA 2012, 109, 19403–19407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alevizos, I.; Zheng, C.; Cotrim, A.P.; Liu, S.; McCullagh, L.; Billings, M.E.; Goldsmith, C.M.; Tandon, M.; Helmerhorst, E.J.; Catalan, M.A.; et al. Late responses to adenoviral-mediated transfer of the aquaporin-1 gene for radiation-induced salivary hypofunction. Gene Ther. 2017, 24, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Alevizos, I.; Zheng, C.; Cotrim, A.P.; Goldsmith, C.M.; McCullagh, L.; Berkowitz, T.; Strobl, S.L.; Malyguine, A.; Kopp, W.C.; Chiorini, J.A.; et al. Immune reactivity after adenoviral-mediated aquaporin-1 cDNA transfer to human parotid glands. Oral Dis. 2017, 23, 337–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Zourelias, L.; Wu, C.; Edwards, P.C.; Trombetta, M.; Passineau, M.J. Ultrasound-assisted nonviral gene transfer of AQP1 to the irradiated minipig parotid gland restores fluid secretion. Gene Ther. 2015, 22, 739–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, M.; Kuroda, S.; Mansjur, K.Q.; Khaliunaa, G.; Nagata, K.; Horiuchi, S.; Inubushi, T.; Yamamura, Y.; Azuma, M.; Tanaka, E. Low-intensity pulsed ultrasound rescues insufficient salivary secretion in autoimmune sialadenitis. Arthritis Res. Ther. 2015, 17, 278. [Google Scholar] [CrossRef] [PubMed]
- Lai, Z.; Yin, H.; Cabrera-Perez, J.; Guimaro, M.C.; Afione, S.; Michael, D.G.; Glenton, P.; Patel, A.; Swaim, W.D.; Zheng, C.; et al. Aquaporin gene therapy corrects Sjogren’s syndrome phenotype in mice. Proc. Natl. Acad. Sci. USA 2016, 113, 5694–5699. [Google Scholar] [CrossRef] [PubMed]
- Hai, B.; Zhao, Q.; Deveau, M.A.; Liu, F. Delivery of Sonic Hedgehog Gene Repressed Irradiation-induced Cellular Senescence in Salivary Glands by Promoting DNA Repair and Reducing Oxidative Stress. Theranostics 2018, 8, 1159–1167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.J.; Lee, Y.J.; Kwon, H.C.; Bae, S.; Kim, S.H.; Min, J.J.; Cho, C.K.; Lee, Y.S. Radioprotective effect of heat shock protein 25 on submandibular glands of rats. Am. J. Pathol. 2006, 169, 1601–1611. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.P.; Sunavala-Dossabhoy, G. Promising Gene Therapeutics for Salivary Gland Radiotoxicity. AIMS Med. Sci. 2016, 3, 329–344. [Google Scholar] [CrossRef] [PubMed]
- Aure, M.H.; Konieczny, S.F.; Ovitt, C.E. Salivary gland homeostasis is maintained through acinar cell self-duplication. Dev. Cell 2015, 33, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Vissink, A.; van Luijk, P.; Langendijk, J.A.; Coppes, R.P. Current ideas to reduce or salvage radiation damage to salivary glands. Oral Dis. 2015, 21, e1–e10. [Google Scholar] [CrossRef] [PubMed]
- Kagami, H. The potential use of cell-based therapies in the treatment of oral diseases. Oral Dis. 2015, 21, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Lombaert, I.; Movahednia, M.M.; Adine, C.; Ferreira, J.N. Concise Review: Salivary Gland Regeneration: Therapeutic Approaches from Stem Cells to Tissue Organoids. Stem Cells 2017, 35, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.N.; Rungarunlert, S.; Urkasemsin, G.; Adine, C.; Souza, G.R. Three-Dimensional Bioprinting Nanotechnologies towards Clinical Application of Stem Cells and Their Secretome in Salivary Gland Regeneration. Stem Cells Int. 2016, 2016, 7564689. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, N.; Honda, Y.; Momota, Y.; Tran, S.D. Dedifferentiated Fat (DFAT) cells: A cell source for oral and maxillofacial tissue engineering. Oral Dis. 2018. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soyfoo, M.S.; Chivasso, C.; Perret, J.; Delporte, C. Involvement of Aquaporins in the Pathogenesis, Diagnosis and Treatment of Sjögren’s Syndrome. Int. J. Mol. Sci. 2018, 19, 3392. https://doi.org/10.3390/ijms19113392
Soyfoo MS, Chivasso C, Perret J, Delporte C. Involvement of Aquaporins in the Pathogenesis, Diagnosis and Treatment of Sjögren’s Syndrome. International Journal of Molecular Sciences. 2018; 19(11):3392. https://doi.org/10.3390/ijms19113392
Chicago/Turabian StyleSoyfoo, Muhammad Shahnawaz, Clara Chivasso, Jason Perret, and Christine Delporte. 2018. "Involvement of Aquaporins in the Pathogenesis, Diagnosis and Treatment of Sjögren’s Syndrome" International Journal of Molecular Sciences 19, no. 11: 3392. https://doi.org/10.3390/ijms19113392
APA StyleSoyfoo, M. S., Chivasso, C., Perret, J., & Delporte, C. (2018). Involvement of Aquaporins in the Pathogenesis, Diagnosis and Treatment of Sjögren’s Syndrome. International Journal of Molecular Sciences, 19(11), 3392. https://doi.org/10.3390/ijms19113392