Hormonal Responses to Plasmodiophora brassicae Infection in Brassica napus Cultivars Differing in Their Pathogen Resistance
Abstract
:1. Introduction
2. Results
2.1. Plasmodiophora Brassicae Infection Responses Differ Between Cultivars and Hormone Treatments
2.2. Growth-Promoting Hormones
2.3. Defence Hormones
2.4. Stress Hormones
2.5. Hormone-Related Gene Expression
2.5.1. Cytokinin-Related Genes
2.5.2. Auxin-Related Genes
2.5.3. Salicylic Acid-Related Genes
2.5.4. Jasmonic Acid-Related Genes
2.5.5. Abscisic Acid-Related Genes
2.5.6. Ethylene-Related Genes
3. Discussion
3.1. The Early Response is Characterized by Changes in Growth Promoting Hormones
3.2. Differences in the Gall Formation of the Susceptible Cultivar Hornet and Resistant Cultivar Alister
3.3. The Impacts of Salicylic Acid and Jasmonic Acid Applications
3.4. Organ Specificity—Leaves vs. Roots and Galls
4. Materials and Methods
4.1. Biological Materials and Pathogen Inoculation
4.2. Experimental Design
4.3. Disease Assessment
4.4. Phytohormone Analysis
4.5. Quantitative RT-PCR
4.6. Statistic Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABA | abscisic acid |
| ACS2 | 1-aminocyclopropane-1-carboxylic acid synthetase 2 |
| AOC | allene oxide cyclase |
| CK cZ | cytokinin cis-zeatin |
| dai | days after inoculation |
| DI | disease Index |
| EIN2 | ethylene insensitive 2 |
| IAA | indole-3-acetic acid |
| ICS1 | isochorismate synthase 1 |
| IPT3 | isopentenyltransferase 3 |
| JA | jasmonic acid |
| JAR1 | jasmonate resistant 1 |
| LOX4 | lipoxygenase 4 |
| NCED3 | 9-cis-epoxycarotenoid dioxygenase |
| NIT1 | nitrilase 1 |
| NPR1 | nonexpresser of PR genes |
| PDF1.2 | plant defensin 1.2 |
| PR | pathogenesis-related protein |
| SA | salicylic acid |
| TAA1 | tryptophan aminotransferase |
| YUC | indole-3-pyruvate monooxygenase |
References
- Dixon, G.R. The occurrence and economic impact of Plasmodiophora brassicae and clubroot disease. J. Plant Growth Regul. 2009, 28, 194–202. [Google Scholar] [CrossRef]
- Ingram, D.S.; Tommerup, I.C. The life history of Plasmodiophora brassicae Woron. Proc. R. Soc. Lond. B 1972, 180, 103–112. [Google Scholar] [CrossRef]
- Wallenhammar, A.C. Prevalence of Plasmodiophora brassicae in a spring oilseed rape growing area in central Sweden and factors influencing soil infestation levels. Plant Pathol. 1996, 45, 710–719. [Google Scholar] [CrossRef]
- Ludwig-Müller, J. Belowground Defence Strategies in Plants. In Belowground Defence Strategies in Plants; Vos, C., Kamal, K., Eds.; Signaling and Communication in Plants Series; Springer: Cham, Switzerland, 2016; pp. 195–219. ISBN 978-3-319-42317-3. [Google Scholar]
- Ludwig-Müller, J.; Prinsen, E.; Rolfe, S.A.; Scholes, J.D. Metabolism and plant hormone action during clubroot disease. J. Plant Growth Regul. 2009, 28, 229–244. [Google Scholar] [CrossRef]
- Siemens, J.; González, M.C.; Wolf, S.; Hofmann, C.; Greiner, S.; Du, Y.; Rausch, T.; Roitsch, T.; Ludwig-Müller, J. Extracellular invertase is involved in the regulation of clubroot disease in Arabidopsis thaliana. Mol. Plant Pathol. 2011, 12, 247–262. [Google Scholar] [CrossRef] [PubMed]
- Dekhuijzen, H.M.; Overeem, J.C. The role of cytokinins in clubroot formation. Physiol. Plant Pathol. 1971, 1, 151–161. [Google Scholar] [CrossRef]
- Dekhuijzen, H.M. The occurrence of free and bound cytokinins in plasmodia of Plasmodiophora brassicae isolated from tissue cultures of clubroots. Plant Cell Rep. 1981, 1, 18–20. [Google Scholar] [CrossRef]
- Müller, P.; Hilgenberg, W. Isomers of zeatin and zeatin riboside in clubroot tissue—Evidence for trans-zeatin biosynthesis by Plasmodiophora brassicae. Physiol. Plant. 1986, 66, 245–250. [Google Scholar] [CrossRef]
- Siemens, J.; Keller, I.; Sarx, J.; Kunz, S.; Schuller, A.; Nagel, W.; Schmülling, T.; Parniske, M.; Ludwig-Müller, J. Transcriptome analysis of Arabidopsis clubroots indicate a key role for cytokinins in disease development. Mol. Plant Microbe Interact. 2006, 19, 480–494. [Google Scholar] [CrossRef]
- Devos, S.; Laukens, K.; Deckers, P.; Van Der Straeten, D.; Beeckman, T.; Inzé, D.; Van Onckelen, H.; Witters, E.; Prinsen, E. A hormone and proteome approach to picturing the initial metabolic events during Plasmodiophora brassicae infection on Arabidopsis. Mol. Plant Microbe Interact. 2006, 19, 1431–1443. [Google Scholar] [CrossRef]
- Malinowski, R.; Novák, O.; Borhan, M.H.; Spíchal, L.; Strnad, M.; Rolfe, S.A. The role of cytokinins in clubroot disease. Eur. J. Plant Pathol. 2016, 145, 543–557. [Google Scholar] [CrossRef] [Green Version]
- Grsic, S.; Kirchheim, B.; Pieper, K.; Fritsch, M.; Hilgenberg, W.; Ludwig-Müller, J. Induction of auxin biosynthetic enzymes by jasmonic acid and in clubroot diseased Chinese cabbage plants. Physiol. Plant. 1999, 105, 521–531. [Google Scholar] [CrossRef]
- Schuller, A.; Kehr, J.; Ludwig-Müller, J. Laser microdissection coupled to transcriptional profiling of Arabidopsis roots inoculated by Plasmodiophora brassicae indicates a role for brassinosteroids in clubroot formation. Plant Cell Physiol. 2014, 55, 392–411. [Google Scholar] [CrossRef] [PubMed]
- Lemarie, S.; Robert-Seilaniantz, A.; Lariagon, C.; Lemoine, J.; Marnet, N.; Jubault, M.; Manzanares-Dauleux, M.J.; Gravot, A. Both the jasmonic acid and the salicylic acid pathways contribute to resistance to the biotrophic clubroot agent Plasmodiophora brassicae in Arabidopsis. Plant Cell Physiol. 2015, 56, 2158–2168. [Google Scholar] [CrossRef] [PubMed]
- Jubault, M.; Lariagon, C.; Taconnat, L.; Renou, J.P.; Gravot, A.; Delourme, R.; Manzanares-Dauleux, M.J. Partial resistance to clubroot in Arabidopsis is based on changes in the host primary metabolism and targeted cell division and expansion capacity. Funct. Integr. Genom. 2013, 13, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Lovelock, D.A.; Sola, I.; Marschollek, S.; Donald, C.E.; Rusak, G.; van Pée, K.-H.; Ludwig-Müller, J.; Cahill, D.M. Analysis of salicylic acid-dependent pathways in Arabidopsis thaliana following infection with Plasmodiophora brassicae and the influence of salicylic acid on disease. Mol. Plant Pathol. 2016, 17, 1237–1251. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.Y.; Spivey, N.W.; Zeng, W.Q.; Liu, P.P.; Fu, Z.Q.; Klessig, D.F.; He, S.Y.; Dong, X.N. Coronatine promotes Pseudomonas syringae virulence in plants by activating a signaling cascade that inhibits salicylic acid accumulation. Cell Host Microbe 2012, 11, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Kobelt, P.; Siemens, J.; Sacristán, M.D. Histological characterisation of the incompatible interaction between Arabidopsis thaliana and the obligate biotrophic pathogen Plasmodiophora brassicae. Mycol. Res. 2000, 104, 220–225. [Google Scholar] [CrossRef]
- Řičařová, V.; Kazda, J.; Baranyk, P.; Ryšánek, P. Greenhouse and field experiments with winter oilseed rape cultivars resistant to Plasmodiophora brassicae Wor. Crop Prot. 2017, 92, 60–69. [Google Scholar] [CrossRef]
- Schwelm, A.; Fogelqvist, J.; Knaust, A.; Jülke, S.; Lilja, T.; Bonilla-Rosso, G.; Karlsson, M.; Shevchenko, A.; Choi, S.R.; Dhandapani, V.; et al. The Plasmodiophora brassicae genome reveals insights in its life cycle and ancestry of chitin synthases. Sci. Rep. 2015, 5, 11153. [Google Scholar] [CrossRef]
- Devos, S.; Vissenberg, K.; Verbelen, J.P.; Prinsen, E. Infection of Chinese cabbage by Plasmodiophora brassicae leads to a stimulation of plant growth: Impacts on cell wall metabolism and hormone balance. New Phytol. 2005, 166, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Ando, S.; Asano, T.; Tsushima, S.; Kamachi, S.; Hagio, T.; Tabei, Y. Changes in gene expression of putative isopentenyltransferase during clubroot development in Chinese cabbage (Brassica rapa L.). Physiol. Mol. Plant Pathol. 2005, 67, 59–67. [Google Scholar] [CrossRef]
- Ludwig-Müller, J.; Bendel, U.; Thermann, P.; Ruppel, M.; Epstein, E.; Hilgenberg, W. Concentrations of indole-3-acetic acid in plants of tolerant and susceptible varieties of Chinese cabbage infected with Plasmodiophora brassicae Woron. New Phytol. 1993, 125, 763–769. [Google Scholar] [CrossRef]
- Xu, L.; Ren, L.; Chen, K.; Liu, F.; Fang, X. Putative role of IAA during the early response of Brassica napus L. to Plasmodiophora brassicae. Eur. J. Plant Pathol. 2016, 145, 601–613. [Google Scholar] [CrossRef]
- Jahn, L.; Mucha, S.; Bergmann, S.; Horn, C.; Siemens, J.; Staswick, P.; Steffens, B.; Ludwig-Müller, J. The clubroot pathogen (Plasmodiophora brassicae) influences auxin signaling to regulate auxin homeostasis. Plants 2013, 2, 726–749. [Google Scholar] [CrossRef]
- Irani, S.; Trost, B.; Waldner, M.; Nayidu, N.; Tu, J.Y.; Kusalik, A.J.; Todd, C.D.; Wei, Y.D.; Bonham-Smith, W.C. Transcriptome analysis of response to Plasmodiophora brassicae infection in the Arabidopsis shoot and root. BMC Genom. 2018, 19, 23. [Google Scholar] [CrossRef]
- Ludwig-Müller, J. Plant defence—What can we learn from clubroots? Austral. Plant Pathol. 2009, 38, 318–324. [Google Scholar] [CrossRef]
- Ishikawa, T.; Okazaki, K.; Kuroda, H.; Itoh, K.; Mitsui, T.; Hori, H. Molecular cloning of Brassica rapa nitrilases and their expression during clubroot development. Mol. Plant Pathol. 2007, 8, 623–637. [Google Scholar] [CrossRef]
- Ando, S.; Tsushima, S.; Kamachi, S.; Konagaya, K.I.; Tabei, Y. Alternative transcription initiation of the nitrilase gene (BrNIT2) caused by infection with Plasmodiophora brassicae Woron. in Chinese cabbage (Brassica rapa L.). Plant Mol. Biol. 2008, 68, 557–569. [Google Scholar] [CrossRef]
- Liu, Y.; Yin, Y.-P.; Wang, Z.-K.; Luo, Y.-L. Expression of nitrilases in Brassica juncea var. tumida Tsen in root galls caused by Plasmodiophora brassicae. J. Integr. Agric. 2012, 11, 100–108. [Google Scholar] [CrossRef]
- Grsic-Rausch, S.; Kobelt, P.; Siemens, J.; Bischoff, M.; Ludwig-Müller, J. Expression and localization of nitrilase during symptom development of the clubroot disease in Arabidopsis thaliana. Plant Physiol. 2000, 122, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Ludwig-Müller, J.; Pieper, K.; Ruppel, M.; Cohen, J.D.; Epstein, E.; Kiddle, G.; Bennett, R. Indole glucosinolate and auxin biosynthesis in Arabidopsis thaliana (L.) Heynh. glucosinolate mutants and the development of the clubroot disease. Planta 1999, 208, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, Y.; Fang, Z.; Li, Z.; Yang, L.; Zhuang, M.; Zhang, Y.; Lv, H. Comparative transcriptome analysis between broccoli (Brassica oleracea var. italica) and wild cabbage (Brassica macrocarpa Guss.) in response to Plasmodiophora brassicae during different infection stages. Front. Plant Sci. 2016, 7, 1929. [Google Scholar] [CrossRef] [PubMed]
- Lebel, E.; Heifetz, P.; Thorne, L.; Uknes, S.; Ryals, J.; Ward, E. Functional analysis of regulatory sequences controlling PR-1 gene expression in Arabidopsis. Plant J. 1998, 16, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Ludwig-Müller, J.; Schubert, B.; Pieper, K.; Ihmig, S.; Hilgenberg, W. Glucosinolate content in susceptible and resistant Chinese cabbage varieties during development of clubroot disease. Phytochemistry 1997, 44, 407–414. [Google Scholar] [CrossRef]
- Knaust, A.; Ludwig-Müller, J. The ethylene signaling pathway is needed to restrict root gall growth in Arabidopsis after infection with the obligate biotrophic protist Plasmodiophora brassicae. J. Plant Growth Regul. 2013, 32, 9–21. [Google Scholar] [CrossRef]
- Agarwal, A.; Kaul, V.; Faggian, R.; Rookes, J.E.; Ludwig-Müller, J.; Cahill, D.M. Analysis of global host gene expression during the primary phase of the Arabidopsis thaliana-Plasmodiophora brassicae interaction. Funct. Plant. Biol. 2011, 38, 462–478. [Google Scholar] [CrossRef]
- Lovelock, D.A.; Donald, C.E.; Conlan, X.A.; Cahill, D.M. Salicylic acid suppression of clubroot in broccoli (Brassicae oleracea var. italica) caused by the obligate biotroph Plasmodiophora brassicae. Austral. Plant Pathol. 2013, 42, 141–153. [Google Scholar] [CrossRef]
- Ludwig-Müller, J.; Jülke, S.; Geiß, K.; Richter, F.; Sola, I.; Rusak, G.; Mithöfer, A.; Keenan, S.; Bulman, S. A novel methyltransferase from the intracellular pathogen Plasmodiophora brassicae methylates salicylic acid. Mol. Plant Pathol. 2015, 16, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Bulman, S.; Richter, F.; Marschollek, S.; Benade, F.; Jülke, S.; Ludwig-Müller, J. Arabidopsis thaliana expressing PbBSMT, a gene encoding a SABATH-type methyltransferase from the plant pathogenic protist Plasmodiophora brassicae, show leaf chlorosis and altered host susceptibility. Plant Biol. J. 2018. [Google Scholar] [CrossRef] [PubMed]
- Djavaheri, M.; Ma, L.; Klessig, D.F.; Mithöfer, A.; Gropp, G.; Borhan, M.H. Mimicking the host regulation of SA: A virulence strategy by the clubroot pathogen Plasmodiophora brassicae. Mol. Plant Microbe Interact. 2018. [Google Scholar] [CrossRef] [PubMed]
- Vlot, A.C.; Dempsey, D.A.; Klessig, D.F. Salicylic acid, a multifaceted hormone to combat disease. Annu. Rev. Phytopathol. 2009, 47, 177–206. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, R.; Shanmugam, A.; Hwang, I.; Park, J.-I.; Nou, I.-S. Expression of salicylic acid-related genes in Brassica oleracea var. capitata during Plasmodiophora brassicae infection. Genome 2016, 59, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.H. A system for the determination of races of Plasmodiophora brassicae that infect cabbage and rutabaga. Phytopathology 1966, 56, 624–626. [Google Scholar]
- Tewari, J.P.; Strelkov, S.E.; Orchard, D.; Hartman, M.; Lange, R.M.; Turkington, T.K. Identification of clubroot of crucifers on canola (Brassica napus) in Alberta. Can. J. Plant Pathol. 2005, 27, 143–144. [Google Scholar] [CrossRef]
- Strelkov, S.E.; Tewari, J.P.; Smith-Degenhardt, E. Characterization of Plasmodiophora brassicae populations from Alberta, Canada. Can. J. Plant Pathol. 2006, 28, 467–474. [Google Scholar] [CrossRef]
- Kuginuki, Y.; Yoshikawa, H.; Hirai, M. Variation in virulence of Plasmodiophora brassicae in Japan tested with clubroot- resistant cultivars of Chinese cabbage (Brassica rapa L. ssp. pekinesis). Eur. J. Plant Pathol. 1999, 105, 327–332. [Google Scholar] [CrossRef]
- Horiuchi, S.; Hori, M. A simple greenhouse technique for obtaining high levels of clubroot incidence. Bull. Chugoku Natl. Agric. Exp. Stn. Ser. E 1980, 17, 33–55. [Google Scholar]
- Dobrev, P.I.; Kaminek, M. Fast and efficient separation of cytokinins from auxin and abscisic acid and their purification using mixed-mode solid-phase extraction. J. Chromatogr. A 2002, 950, 21–29. [Google Scholar] [CrossRef]
- Dobrev, P.I.; Vankova, R. Quantification of abscisic acid, cytokinin, and auxin content in salt-stressed plant tissues. In Plant Salt Tolerance. Methods in Molecular Biology (Methods and Protocols); Shabala, S., Cuin, T., Eds.; Humana Press: Totowa, NJ, USA, 2012; Volume 913, pp. 251–261. ISBN 978-1-61779-985-3. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Joshi, R.K.; Megha, S.; Rahman, M.H.; Basu, U.; Kav, N.N. A global study of transcriptome dynamics in canola (Brassica napus L.) responsive to Sclerotinia sclerotiorum infection using RNA-Seq. Gene 2016, 590, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Strelkov, S.E.; Kav, N.N.V. Oxalic acid-mediated stress responses in Brassica napus L. Proteomics 2009, 9, 3156–3173. [Google Scholar] [CrossRef]
- Liu, F.; Li, X.; Wang, M.; Wen, J.; Yi, B.; Shen, J.; Ma, C.; Fu, T.; Tu, J. Interactions of WRKY 15 and WRKY 33 transcription factors and their roles in the resistance of oilseed rape to Sclerotinia infection. Plant Biotechnol. J. 2018, 16, 911–925. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Sanz, H.; Solis, M.T.; Lopez, M.F.; Gomez-Cadenas, A.; Risueno, M.C.; Testillano, P.S. Auxin biosynthesis, accumulation, action and transport are involved in stress-induced microspore embryogenesis initiation and progression in Brassica napus. Plant Cell Physiol. 2015, 56, 1401–1417. [Google Scholar] [CrossRef]
- Šasek, V.; Novakova, M.; Jindrichova, B.; Boka, K.; Valentova, O.; Burketova, L. Recognition of avirulence gene AvrLm1 from hemibiotrophic ascomycete Leptosphaeria maculans triggers salicylic acid and ethylene signaling in Brassica napus. Mol. Plant Microbe Interact. 2012, 25, 1238–1250. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Jiang, L.; Jameson, P.E. Expression patterns of Brassica napus genes implicate IPT, CKX, sucrose transporter, cell wall invertase, and amino acid permease gene family members in leaf, flower, silique, and seed development. J. Exp. Bot. 2015, 66, 5067–5082. [Google Scholar] [CrossRef]
- Zhou, T.; Hua, Y.; Huang, Y.; Ding, G.; Shi, L.; Xu, F. Physiological and transcriptional analyses reveal differential phytohormone responses to boron deficiency in Brassica napus genotypes. Front. Plant Sci. 2016, 7, 221. [Google Scholar] [CrossRef]
- Pontius, J.; Wagner, L.; Schuler, G. The NCBI Handbook; National Center for Biotechnology Information: Bethesda, MD, USA, 2003. [Google Scholar]
- Untergasser, A.; Nijveen, H.; Rao, X.; Bisseling, T.; Geurts, R.; Leunissen, J.A. Primer3Plus, an enhanced web interface to Primer3. Nucleic Acids Res. 2007, 35, W71–W74. [Google Scholar] [CrossRef] [PubMed]
- Zuker, M.; Mathews, D.H.; Turner, D.H. Algorithms and thermodynamics for RNA secondary structure prediction: A practical guide. In RNA Biochemistry and Biotechnology; Barciszewski, J., Clark, B.F.C., Eds.; Springer: Dordrecht, The Netherlands, 1999; Volume 70, ISBN 978-0-7923-5862-6. [Google Scholar]
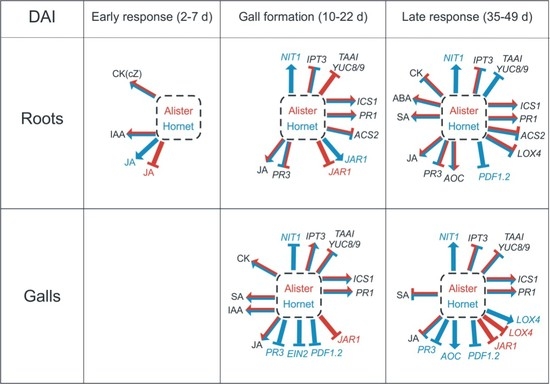
 ) by foliar spray with 1 mM salicylic acid (SA)—once (SA + Plasmodiophora) or twice (two SA + Plasmodiophora); or with 1 mM jasmonic acid (JA) (JA + Plasmodiophora). Samples were collected 2–49 days after inoculation (dai) at the days indicated in the scheme.
) by foliar spray with 1 mM salicylic acid (SA)—once (SA + Plasmodiophora) or twice (two SA + Plasmodiophora); or with 1 mM jasmonic acid (JA) (JA + Plasmodiophora). Samples were collected 2–49 days after inoculation (dai) at the days indicated in the scheme.
 ) by foliar spray with 1 mM salicylic acid (SA)—once (SA + Plasmodiophora) or twice (two SA + Plasmodiophora); or with 1 mM jasmonic acid (JA) (JA + Plasmodiophora). Samples were collected 2–49 days after inoculation (dai) at the days indicated in the scheme.
) by foliar spray with 1 mM salicylic acid (SA)—once (SA + Plasmodiophora) or twice (two SA + Plasmodiophora); or with 1 mM jasmonic acid (JA) (JA + Plasmodiophora). Samples were collected 2–49 days after inoculation (dai) at the days indicated in the scheme.







| Disease Index (%) | |||||
|---|---|---|---|---|---|
| dai | Experimental Variant | Hornet | Alister | ||
| 2016 | 2017 | 2016 | 2017 | ||
| 10 | Plasmodiophora | 0 | 0 | 0 | 0 |
| SA + Plasmodiophora | 0 | 0 | |||
| 2 SA + Plasmodiophora | 0 | 0 | 0 | 0 | |
| JA + Plasmodiophora | 0 | 0 | |||
| 15 | Plasmodiophora | 0 | 0 | 0 | 0 |
| SA + Plasmodiophora | 0 | 0 | |||
| 2 SA + Plasmodiophora | 0 | 19.0 | 0 | 0 | |
| JA + Plasmodiophora | 55.6 | 0 | |||
| 22 | Plasmodiophora | 100.0 | 40 | 6.7 | 6.7 |
| SA + Plasmodiophora | 46.7 | 6.7 | |||
| 2 SA + Plasmodiophora | 40.0 | 53.3 | 0 | 0 | |
| JA + Plasmodiophora | 60.0 | 13.3 | |||
| 35 | Plasmodiophora | 100.0 | 33.3 | 6.7 | 0 |
| SA + Plasmodiophora | 53.3 | 13.3 | |||
| 2 SA + Plasmodiophora | 46.7 | 60.0 | 0 | 0 | |
| JA + Plasmodiophora | 83.3 | 0 | |||
| 42 | Plasmodiophora | 100.0 | 53.3 | 40.0 | 0 |
| SA + Plasmodiophora | 86.7 | 6.7 | |||
| 2 SA + Plasmodiophora | 60.0 | 58.3 | 0 | 0 | |
| JA + Plasmodiophora | 88.9 | 3.7 | |||
| 49 | Plasmodiophora | 100 | 0 | ||
| SA + Plasmodiophora | |||||
| 2 SA + Plasmodiophora | 66.7 | 0 | |||
| JA + Plasmodiophora | 75.0 | 0 | |||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prerostova, S.; Dobrev, P.I.; Konradyova, V.; Knirsch, V.; Gaudinova, A.; Kramna, B.; Kazda, J.; Ludwig-Müller, J.; Vankova, R. Hormonal Responses to Plasmodiophora brassicae Infection in Brassica napus Cultivars Differing in Their Pathogen Resistance. Int. J. Mol. Sci. 2018, 19, 4024. https://doi.org/10.3390/ijms19124024
Prerostova S, Dobrev PI, Konradyova V, Knirsch V, Gaudinova A, Kramna B, Kazda J, Ludwig-Müller J, Vankova R. Hormonal Responses to Plasmodiophora brassicae Infection in Brassica napus Cultivars Differing in Their Pathogen Resistance. International Journal of Molecular Sciences. 2018; 19(12):4024. https://doi.org/10.3390/ijms19124024
Chicago/Turabian StylePrerostova, Sylva, Petre I. Dobrev, Veronika Konradyova, Vojtech Knirsch, Alena Gaudinova, Barbara Kramna, Jan Kazda, Jutta Ludwig-Müller, and Radomira Vankova. 2018. "Hormonal Responses to Plasmodiophora brassicae Infection in Brassica napus Cultivars Differing in Their Pathogen Resistance" International Journal of Molecular Sciences 19, no. 12: 4024. https://doi.org/10.3390/ijms19124024
APA StylePrerostova, S., Dobrev, P. I., Konradyova, V., Knirsch, V., Gaudinova, A., Kramna, B., Kazda, J., Ludwig-Müller, J., & Vankova, R. (2018). Hormonal Responses to Plasmodiophora brassicae Infection in Brassica napus Cultivars Differing in Their Pathogen Resistance. International Journal of Molecular Sciences, 19(12), 4024. https://doi.org/10.3390/ijms19124024