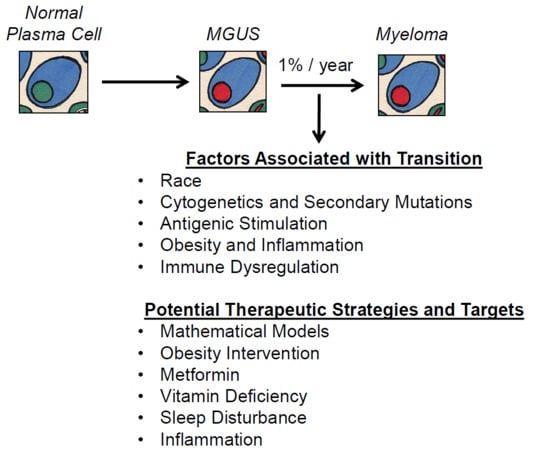
Prevention Is the Best Treatment: The Case for Understanding the Transition from Monoclonal Gammopathy of Undetermined Significance to Myeloma
Abstract
:1. Multiple Myeloma Is a Terminal Disease that Proceeds Through a Requisite Pre-Malignant Stage
2. MGUS Itself Is Not a Benign Condition
3. Factors Associated with the MGUS to MM Transition
3.1. Race as a Risk Factor in MM
3.2. Prognostic Value of Cytogenetics and Role of Secondary Mutations in Driving the MGUS to MM Transition
3.3. Uniquely Dysregulated Genes in Plasma Cells of MGUS
3.4. Antigenic Stimulation
3.5. Obesity and Inflammation
3.6. Immune Dysregulation
4. Potential Therapeutic Targets and Strategies in Preventing Myeloma
4.1. Use of Mathematical Models in Studying Prevention
4.2. Obesity Intervention
4.3. Metformin
4.4. Vitamin Deficiency
4.5. Sleep Disturbance and Intermittent Hypoxia
4.6. Additional Modifiers of the Inflammatory Response
5. If Treatments Are Improving, Why Study Prevention in Myeloma?
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Rajkumar, S.V.; Dimopoulos, M.A.; Palumbo, A.; Blade, J.; Merlini, G.; Mateos, M.V.; Kumar, S.; Hillengass, J.; Kastritis, E.; Richardson, P.; et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014, 15, e538–e548. [Google Scholar] [CrossRef] [Green Version]
- Ravi, P.; Kumar, S.K.; Cerhan, J.R.; Maurer, M.J.; Dingli, D.; Ansell, S.M.; Rajkumar, S.V. Defining cure in multiple myeloma: A comparative study of outcomes of young individuals with myeloma and curable hematologic malignancies. Blood Cancer J. 2018, 8, 26. [Google Scholar] [CrossRef] [PubMed]
- Blimark, C.H.; Turesson, I.; Genell, A.; Ahlberg, L.; Björkstrand, B.; Carlson, K.; Forsberg, K.; Juliusson, G.; Linder, O.; Mellqvist, U.-H.; et al. Outcome and survival of myeloma patients diagnosed 2008–2015. Real-world data on 4904 patients from the Swedish Myeloma Registry. Haematologica 2018, 103, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Pulte, D.; Jansen, L.; Castro, F.A.; Emrich, K.; Katalinic, A.; Holleczek, B.; Brenner, H. Trends in survival of multiple myeloma patients in Germany and the United States in the first decade of the 21st century. Br. J. Haematol. 2015, 171, 189–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turesson, I.; Bjorkholm, M.; Blimark, C.H.; Kristinsson, S.; Velez, R.; Landgren, O. Rapidly changing myeloma epidemiology in the general population: Increased incidence, older patients, and longer survival. Eur. J. Haematol. 2018. Available online: https://onlinelibrary.wiley.com/doi/full/10.1111/ejh.13083 (accessed on 20 April 2018).
- Rajkumar, S.V. Prevention of progression in monoclonal gammopathy of undetermined significance. Clin. Cancer Res. 2009, 15, 5606–5608. [Google Scholar] [CrossRef] [PubMed]
- Meattini, I.; Pasinetti, N.; Meduri, B.; De Rose, F.; De Santis, M.C.; Franco, P.; Lancellotta, V.; Rossi, F.; Saieva, C.; Desideri, I.; et al. A national multicenter study on 1072 DCIS patients treated with breast-conserving surgery and whole breast radiotherapy (COBCG-01 study). Radiother. Oncol. 2018. Available online: https://www.sciencedirect.com/science/article/pii/S0167814018334145 (accessed on 31 July 2018).
- Cuzick, J.; Sestak, I.; Forbes, J.F.; Dowsett, M.; Knox, J.; Cawthorn, S.; Saunders, C.; Roche, N.; Mansel, R.E.; von Minckwitz, G.; et al. Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): An international, double-blind, randomised placebo-controlled trial. The Lancet 2014, 383, 1041–1048. [Google Scholar] [CrossRef]
- Cuzick, J.; Sestak, I.; Cawthorn, S.; Hamed, H.; Holli, K.; Howell, A.; Forbes, J.F. Tamoxifen for prevention of breast cancer: Extended long-term follow-up of the IBIS-I breast cancer prevention trial. Lancet Oncol. 2015, 16, 67–75. [Google Scholar] [CrossRef]
- Landgren, O.; Graubard, B.I.; Kumar, S.; Kyle, R.A.; Katzmann, J.A.; Murata, K.; Costello, R.; Dispenzieri, A.; Caporaso, N.; Mailankody, S.; et al. Prevalence of myeloma precursor state monoclonal gammopathy of undetermined significance in 12372 individuals 10-49 years old: A population-based study from the National Health and Nutrition Examination Survey. Blood Cancer J. 2017, 7, e618. [Google Scholar] [CrossRef] [PubMed]
- Vernocchi, A.; Longhi, E.; Lippi, G.; Gelsumini, S. Increased Monoclonal Components: Prevalence in an Italian Population of 44 474 Outpatients Detected by Capillary Electrophoresis. J. Med. Biochem. 2016, 35, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Kyle, R.A.; Rajkumar, S.V. Monoclonal gammopathy of undetermined significance. Br. J. Haematol. 2006, 134, 573–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kyle, R.A.; San-Miguel, J.F.; Mateos, M.V.; Rajkumar, S.V. Monoclonal gammopathy of undetermined significance and smoldering multiple myeloma. Hematology/oncology Clin. N. Am. 2014, 28, 775–790. [Google Scholar] [CrossRef] [PubMed]
- Go, R.S.; Rajkumar, S.V. How I manage monoclonal gammopathy of undetermined significance. Blood 2017, 13, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, H.M.; Mauermann, M.L.; Rajkumar, S.V. Monoclonal Gammopathy–Associated Peripheral Neuropathy: Diagnosis and Management. Mayo Clin. Proc. 2017, 92, 838–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishida, Y.; Iwama, K.; Yamakura, M.; Takeuchi, M.; Matsue, K. Renal Fanconi syndrome as a cause of chronic kidney disease in patients with monoclonal gammopathy of undetermined significance: Partially reversed renal function by high-dose dexamethasone with bortezomib. Leuk. Lymphoma 2012, 53, 1804–1806. [Google Scholar] [CrossRef] [PubMed]
- Bridoux, F.; Hugue, V.; Coldefy, O.; Goujon, J.M.; Bauwens, M.; Sechet, A.; Preud’Homme, J.L.; Touchard, G. Fibrillary glomerulonephritis and immunotactoid (microtubular) glomerulopathy are associated with distinct immunologic features. Kidney Int. 2002, 62, 1764–1775. [Google Scholar] [CrossRef] [PubMed]
- Nasr, S.H.; Valeri, A.M.; Cornell, L.D.; Fidler, M.E.; Sethi, S.; Leung, N.; Fervenza, F.C. Fibrillary Glomerulonephritis: A Report of 66 Cases from a Single Institution. Clin. J. Am. Soc. Nephrol. 2011, 6, 775–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dispenzieri, A. POEMS syndrome: 2017 Update on diagnosis, risk stratification, and management. Am. J. Hematol. 2017, 92, 814–829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Druey, K.M.; Parikh, S.M. Idiopathic systemic capillary leak syndrome (Clarkson disease). J. Allergy Clin. Immunol. 2017, 140, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Balderman, S.R.; Lichtman, M.A. Unusual Manifestations of Monoclonal Gammopathy: I. Ocular Disease. Rambam Maimonides Med. J. 2015, 6, e0026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiang, H.H.; Wieland, R.S.; Rogers, T.S.; Gibson, P.C.; Atweh, G.; McCormick, G. Paraproteinemic keratopathy in monoclonal gammopathy of undetermined significance treated with primary keratoprosthesis: Case report, histopathologic findings, and world literature review. Medicine 2017, 96, e8649. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Liu, J.; Bai, C.; Wang, J.; Song, X. Bortezomib combined with thalidomide and dexamethasone is effective for patient with crystal-storing histiocytosis associated with monoclonal gammopathy of undermined significance. Eur. J. Haematol. 2012, 89, 183–184. [Google Scholar] [CrossRef] [PubMed]
- de Alba Campomanes, A.G.; Rutar, T.; Crawford, J.B.; Seiff, S.; Goodman, D.; Grenert, J. Crystal-storing histiocytosis and crystalline keratopathy caused by monoclonal gammopathy of undetermined significance. Cornea 2009, 28, 1081–1084. [Google Scholar] [CrossRef] [PubMed]
- Kanagal-Shamanna, R.; Xu-Monette, Z.Y.; Miranda, R.N.; Dogan, A.; Zou, D.; Luthra, R.; Weber, D.M.; O’Malley, D.P.; Jorgensen, J.L.; Khoury, J.D.; et al. Crystal-storing histiocytosis: A clinicopathological study of 13 cases. Histopathology 2015, 68, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.; Braggio, E.; Jacobus, S.; Jung, S.; Larson, D.; Therneau, T.; Dispenzieri, A.; Van Wier, S.A.; Ahmann, G.; Levy, J.; et al. Uncovering the biology of multiple myeloma among African Americans: A comprehensive genomics approach. Blood 2013, 121, 3147–3152. [Google Scholar] [CrossRef] [PubMed]
- McFarlane, H. Multiple myeloma in Jamaica: A study of 40 cases with special reference to the incidence and laboratory diagnosis. J. Clin. Pathol. 1966, 19, 268–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenberg, A.J.; Vachon, C.M.; Rajkumar, S.V. Disparities in the prevalence, pathogenesis and progression of monoclonal gammopathy of undetermined significance and multiple myeloma between blacks and whites. Leukemia 2012, 26, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.M.; Gridley, G.; Check, D.; Landgren, O. Risk of multiple myeloma and monoclonal gammopathy of undetermined significance among white and black male United States veterans with prior autoimmune, infectious, inflammatory, and allergic disorders. Blood 2008, 111, 3388–3394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landgren, O.; Gridley, G.; Turesson, I.; Caporaso, N.E.; Goldin, L.R.; Baris, D.; Fears, T.R.; Hoover, R.N.; Linet, M.S. Risk of monoclonal gammopathy of undetermined significance (MGUS) and subsequent multiple myeloma among African American and white veterans in the United States. Blood 2006, 107, 904–906. [Google Scholar] [CrossRef] [PubMed]
- Cohen, H.J.; Crawford, J.; Rao, M.K.; Pieper, C.F.; Currie, M.S. Racial differences in the prevalence of monoclonal gammopathy in a community-based sample of the elderly. Am. J. Med. 1998, 104, 439–444. [Google Scholar] [CrossRef]
- Landgren, O.; Graubard, B.I.; Katzmann, J.A.; Kyle, R.A.; Ahmadizadeh, I.; Clark, R.; Kumar, S.K.; Dispenzieri, A.; Greenberg, A.J.; Therneau, T.M.; et al. Racial disparities in the prevalence of monoclonal gammopathies: A population-based study of 12,482 persons from the National Health and Nutritional Examination Survey. Leukemia 2014, 28, 1537–1542. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, H.; Mayr, W. Genetic aspects of susceptibility to multiple myeloma. Blood 1982, 59, 1286–1291. [Google Scholar] [PubMed]
- Patel, M.; Wadee, A.A.; Galpin, J.; Gavalakis, C.; Fourie, A.M.; Kuschke, R.H.; Philip, V. HLA class I and class II antigens associated with multiple myeloma in southern Africa. Clin. Lab. Haematol. 2002, 24, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Beksac, M.; Gragert, L.; Fingerson, S.; Maiers, M.; Zhang, M.J.; Albrecht, M.; Zhong, X.; Cozen, W.; Dispenzieri, A.; Lonial, S.; et al. HLA polymorphism and risk of multiple myeloma. Leukemia 2016, 30, 2260–2264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warren, J.L.; Harlan, L.C.; Stevens, J.; Little, R.F.; Abel, G.A. Multiple myeloma treatment transformed: A population-based study of changes in initial management approaches in the United States. J. Clin. Oncol. 2013, 31, 1984–1989. [Google Scholar] [CrossRef] [PubMed]
- Ailawadhi, S.; Frank, R.D.; Advani, P.; Swaika, A.; Temkit, M.; Menghani, R.; Sharma, M.; Meghji, Z.; Paulus, S.; Khera, N.; et al. Racial disparity in utilization of therapeutic modalities among multiple myeloma patients: A SEER-medicare analysis. Cancer Med. 2017, 6, 2876–2885. [Google Scholar] [CrossRef] [PubMed]
- Ailawadhi, S.; Jacobus, S.; Sexton, R.; Stewart, A.K.; Dispenzieri, A.; Hussein, M.A.; Zonder, J.A.; Crowley, J.; Hoering, A.; Barlogie, B.; et al. Disease and outcome disparities in multiple myeloma: Exploring the role of race/ethnicity in the Cooperative Group clinical trials. Blood Cancer J. 2018, 8, 67. [Google Scholar] [CrossRef] [PubMed]
- Rajan, A.M.; Rajkumar, S.V. Interpretation of cytogenetic results in multiple myeloma for clinical practice. Blood Cancer J. 2015, 5, e365. [Google Scholar] [CrossRef] [PubMed]
- Bergsagel, P.L.; Chesi, M.; Nardini, E.; Brents, L.A.; Kirby, S.L.; Kuehl, W.M. Promiscuous translocations into immunoglobulin heavy chain switch regions in multiple myeloma. Proc. Natl. Acad. Sci. USA 1996, 93, 13931–13936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tricot, G.; Barlogie, B.; Jagannath, S.; Bracy, D.; Mattox, S.; Vesole, D.H.; Naucke, S.; Sawyer, J.R. Poor prognosis in multiple myeloma is associated only with partial or complete deletions of chromosome 13 or abnormalities involving 11q and not with other karyotype abnormalities. Blood 1995, 86, 4250–4256. [Google Scholar] [PubMed]
- Hanamura, I.; Stewart, J.P.; Huang, Y.; Zhan, F.; Santra, M.; Sawyer, J.R.; Hollmig, K.; Zangarri, M.; Pineda-Roman, M.; van Rhee, F.; et al. Frequent gain of chromosome band 1q21 in plasma-cell dyscrasias detected by fluorescence in situ hybridization: Incidence increases from MGUS to relapsed myeloma and is related to prognosis and disease progression following tandem stem-cell transplantation. Blood 2006, 108, 1724–1732. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.V.; Gupta, V.; Fonseca, R.; Dispenzieri, A.; Gonsalves, W.I.; Larson, D.; Ketterling, R.P.; Lust, J.A.; Kyle, R.A.; Kumar, S.K. Impact of primary molecular cytogenetic abnormalities and risk of progression in smoldering multiple myeloma. Leukemia 2013, 27, 1738–1744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neben, K.; Jauch, A.; Hielscher, T.; Hillengass, J.; Lehners, N.; Seckinger, A.; Granzow, M.; Raab, M.S.; Ho, A.D.; Goldschmidt, H.; et al. Progression in smoldering myeloma is independently determined by the chromosomal abnormalities del(17p), t(4;14), gain 1q, hyperdiploidy, and tumor load. J. Clin. Oncol. 2013, 31, 4325–4332. [Google Scholar] [CrossRef] [PubMed]
- Amend, S.R.; Wilson, W.C.; Chu, L.; Lu, L.; Liu, P.; Serie, D.; Su, X.; Xu, Y.; Wang, D.; Gramolini, A.; et al. Whole Genome Sequence of Multiple Myeloma-Prone C57BL/KaLwRij Mouse Strain Suggests the Origin of Disease Involves Multiple Cell Types. PLoS ONE 2015, 10, e0127828. [Google Scholar] [CrossRef] [PubMed]
- Chesi, M.; Robbiani, D.F.; Sebag, M.; Chng, W.J.; Affer, M.; Tiedemann, R.; Valdez, R.; Palmer, S.E.; Haas, S.S.; Stewart, A.K.; et al. AID-dependent activation of a MYC transgene induces multiple myeloma in a conditional mouse model of post-germinal center malignancies. Cancer cell 2008, 13, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Masuzaki, H.; Paterson, J.; Shinyama, H.; Morton, N.M.; Mullins, J.J.; Seckl, J.R.; Flier, J.S. A transgenic model of visceral obesity and the metabolic syndrome. Science 2001, 294, 2166–2170. [Google Scholar] [CrossRef] [PubMed]
- Lionetti, M.; Barbieri, M.; Todoerti, K.; Agnelli, L.; Marzorati, S.; Fabris, S.; Ciceri, G.; Galletti, S.; Milesi, G.; Manzoni, M.; et al. Molecular spectrum of BRAF, NRAS and KRAS gene mutations in plasma cell dyscrasias: Implication for MEK-ERK pathway activation. Oncotarget 2015, 6, 24205–24217. [Google Scholar] [CrossRef] [PubMed]
- Chng, W.J.; Gonzalez-Paz, N.; Price-Troska, T.; Jacobus, S.; Rajkumar, S.V.; Oken, M.M.; Kyle, R.A.; Henderson, K.J.; Van Wier, S.; Greipp, P.; et al. Clinical and biological significance of RAS mutations in multiple myeloma. Leukemia 2008, 22, 2280–2284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mullins, C.D.; Su, M.Y.; Hucthagowder, V.; Chu, L.; Lu, L.; Kulkarni, S.; Novack, D.; Vij, R.; Tomasson, M.H. Germinal center B-cells resist transformation by Kras independently of tumor suppressor Arf. PLoS ONE 2013, 8, e67941. [Google Scholar] [CrossRef] [PubMed]
- Zhan, F.; Barlogie, B.; Arzoumanian, V.; Huang, Y.; Williams, D.R.; Hollmig, K.; Pineda-Roman, M.; Tricot, G.; van Rhee, F.; Zangari, M.; et al. Gene-expression signature of benign monoclonal gammopathy evident in multiple myeloma is linked to good prognosis. Blood 2007, 109, 1692–1700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guikema, J.E.; Hovenga, S.; Vellenga, E.; Conradie, J.J.; Abdulahad, W.H.; Bekkema, R.; Smit, J.W.; Zhan, F.; Shaughnessy, J., Jr.; Bos, N.A. CD27 is heterogeneously expressed in multiple myeloma: Low CD27 expression in patients with high-risk disease. Br. J. Haematol. 2003, 121, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Branagan, A.R.; Liu, J.; Boddupalli, C.S.; Mistry, P.K.; Dhodapkar, M.V. Clonal Immunoglobulin against Lysolipids in the Origin of Myeloma. N. Engl. J. Med. 2016, 374, 555–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bosseboeuf, A.; Feron, D.; Tallet, A.; Rossi, C.; Charlier, C.; Garderet, L.; Caillot, D.; Moreau, P.; Cardo-Vila, M.; Pasqualini, R.; et al. Monoclonal IgG in MGUS and multiple myeloma targets infectious pathogens. JCI Insight 2017, 2, e95367. [Google Scholar] [CrossRef] [PubMed]
- Carson, K.R.; Bates, M.L.; Tomasson, M.H. The skinny on obesity and plasma cell myeloma: A review of the literature. Bone marrow transplant. 2014, 49, 1009–1015. [Google Scholar] [CrossRef] [PubMed]
- Landgren, O.; Rajkumar, S.V.; Pfeiffer, R.M.; Kyle, R.A.; Katzmann, J.A.; Dispenzieri, A.; Cai, Q.; Goldin, L.R.; Caporaso, N.E.; Fraumeni, J.F.; et al. Obesity is associated with an increased risk of monoclonal gammopathy of undetermined significance among black and white women. Blood 2010, 116, 1056–1059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marinac, C.R.; Birmann, B.M.; Lee, I.M.; Rosner, B.A.; Townsend, M.K.; Giovannucci, E.; Rebbeck, T.R.; Buring, J.E.; Colditz, G.A. Body mass index throughout adulthood, physical activity, and risk of multiple myeloma: A prospective analysis in three large cohorts. Br. J. Cancer 2018, 118, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Birmann, B.M.; Andreotti, G.; De Roos, A.J.; Camp, N.J.; Chiu, B.C.H.; Spinelli, J.J.; Becker, N.; Benhaim-Luzon, V.; Bhatti, P.; Boffetta, P.; et al. Young Adult and Usual Adult Body Mass Index and Multiple Myeloma Risk: A pooled analysis in the international multiple myeloma consortium (IMMC). Cancer Epidemiol. Prev. Biomark. 2017, 26, 876–885. [Google Scholar] [CrossRef] [PubMed]
- Teras, L.R.; Kitahara, C.M.; Birmann, B.M.; Hartge, P.A.; Wang, S.S.; Robien, K.; Patel, A.V.; Adami, H.-O.; Weiderpass, E.; Giles, G.G.; et al. Body size and multiple myeloma mortality: A pooled analysis of 20 prospective studies. Br. J. Haematol. 2014, 166, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, J.N.; Mailankody, S.; Korde, N.; Wang, Y.; Tageja, N.; Costello, R.; Zingone, A.; Hultcrantz, M.; Pollak, M.N.; Purdue, M.P.; et al. Circulating Adiponectin Levels Differ Between Patients with Multiple Myeloma and its Precursor Disease. Obesity 2017, 25, 1317–1320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medina, E.A.; Oberheu, K.; Polusani, S.R.; Ortega, V.; Velagaleti, G.V.; Oyajobi, B.O. PKA/AMPK signaling in relation to adiponectin’s antiproliferative effect on multiple myeloma cells. Leukemia 2014, 28, 2080–2089. [Google Scholar] [CrossRef] [PubMed]
- Baumann, P.; Mandl-Weber, S.; Emmerich, B.; Straka, C.; Schmidmaier, R. Activation of adenosine monophosphate activated protein kinase inhibits growth of multiple myeloma cells. Exp. Cell Res. 2007, 313, 3592–3603. [Google Scholar] [CrossRef] [PubMed]
- Lwin, S.T.; Olechnowicz, S.W.Z.; Fowler, J.A.; Edwards, C.M. Diet-induced obesity promotes a myeloma-like condition in vivo. Leukemia 2014, 29, 507. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Cai, Z.; Wang, S.; Zhang, X.; Qian, J.; Hong, S.; Li, H.; Wang, M.; Yang, J.; Yi, Q. Macrophages are an abundant component of myeloma microenvironment and protect myeloma cells from chemotherapy drug–induced apoptosis. Blood 2009, 114, 3625. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, X.; Zheng, Y. The Role of Tumor Associated Macrophages in Multiple Myeloma and Its Pathophysiological Effect on Myeloma Cells Survival, Apopotosis and Angiogenesis. Blood 2015, 126, 4204. [Google Scholar]
- Asimakopoulos, F.; Kim, J.; Denu, R.A.; Hope, C.; Jensen, J.L.; Ollar, S.J.; Hebron, E.; Flanagan, C.; Callander, N.; Hematti, P. Macrophages in multiple myeloma: Emerging concepts and therapeutic implications. Leuk. Lymphoma 2013, 54, 2112–2121. [Google Scholar] [CrossRef] [PubMed]
- Hope, C.; Ollar, S.J.; Heninger, E.; Hebron, E.; Jensen, J.L.; Kim, J.; Maroulakou, I.; Miyamoto, S.; Leith, C.; Yang, D.T.; et al. TPL2 kinase regulates the inflammatory milieu of the myeloma niche. Blood 2014, 123, 3305–3315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rutsch, S.; Neppalli, V.T.; Shin, D.M.; DuBois, W.; Morse, H.C., 3rd; Goldschmidt, H.; Janz, S. IL-6 and MYC collaborate in plasma cell tumor formation in mice. Blood 2010, 115, 1746–1754. [Google Scholar] [CrossRef] [PubMed]
- Duncan, K.; Rosean, T.R.; Tompkins, V.S.; Olivier, A.; Sompallae, R.; Zhan, F.; Tricot, G.; Acevedo, M.R.; Ponto, L.L.; Walsh, S.A.; et al. 18F-FDG-PET/CT imaging in an IL-6- and MYC-driven mouse model of human multiple myeloma affords objective evaluation of plasma cell tumor progression and therapeutic response to the proteasome inhibitor ixazomib. Blood Cancer J. 2013, 3, e165. [Google Scholar] [CrossRef] [PubMed]
- Dechow, T.; Steidle, S.; Gotze, K.S.; Rudelius, M.; Behnke, K.; Pechloff, K.; Kratzat, S.; Bullinger, L.; Fend, F.; Soberon, V.; et al. GP130 activation induces myeloma and collaborates with MYC. J. Clin. Investig. 2014, 124, 5263–5274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burger, R.; Gunther, A.; Klausz, K.; Staudinger, M.; Peipp, M.; Penas, E.M.; Rose-John, S.; Wijdenes, J.; Gramatzki, M. Due to interleukin-6 type cytokine redundancy only glycoprotein 130 receptor blockade efficiently inhibits myeloma growth. Haematologica 2017, 102, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Kawano, Y.; Moschetta, M.; Kokubun, K.; Lukyanchykov, P.; Sula Karreci, E.; Manier, S.; Tsukamoto, S.; Takagi, S.; Shi, J.; Reagan, M.R.; et al. Characterization of the role of regulatory T cells (Tregs) in inducing progression of multiple Myeloma. Blood 2015, 126, 502. [Google Scholar]
- Altrock, P.M.; Ferlic, J.; Galla, T.; Tomasson, M.H.; Michor, F. Computational Model of Progression to Multiple Myeloma Identifies Optimum Screening Strategies. JCO. Clin. Cancer Inform. 2018, 2, 1–12. [Google Scholar] [CrossRef]
- Tang, M.; Zhao, R.; van de Velde, H.; Tross, J.G.; Mitsiades, C.; Viselli, S.; Neuwirth, R.; Esseltine, D.-L.; Anderson, K.; Ghobrial, I.M.; et al. Myeloma cell dynamics in response to treatment supports a model of hierarchical differentiation and clonal evolution. Clin. Cancer Res. 2016, 22, 4206–4214. [Google Scholar] [CrossRef] [PubMed]
- Thorsteinsdottir, S.; Lund, S.H.; Lindqvist, E.K.; Thordardottir, M.; Sigurdsson, G.; Costello, R.; Burton, D.; Steingrimsdottir, H.; Gudnason, V.; Eiriksdottir, G.; et al. Bone disease in monoclonal gammopathy of undetermined significance: Results from a screened population-based study. Blood Adv. 2017, 1, 2790–2798. [Google Scholar] [CrossRef] [PubMed]
- Kotsis, V.; Tsioufis, K.; Antza, C.; Seravalle, G.; Coca, A.; Sierra, C.; Lurbe, E.; Stabouli, S.; Jelakovic, B.; Redon, J.; et al. Obesity and cardiovascular risk: A call for action from the European society of hypertension working group of obesity, diabetes and the high-risk patient and european association for the study of obesity part b obesity-induced cardiovascular disease, early prevention strategies and future research directions. J. Hypertens. 2018, 36, 1441–1455. [Google Scholar] [PubMed]
- Fontana, L. Interventions to promote cardiometabolic health and slow cardiovascular ageing. Nat. Rev. Cardiol. 2018, 15, 566–577. [Google Scholar] [CrossRef] [PubMed]
- Jarlenski, M.P.; Gudzune, K.A.; Bennett, W.L.; Cooper, L.A.; Bleich, S.N. Insurance coverage for weight loss: Overweight adults’ views. Am. J. Prev. Med. 2013, 44, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Appel, L.J.; Clark, J.M.; Yeh, H.C.; Wang, N.Y.; Coughlin, J.W.; Daumit, G.; Miller, E.R., 3rd; Dalcin, A.; Jerome, G.J.; Geller, S.; Noronha, G.; et al. Comparative effectiveness of weight-loss interventions in clinical practice. N. Engl. J. Med. 2011, 365, 1959–1968. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.H.; Stoll, C.R.; Colditz, G.A. Cost-effectiveness of bariatric surgery: Should it be universally available? Maturitas 2011, 69, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Manini, T.M.; Carr, L.J.; King, A.C.; Marshall, S.; Robinson, T.N.; Rejeski, W.J. Interventions to reduce sedentary behavior. Med. Sci. Sports Exerc. 2015, 47, 1306–1310. [Google Scholar] [CrossRef] [PubMed]
- Kruse, N.T.; Hughes, W.E.; Benzo, R.M.; Carr, L.J.; Casey, D.P. Workplace Strategies to Prevent Sitting-induced Endothelial Dysfunction. Med. Sci. Sports Exerc. 2018, 50, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Jao, Y.L.; Gardner, S.E.; Carr, L.J. Measuring weight-bearing activities in patients with previous diabetic foot ulcers. J. Wound Ostomy Continence Nurs. 2017, 44, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Tucker, S.J.; Carr, L.J. Translating physical activity evidence to hospital settings: A call for culture change. Clin. Nurse Spec. 2016, 30, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.H.; Luo, S.; O’Brian, K.K.; Thomas, T.S.; Colditz, G.A.; Carlsson, N.P.; Carson, K.R. Association between metformin use and progression of monoclonal gammopathy of undetermined significance to multiple myeloma in US veterans with diabetes mellitus: A population-based retrospective cohort study. Lancet Haematol. 2015, 2, e30–e36. [Google Scholar] [CrossRef]
- Boursi, B.; Mamtani, R.; Yang, Y.-X.; Weiss, B.M. Impact of metformin on the progression of MGUS to multiple myeloma. Leuk. Lymphoma 2017, 58, 1265–1267. [Google Scholar] [CrossRef] [PubMed]
- Zi, F.M.; He, J.S.; Li, Y.; Wu, C.; Yang, L.; Yang, Y.; Wang, L.J.; He, D.H.; Zhao, Y.; Wu, W.J.; et al. Metformin displays anti-myeloma activity and synergistic effect with dexamethasone in in vitro and in vivo xenograft models. Cancer Lett. 2015, 356, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Jagannathan, S.; Abdel-Malek, M.A.; Malek, E.; Vad, N.; Latif, T.; Anderson, K.C.; Driscoll, J.J. Pharmacologic screens reveal metformin that suppresses GRP78-dependent autophagy to enhance the anti-myeloma effect of bortezomib. Leukemia 2015, 29, 2184–2191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalva-Aydemir, S.; Bajpai, R.; Martinez, M.; Adekola, K.U.; Kandela, I.; Wei, C.; Singhal, S.; Koblinski, J.E.; Raje, N.S.; Rosen, S.T.; et al. Targeting the metabolic plasticity of multiple myeloma with FDA-approved ritonavir and metformin. Clin. Cancer Res. 2015, 21, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Keats, J.J.; Maxwell, C.A.; Taylor, B.J.; Hendzel, M.J.; Chesi, M.; Bergsagel, P.L.; Larratt, L.M.; Mant, M.J.; Reiman, T.; Belch, A.R.; et al. Overexpression of transcripts originating from the MMSET locus characterizes all t(4;14)(p16;q32)-positive multiple myeloma patients. Blood 2005, 105, 4060–4069. [Google Scholar] [CrossRef] [PubMed]
- White-Al Habeeb, N.M.; Garcia, J.; Fleshner, N.; Bapat, B. Metformin elicits antitumor effects and downregulates the histone methyltransferase multiple myeloma SET Domain (MMSET) in prostate cancer cells. Prostate 2016, 76, 1507–1518. [Google Scholar] [CrossRef] [PubMed]
- Long-Term Safety, Tolerability, and weight loss associated with metformin in the diabetes prevention program outcomes study. Diabetes Care 2012, 35, 731–737. [CrossRef] [PubMed]
- Drake, M.T.; Ng, A.C. Vitamin D deficiency in multiple myeloma. Eur. J. Clin. Med. Oncol. 2010, 2, 1–4. [Google Scholar]
- Ravenborg, N.; Udd, K.; Berenson, A.; Costa, F.; Berenson, J.R. Vitamin D levels are frequently below normal in multiple myeloma patients and are infrequently assessed by their treating physicians. Blood 2014, 124, 5769. [Google Scholar]
- Badros, A.; Goloubeva, O.; Terpos, E.; Milliron, T.; Baer, M.R.; Streeten, E. Prevalence and significance of vitamin D deficiency in multiple myeloma patients. Br. J. Haematol. 2008, 142, 492–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hudzik, S.; Snoad, B.; Mousa, L.; Sborov, D.W.; Williams, N.; Jones, D.; Hofmeister, C.C. The majority of myeloma patients are vitamin D deficient, unrelated to survival or cytogenetics. Blood 2015, 126, 5336. [Google Scholar]
- Maier, G.S.; Horas, K.; Kurth, A.A.; Lazovic, D.; Seeger, J.B.; Maus, U. Prevalence of vitamin D deficiency in patients with bone metastases and multiple myeloma. Anticancer Res. 2015, 35, 6281–6285. [Google Scholar] [PubMed]
- Lipe, B.; Kambhampati, S.; Veldhuizen, P.V.; Yacoub, A.; Aljitawi, O.; Mikhael, J. Correlation between markers of bone metabolism and vitamin D levels in patients with monoclonal gammopathy of undetermined significance (MGUS). Blood Cancer J. 2017, 7, 646. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Gu, F.; Caporaso, N.; Matthews, C.E. Relationship between sleep characteristics and measures of body size and composition in a nationally-representative sample. BMC Obes. 2016, 3, 48. [Google Scholar] [CrossRef] [PubMed]
- Rahe, C.; Czira, M.E.; Teismann, H.; Berger, K. Associations between poor sleep quality and different measures of obesity. Sleep Med. 2015, 16, 1225–1228. [Google Scholar] [CrossRef] [PubMed]
- Knutson, K.L. Does inadequate sleep play a role in vulnerability to obesity? Am. J. Hum. Biol. 2012, 24, 361–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, F.; Xiao, Q.; Chu, L.W.; Yu, K.; Matthews, C.E.; Hsing, A.W.; Caporaso, N.E. Sleep duration and cancer in the NIH-AARP diet and health study cohort. PLoS ONE 2016, 11, e0161561. [Google Scholar] [CrossRef] [PubMed]
- Young, T.; Palta, M.; Dempsey, J.; Peppard, P.E.; Nieto, F.J.; Hla, K.M. Burden of sleep apnea: Rationale, design, and major findings of the wisconsin sleep cohort study. WMJ 2009, 108, 246–249. [Google Scholar] [PubMed]
- Hudgel, D.W. Sleep apnea severity classification—Revisited. Sleep 2016, 39, 1165–1166. [Google Scholar] [CrossRef] [PubMed]
- Peppard, P.E.; Nieto, F.J. Here Come the Sleep Apnea-Cancer Studies. Sleep 2013, 36, 1409–1411. [Google Scholar] [CrossRef] [PubMed]
- Miller, Y.E.; Karoor, V.; Dempsey, E.C.; Fagan, K.A. Sleep-disordered breathing, hypoxemia, and cancer mortality. Am. J. Respir. Crit. Care Med. 2013, 187, 330–331. [Google Scholar] [CrossRef] [PubMed]
- Nieto, F.J.; Peppard, P.E.; Young, T.; Finn, L.; Hla, K.M.; Farre, R. Sleep-disordered breathing and cancer mortality: Results from the Wisconsin Sleep Cohort Study. Am. J. Respir. Crit. Care Med. 2012, 186, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Vilaseca, A.; Campillo, N.; Torres, M.; Musquera, M.; Gozal, D.; Montserrat, J.M.; Alcaraz, A.; Touijer, K.A.; Farre, R.; Almendros, I. Intermittent hypoxia increases kidney tumor vascularization in a murine model of sleep apnea. PLoS ONE 2017, 12, e0179444. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ren, F.; Qi, C.; Xu, L.; Fang, Y.; Liang, M.; Feng, J.; Chen, B.; Ning, W.; Cao, J. Intermittent hypoxia promotes melanoma lung metastasis via oxidative stress and inflammation responses in a mouse model of obstructive sleep apnea. Respir. Res. 2018, 19, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almendros, I.; Wang, Y.; Becker, L.; Lennon, F.E.; Zheng, J.; Coats, B.R.; Schoenfelt, K.S.; Carreras, A.; Hakim, F.; Zhang, S.X.; et al. Intermittent hypoxia-induced changes in tumor-associated macrophages and tumor malignancy in a mouse model of sleep apnea. Am. J. Respir. Crit. Care Med. 2014, 189, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Garcia, M.A.; Campos-Rodriguez, F.; Nagore, E.; Martorell, A.; Rodriguez-Peralto, J.L.; Riveiro-Falkenbach, E.; Hernandez, L.; Banuls, J.; Arias, E.; Ortiz, P.; et al. Sleep-Disordered breathing is independently associated with increased aggressiveness of cutaneous melanoma: A multicenter observational study in 443 patients. Chest 2018. Available online: https://www.sciencedirect.com/science/article/pii/S0012369218311097 (accessed on 27 July 2018).
- Alvarez-Martins, I.; Remedio, L.; Matias, I.; Diogo, L.N.; Monteiro, E.C.; Dias, S. The impact of chronic intermittent hypoxia on hematopoiesis and the bone marrow microenvironment. Pflugers Arch-Eur. J. Physiol. 2016, 468, 919–932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Wang, C. Immune status of children with obstructive sleep apnea/hypopnea syndrome. Pak. J. Med. Sci. 2017, 33, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Netzer, N.C.; Stoohs, R.A.; Netzer, C.M.; Clark, K.; Strohl, K.P. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann. Intern. Med. 1999, 131, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Johns, M.W. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep 1992, 15, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Ittaman, S.V.; VanWormer, J.J.; Rezkalla, S.H. The role of aspirin in the prevention of cardiovascular disease. Clin. Med. Res. 2014, 12, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Campbell, C.L.; Smyth, S.; Montalescot, G.; Steinhubl, S.R. Aspirin dose for the prevention of cardiovascular disease: A systematic review. JAMA 2007, 297, 2018–2024. [Google Scholar] [CrossRef] [PubMed]
- Ajani, U.A.; Ford, E.S.; Greenland, K.J.; Giles, W.H.; Mokdad, A.H. Aspirin use among U.S. adults: Behavioral Risk Factor Surveillance System. Am. J. Prev. Med. 2006, 30, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Birmann, B.M.; Giovannucci, E.L.; Rosner, B.A.; Colditz, G.A. Regular aspirin use and risk of multiple myeloma: A prospective analysis in the health professionals follow-up study and nurses’ health study. Cancer Prev. Res. 2014, 7, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.F.; Ng, T.Y.; Wong, F.C.; Tung, S.Y. Aspirin and risk of multiple myeloma in adults: A systematic review and meta-analysis. Leuk. Res. Rep. 2017, 7, 23–28. [Google Scholar] [CrossRef] [PubMed]
- McNeil, J.J.; Nelson, M.R.; Woods, R.L.; Lockery, J.E.; Wolfe, R.; Reid, C.M.; Kirpach, B.; Shah, R.C.; Ives, D.G.; Storey, E.; et al. Effect of aspirin on all-cause mortality in the healthy elderly. N. Engl. J. Med. 2018, 379, 1519–1528. [Google Scholar] [CrossRef] [PubMed]
- McNeil, J.J.; Wolfe, R.; Woods, R.L.; Tonkin, A.M.; Donnan, G.A.; Nelson, M.R.; Reid, C.M.; Lockery, J.E.; Kirpach, B.; Storey, E.; et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N. Engl. J. Med. 2018, 379, 1509–1518. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, P.M.; Cook, N.R.; Gaziano, J.M.; Price, J.F.; Belch, J.F.F.; Roncaglioni, M.C.; Morimoto, T.; Mehta, Z. Effects of aspirin on risks of vascular events and cancer according to bodyweight and dose: Analysis of individual patient data from randomised trials. Lancet 2018, 392, 387–399. [Google Scholar] [CrossRef]
- Orlowski, R.Z.; Gercheva, L.; Williams, C.; Sutherland, H.; Robak, T.; Masszi, T.; Goranova-Marinova, V.; Dimopoulos, M.A.; Cavenagh, J.D.; Spicka, I.; et al. A phase 2, randomized, double-blind, placebo-controlled study of siltuximab (anti-IL-6 mAb) and bortezomib versus bortezomib alone in patients with relapsed or refractory multiple myeloma. Am. J. Hematol. 2015, 90, 42–49. [Google Scholar] [CrossRef] [PubMed]
- San-Miguel, J.; Blade, J.; Shpilberg, O.; Grosicki, S.; Maloisel, F.; Min, C.K.; Polo Zarzuela, M.; Robak, T.; Prasad, S.V.; Tee Goh, Y.; et al. Phase 2 randomized study of bortezomib-melphalan-prednisone with or without siltuximab (anti-IL-6) in multiple myeloma. Blood 2014, 123, 4136–4142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, T.; Hishitani, Y.; Ogata, A. Monoclonal antibodies in rheumatoid arthritis: Comparative effectiveness of tocilizumab with tumor necrosis factor inhibitors. Biol. Targets Ther. 2014, 8, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Bijlsma, J.W.J.; Welsing, P.M.J.; Woodworth, T.G.; Middelink, L.M.; Petho-Schramm, A.; Bernasconi, C.; Borm, M.E.A.; Wortel, C.H.; Ter Borg, E.J.; Jahangier, Z.N.; et al. Early rheumatoid arthritis treated with tocilizumab, methotrexate, or their combination (U-Act-Early): A multicentre, randomised, double-blind, double-dummy, strategy trial. Lancet 2016, 388, 343–355. [Google Scholar] [CrossRef]
- Schutz, N.; Marker-Hermann, E. Rheumatoid arthritis and multiple myeloma as comorbidity. Is tocilizumab a therapy option? Z. Rheumatol. 2012, 71, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, Y.; Nagashima, T.; Honne, K.; Kamata, Y.; Iwamoto, M.; Okazaki, H.; Sato, K.; Ozawa, K.; Minota, S. Successful treatment of a patient with rheumatoid arthritis and IgA-kappa multiple myeloma with tocilizumab. Int. Med. 2011, 50, 639–642. [Google Scholar] [CrossRef]
- Goodwin, J.A.; Coleman, E.A.; Sullivan, E.; Easley, R.; McNatt, P.K.; Chowdhury, N.; Stewart, C.B. Personal Financial Effects of Multiple Myeloma and its Treatment. Cancer Nurs. 2013, 36, 301–308. [Google Scholar] [CrossRef] [PubMed]
- MacEwan, J.P.; Batt, K.; Yin, W.; Peneva, D.; Sison, S.; Vine, S.; Chen, C. Economic burden of multiple myeloma among patients in successive lines of therapy in the United States. Leuk. Lymphoma 2018, 59, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.V. Value and cost of myeloma therapy. Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 662–666. [Google Scholar] [CrossRef] [PubMed]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomasson, M.H.; Ali, M.; De Oliveira, V.; Xiao, Q.; Jethava, Y.; Zhan, F.; Fitzsimmons, A.M.; Bates, M.L. Prevention Is the Best Treatment: The Case for Understanding the Transition from Monoclonal Gammopathy of Undetermined Significance to Myeloma. Int. J. Mol. Sci. 2018, 19, 3621. https://doi.org/10.3390/ijms19113621
Tomasson MH, Ali M, De Oliveira V, Xiao Q, Jethava Y, Zhan F, Fitzsimmons AM, Bates ML. Prevention Is the Best Treatment: The Case for Understanding the Transition from Monoclonal Gammopathy of Undetermined Significance to Myeloma. International Journal of Molecular Sciences. 2018; 19(11):3621. https://doi.org/10.3390/ijms19113621
Chicago/Turabian StyleTomasson, Michael H., Mahmoud Ali, Vanessa De Oliveira, Qian Xiao, Yogesh Jethava, Fenghuang Zhan, Adam M. Fitzsimmons, and Melissa L. Bates. 2018. "Prevention Is the Best Treatment: The Case for Understanding the Transition from Monoclonal Gammopathy of Undetermined Significance to Myeloma" International Journal of Molecular Sciences 19, no. 11: 3621. https://doi.org/10.3390/ijms19113621
APA StyleTomasson, M. H., Ali, M., De Oliveira, V., Xiao, Q., Jethava, Y., Zhan, F., Fitzsimmons, A. M., & Bates, M. L. (2018). Prevention Is the Best Treatment: The Case for Understanding the Transition from Monoclonal Gammopathy of Undetermined Significance to Myeloma. International Journal of Molecular Sciences, 19(11), 3621. https://doi.org/10.3390/ijms19113621