Ultrastructural and Molecular Analysis of Ribose-Induced Glycated Reconstructed Human Skin
Abstract
:1. Introduction
2. Results
2.1. Selection of Glycation Agent
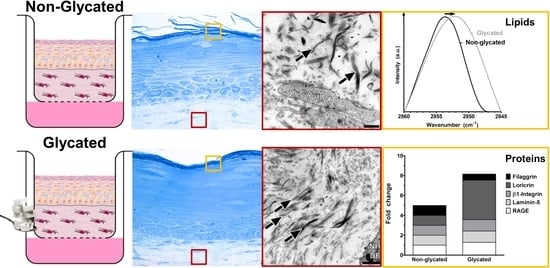
2.2. RHS Morphology
2.3. Dermal Ultrastructure
2.4. Epidermal Ultrastructure
2.5. Molecular Analysis
2.6. Skin Barrier Function
3. Discussion
4. Materials and Methods
4.1. Collagen Glycation
4.2. Cell Culture and Viability
4.3. Reconstructed Human Skin (RHS)
4.4. Reflectance Confocal Microscopy (RCM) and Immunolocalization of Proteins
4.5. Transmission Electron Microscopy (TEM)
4.6. Carboxymethyl Lysine Quantification
4.7. Stratum Corneum Lipid Analysis
4.8. Skin Permeation
4.9. Penetration of Bodipy Loaded Dendritic Core-Multishell Nanotransporters (Bodipy-CMS)
4.10. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AGEs | Advanced Glycated End-products |
| CML | N-CarboxyMethyl-Lysine |
| CMS | Dendritic Core-Multishell |
| DAPI | 4′,6-DiAmidino-2-PhenylIndole |
| ECM | ExtraCellular Matrix |
| HPTLC | High-Performance Thin-Layer Chromatography |
| NHDF | Normal Human Dermal Fibroblast |
| NHK | Normal Human Keratinocytes |
| PBS | Phosphate-Buffered Saline |
| RAGE | Receptor of AGEs |
| RHS | Reconstructed Human Skin |
| SDS | Sodium Dodecyl Sulphate |
| TEM | Transmission Electron Microscopy |
References
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Reeve, E.; Trenaman, S.C.; Rockwood, K.; Hilmer, S.N. Pharmacokinetic and pharmacodynamic alterations in older people with dementia. Expert Opin. Drug Metab. Toxicol. 2017, 13, 651–668. [Google Scholar] [CrossRef] [PubMed]
- Papagrigoraki, A.; Del Giglio, M.; Cosma, C.; Maurelli, M.; Girolomoni, G.; Lapolla, A. Advanced Glycation End Products are Increased in the Skin and Blood of Patients with Severe Psoriasis. Acta Derm.-Venereol. 2017, 97, 782–787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lima, A.L.; Illing, T.; Schliemann, S.; Elsner, P. Cutaneous Manifestations of Diabetes Mellitus: A Review. Am. J. Clin. Dermatol. 2017, 18, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Verzijl, N.; DeGroot, J.; Thorpe, S.R.; Bank, R.A.; Shaw, J.N.; Lyons, T.J.; Bijlsma, J.W.; Lafeber, F.P.; Baynes, J.W.; TeKoppele, J.M. Effect of collagen turnover on the accumulation of advanced glycation end products. J. Biol. Chem. 2000, 275, 39027–39031. [Google Scholar] [CrossRef] [PubMed]
- Pageon, H.; Zucchi, H.; Dai, Z.; Sell, D.R.; Strauch, C.M.; Monnier, V.M.; Asselineau, D. Biological Effects Induced by Specific Advanced Glycation End Products in the Reconstructed Skin Model of Aging. BioRes. Open Access 2015, 4, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Yokota, M.; Tokudome, Y. The Effect of Glycation on Epidermal Lipid Content, Its Metabolism and Change in Barrier Function. Skin Pharmacol. Physiol. 2016, 29, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Cadau, S.; Leoty-Okombi, S.; Pain, S.; Bechetoille, N.; Andre-Frei, V.; Berthod, F. In vitro glycation of an endothelialized and innervated tissue-engineered skin to screen anti-AGE molecules. Biomaterials 2015, 51, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Pennacchi, P.C.; de Almeida, M.E.; Gomes, O.L.; Faiao-Flores, F.; de Araujo Crepaldi, M.C.; Dos Santos, M.F.; de Moraes Barros, S.B.; Maria-Engler, S.S. Glycated Reconstructed Human Skin as a Platform to Study the Pathogenesis of Skin Aging. Tissue Eng. Part A 2015, 21, 2417–2425. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, H.; Gu, L.; Zeng, H.; Maeda, K. Amino Carbonylation of Epidermal Basement Membrane Inhibits Epidermal Cell Function and Is Suppressed by Methylparaben. Cosmetics 2017, 4, 38. [Google Scholar] [CrossRef]
- Käßmeyer, S.; Sehl, J.; Khiao In, M.; Merle, R.; Richardson, K.; Plendl, J. Subcellular interactions during vascular morphogenesis in 3D cocultures between endothelial cells and fibroblasts. Int. J. Mol. Sci. 2017, 18, 2590. [Google Scholar] [CrossRef] [PubMed]
- Longo, C.; Casari, A.; De Pace, B.; Simonazzi, S.; Mazzaglia, G.; Pellacani, G. Proposal for an in vivo histopathologic scoring system for skin aging by means of confocal microscopy. Skin Res. Technol. 2013, 19, e167–e173. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, M.; Lange-Asschenfeldt, S.; Gonzalez, S. Clinical applicability of in vivo reflectance confocal microscopy in dermatology. G. Ital. Dermatol. Venereol. 2012, 147, 171–178. [Google Scholar] [PubMed]
- Lewis, R.N.; McElhaney, R.N. Fourier transform infrared spectroscopy in the study of lipid phase transitions in model and biological membranes: Practical considerations. Methods Mol. Biol. 2007, 400, 207–226. [Google Scholar] [CrossRef] [PubMed]
- Zoschke, C.; Ulrich, M.; Sochorova, M.; Wolff, C.; Vavrova, K.; Ma, N.; Ulrich, C.; Brandner, J.M.; Schafer-Korting, M. The barrier function of organotypic non-melanoma skin cancer models. J. Control. Release 2016, 233, 10–18. [Google Scholar] [CrossRef] [PubMed]
- van den Bogaard, E.H.; Tjabringa, G.S.; Joosten, I.; Vonk-Bergers, M.; van Rijssen, E.; Tijssen, H.J.; Erkens, M.; Schalkwijk, J.; Koenen, H.J. Crosstalk between keratinocytes and T cells in a 3D microenvironment: A model to study inflammatory skin diseases. J. Investig. Dermatol. 2014, 134, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Danso, M.O.; van Drongelen, V.; Mulder, A.; van Esch, J.; Scott, H.; van Smeden, J.; El Ghalbzouri, A.; Bouwstra, J.A. TNF-alpha and Th2 cytokines induce atopic dermatitis-like features on epidermal differentiation proteins and stratum corneum lipids in human skin equivalents. J. Investig. Dermatol. 2014, 134, 1941–1950. [Google Scholar] [CrossRef] [PubMed]
- Khalifah, R.G.; Todd, P.; Booth, A.A.; Yang, S.X.; Mott, J.D.; Hudson, B.G. Kinetics of nonenzymatic glycation of ribonuclease A leading to advanced glycation end products. Paradoxical inhibition by ribose leads to facile isolation of protein intermediate for rapid post-Amadori studies. Biochemistry 1996, 35, 4645–4654. [Google Scholar] [CrossRef] [PubMed]
- Luers, L.; Rysiewski, K.; Dumpitak, C.; Birkmann, E. Kinetics of advanced glycation end products formation on bovine serum albumin with various reducing sugars and dicarbonyl compounds in equimolar ratios. Rejuv. Res. 2012, 15, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Pageon, H.; Techer, M.P.; Asselineau, D. Reconstructed skin modified by glycation of the dermal equivalent as a model for skin aging and its potential use to evaluate anti-glycation molecules. Exp. Gerontol. 2008, 43, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Brings, S.; Fleming, T.; Freichel, M.; Muckenthaler, M.; Herzig, S.; Nawroth, P. Dicarbonyls and Advanced Glycation End-Products in the Development of Diabetic Complications and Targets for Intervention. Int. J. Mol. Sci. 2017, 18, 984. [Google Scholar] [CrossRef] [PubMed]
- Rinnerthaler, M.; Streubel, M.K.; Bischof, J.; Richter, K. Skin aging, gene expression and calcium. Exp. Gerontol. 2015, 68, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Pageon, H.; Bakala, H.; Monnier, V.M.; Asselineau, D. Collagen glycation triggers the formation of aged skin in vitro. Eur. J. Dermatol. 2007, 17, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Maas-Szabowski, N.; Szabowski, A.; Stark, H.J.; Andrecht, S.; Kolbus, A.; Schorpp-Kistner, M.; Angel, P.; Fusenig, N.E. Organotypic cocultures with genetically modified mouse fibroblasts as a tool to dissect molecular mechanisms regulating keratinocyte growth and differentiation. J. Investig. Dermatol. 2001, 116, 816–820. [Google Scholar] [CrossRef] [PubMed]
- Löwa, A.; Vogt, A.; Kaessmeyer, S.; Hedtrich, S. Generation of full-thickness skin equivalents using hair follicle-derived primary human keratinocytes and fibroblasts. J. Tissue Eng. Regener. Med. 2018, 12, e2134–e2146. [Google Scholar] [CrossRef] [PubMed]
- Breitkreutz, D.; Koxholt, I.; Thiemann, K.; Nischt, R. Skin basement membrane: The foundation of epidermal integrity—BM functions and diverse roles of bridging molecules nidogen and perlecan. BioMed Res. Int. 2013, 2013, 179784. [Google Scholar] [CrossRef] [PubMed]
- Candiello, J.; Cole, G.J.; Halfter, W. Age-dependent changes in the structure, composition and biophysical properties of a human basement membrane. Matrix Biol. 2010, 29, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Wolberink, E.A.; van Erp, P.E.; Teussink, M.M.; van de Kerkhof, P.C.; Gerritsen, M.J. Cellular features of psoriatic skin: Imaging and quantification using in vivo reflectance confocal microscopy. Cytom. Part B Clin. Cytom. 2011, 80, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Brody, I. The ultrastructure of the epidermis in psoriasis vulgaris as revealed by electron microscopy. 1. The dermo-epidermal junction and the stratum basale in parakeratosis without keratohyalin. J. Ultrastruct. Res. 1962, 6, 304–323. [Google Scholar] [CrossRef]
- Steven, A.C.; Bisher, M.E.; Roop, D.R.; Steinert, P.M. Biosynthetic pathways of filaggrin and loricrin—Two major proteins expressed by terminally differentiated epidermal keratinocytes. J. Struct. Biol. 1990, 104, 150–162. [Google Scholar] [CrossRef]
- Alnasif, N.; Zoschke, C.; Fleige, E.; Brodwolf, R.; Boreham, A.; Rühl, E.; Eckl, K.M.; Merk, H.F.; Hennies, H.C.; Alexiev, U.; et al. Penetration of normal, damaged and diseased skin—An in vitro study on dendritic core-multishell nanotransporters. J. Control. Release 2014, 185c, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Pageon, H.; Asselineau, D. An in vitro approach to the chronological aging of skin by glycation of the collagen: The biological effect of glycation on the reconstructed skin model. Ann. N. Y. Acad. Sci. 2005, 1043, 529–532. [Google Scholar] [CrossRef] [PubMed]
- Turksen, K.; Kupper, T.; Degenstein, L.; Williams, I.; Fuchs, E. Interleukin 6: Insights to its function in skin by overexpression in transgenic mice. Proc. Natl. Acad. Sci. USA 1992, 89, 5068–5072. [Google Scholar] [CrossRef] [PubMed]
- Sakai, T.; Hatano, Y.; Zhang, W.; Fujiwara, S.; Nishiyori, R. Knockdown of either filaggrin or loricrin increases the productions of interleukin (IL)-1alpha, IL-8, IL-18 and granulocyte macrophage colony-stimulating factor in stratified human keratinocytes. J. Dermatol. Sci. 2015, 80, 158–160. [Google Scholar] [CrossRef] [PubMed]
- Brody, I. The ultrastructure of the epidermis in psoriasis vulgaris as revealed by electron microscopy: 4. Stratum corneum in parakeratosis without keretohyalin. J. Ultrastruct. Res. 1962, 6, 354–367. [Google Scholar] [CrossRef]
- Gould, A.R.; Sharp, P.J.; Smith, D.R.; Stegink, A.J.; Chase, C.J.; Kovacs, J.C.; Penglis, S.; Chatterton, B.E.; Bunn, C.L. Increased permeability of psoriatic skin to the protein, plasminogen activator inhibitor 2. Arch. Dermatol. Res. 2003, 295, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Papagrigoraki, A.; Maurelli, M.; del Giglio, M.; Gisondi, P.; Girolomoni, G. Advanced Glycation End Products in the Pathogenesis of Psoriasis. Int. J. Mol. Sci. 2017, 18, 2471. [Google Scholar] [CrossRef] [PubMed]
- Gerecke, C.; Edlich, A.; Giulbudagian, M.; Schumacher, F.; Zhang, N.; Said, A.; Yealland, G.; Lohan, S.B.; Neumann, F.; Meinke, M.C.; et al. Biocompatibility and characterization of polyglycerol-based thermoresponsive nanogels designed as novel drug-delivery systems and their intracellular localization in keratinocytes. Nanotoxicology 2017, 11, 267–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richardson, K.C.; Jarett, L.; Finke, E.H. Embedding in epoxy resins for ultrathin sectioning in electron microscopy. Stain Technol. 1960, 35, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Vavrova, K.; Henkes, D.; Struver, K.; Sochorova, M.; Skolova, B.; Witting, M.Y.; Friess, W.; Schreml, S.; Meier, R.J.; Schafer-Korting, M.; et al. Filaggrin deficiency leads to impaired lipid profile and altered acidification pathways in a 3D skin construct. J. Investig. Dermatol. 2014, 134, 746–753. [Google Scholar] [CrossRef] [PubMed]
- Opálka, L.; Kováčik, A.; Sochorová, M.; Roh, J.; Kuneš, J.; Lenčo, J.; Vávrová, K. Scalable synthesis of human ultralong chain ceramides. Org. Lett. 2015, 17, 5456–5459. [Google Scholar] [CrossRef] [PubMed]
- Kováčik, A.; Opálka, L.; Šilarová, M.; Roh, J.; Vávrová, K. Synthesis of 6-hydroxyceramide using ruthenium-catalyzed hydrosilylation-protodesilylation. Unexpected formation of a long periodicity lamellar phase in skin lipid membranes. RSC Adv. 2016, 6, 73343–73350. [Google Scholar] [CrossRef]
- Radowski, M.R.; Shukla, A.; von Berlepsch, H.; Bottcher, C.; Pickaert, G.; Rehage, H.; Haag, R. Supramolecular aggregates of dendritic multishell architectures as universal nanocarriers. Angew. Chem. Int. Ed. Engl. 2007, 46, 1265–1269. [Google Scholar] [CrossRef] [PubMed]









| Lipid Standard | Calibration Curve Range [µg] | |
|---|---|---|
| FFA | Lignoceric acid | 0.5–12.5 |
| Chol | Cholesterol | 0.5–12.5 |
| Cer | Ceramide EOS | 0.05–1.25 |
| Ceramide NS | 0.2–5 | |
| Ceramide NP | 0.4–9.5 | |
| Ceramide AS | 0.1–2.5 | |
| Ceramide NH | 0.15–4 | |
| Ceramide AP | 0.1–2.5 | |
| CholS | Cholesterol sulphate | 0.105–2.474 |
| SM | Sphingomyelin | 0.211–4.947 |
| GCer | Glucosylceramide | 0.263–6.184 |
| PL | Phospholipid | 0.421–9.895 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balansin Rigon, R.; Kaessmeyer, S.; Wolff, C.; Hausmann, C.; Zhang, N.; Sochorová, M.; Kováčik, A.; Haag, R.; Vávrová, K.; Ulrich, M.; et al. Ultrastructural and Molecular Analysis of Ribose-Induced Glycated Reconstructed Human Skin. Int. J. Mol. Sci. 2018, 19, 3521. https://doi.org/10.3390/ijms19113521
Balansin Rigon R, Kaessmeyer S, Wolff C, Hausmann C, Zhang N, Sochorová M, Kováčik A, Haag R, Vávrová K, Ulrich M, et al. Ultrastructural and Molecular Analysis of Ribose-Induced Glycated Reconstructed Human Skin. International Journal of Molecular Sciences. 2018; 19(11):3521. https://doi.org/10.3390/ijms19113521
Chicago/Turabian StyleBalansin Rigon, Roberta, Sabine Kaessmeyer, Christopher Wolff, Christian Hausmann, Nan Zhang, Michaela Sochorová, Andrej Kováčik, Rainer Haag, Kateřina Vávrová, Martina Ulrich, and et al. 2018. "Ultrastructural and Molecular Analysis of Ribose-Induced Glycated Reconstructed Human Skin" International Journal of Molecular Sciences 19, no. 11: 3521. https://doi.org/10.3390/ijms19113521
APA StyleBalansin Rigon, R., Kaessmeyer, S., Wolff, C., Hausmann, C., Zhang, N., Sochorová, M., Kováčik, A., Haag, R., Vávrová, K., Ulrich, M., Schäfer-Korting, M., & Zoschke, C. (2018). Ultrastructural and Molecular Analysis of Ribose-Induced Glycated Reconstructed Human Skin. International Journal of Molecular Sciences, 19(11), 3521. https://doi.org/10.3390/ijms19113521