Innovative Clinical Perspectives for CIK Cells in Cancer Patients
Abstract
:1. Introduction
1.1. CIK Cell Production: Cell Factory and Protocol
1.2. CIK Cell Cultures Heterogeneity
1.3. Mechanism of Action: Cytotoxic Activity of CIK Cells
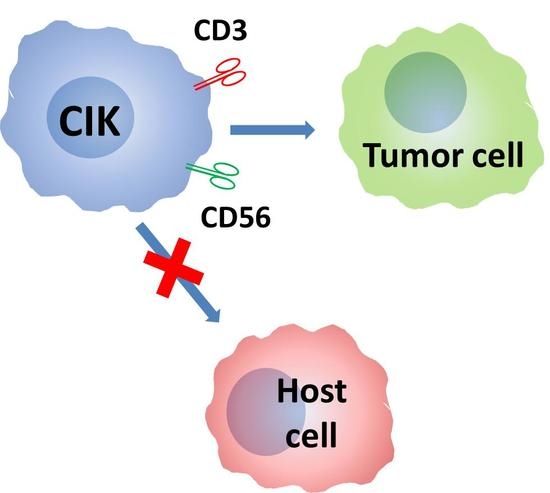
1.4. Mechanism of Action: Receptors Involved in Activity of CIK Cells
1.5. Antitumoral Activity of Human CIK Cells In Vitro
1.6. Antitumoral and Absence of GvHD Activity by Mouse CIK Cells in Mouse Models
1.7. Antitumoral Activity of Human CIK In Vivo in Animal Models
1.8. Studies in Patients
1.9. CIK in the Autologous Setting
1.10. CIK in the Allogeneic Setting
1.11. CIK in the Haploidentical Setting
2. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lanier, L.L.; Spits, H.; Phillips, J.H. The developmental relationship between NK cells and T cells. Immunol. Today 1992, 13, 392–395. [Google Scholar] [CrossRef]
- Lu, P.H.; Negrin, R.S. A novel population of expanded human CD3+CD56+ cells derived from T cells with potent in vivo antitumor activity in mice with severe combined immunodeficiency. J. Immunol. 1994, 153, 1687–1696. [Google Scholar] [PubMed]
- Schmidt-Wolf, I.G.; Lefterova, P.; Mehta, B.A.; Fernandez, L.P.; Huhn, D.; Blume, K.G.; Weissman, I.L.; Negrin, R.S. Phenotypic characterization and identification of effector cells involved in tumor cell recognition of cytokine-induced killer cells. Exp. Hematol. 1993, 21, 1673–1679. [Google Scholar] [PubMed]
- Franceschetti, M.; Pievani, A.; Borleri, G.; Vago, L.; Fleischhauer, K.; Golay, J.; Introna, M. Cytokine-induced killer cells are terminally differentiated activated CD8 cytotoxic T-EMRA lymphocytes. Exp. Hematol. 2009, 37, 616–628.e2. [Google Scholar] [CrossRef] [PubMed]
- Linn, Y.C.; Lau, S.K.; Liu, B.H.; Ng, L.H.; Yong, H.X.; Hui, K.M. Characterization of the recognition and functional heterogeneity exhibited by cytokine-induced killer cell subsets against acute myeloid leukaemia target cell. Immunology 2009, 126, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Alvarnas, J.C.; Linn, Y.C.; Hope, E.G.; Negrin, R.S. Expansion of cytotoxic CD3+CD56+ cells from peripheral blood progenitor cells of patients undergoing autologous hematopoietic cell transplantation. Biol. Blood Marrow Transplant. 2001, 7, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Linn, Y.C.; Lau, L.C.; Hui, K.M. Generation of cytokine-induced killer cells from leukaemic samples with in vitro cytotoxicity against autologous and allogeneic leukaemic blasts. Br. J. Haematol. 2002, 116, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Hoyle, C.; Bangs, C.D.; Chang, P.; Kamel, O.; Mehta, B.; Negrin, R.S. Expansion of Philadelphia chromosome-negative CD3+CD56+ cytotoxic cells from chronic myeloid leukemia patients: In vitro and in vivo efficacy in severe combined immunodeficiency disease mice. Blood 1998, 92, 3318–3327. [Google Scholar] [PubMed]
- Scheffold, C.; Brandt, K.; Johnston, V.; Lefterova, P.; Degen, B.; Schontube, M.; Huhn, D.; Neubauer, A.; Schmidt-Wolf, I.G. Potential of autologous immunologic effector cells for bone marrow purging in patients with chronic myeloid leukemia. Bone Marrow Transplant. 1995, 15, 33–39. [Google Scholar] [PubMed]
- Sconocchia, G.; Lau, M.; Provenzano, M.; Rezvani, K.; Wongsena, W.; Fujiwara, H.; Hensel, N.; Melenhorst, J.; Li, J.; Ferrone, S.; et al. The antileukemia effect of HLA-matched NK and NK-T cells in chronic myelogenous leukemia involves NKG2D-target-cell interactions. Blood 2005, 106, 3666–3672. [Google Scholar] [CrossRef] [PubMed]
- Kornacker, M.; Moldenhauer, G.; Herbst, M.; Weilguni, E.; Tita-Nwa, F.; Harter, C.; Hensel, M.; Ho, A.D. Cytokine-induced killer cells against autologous CLL: Direct cytotoxic effects and induction of immune accessory molecules by interferon-gamma. Int. J. Cancer 2006, 119, 1377–1382. [Google Scholar] [CrossRef] [PubMed]
- Lefterova, P.; Schakowski, F.; Buttgereit, P.; Scheffold, C.; Huhn, D.; Schmidt-Wolf, I.G. Expansion of CD3+CD56+ cytotoxic cells from patients with chronic lymphocytic leukemia: In vitro efficacy. Haematologica 2000, 85, 1108–1109. [Google Scholar] [PubMed]
- Linn, Y.C.; Wang, S.M.; Hui, K.M. Comparative gene expression profiling of cytokine-induced killer cells in response to acute myloid leukemic and acute lymphoblastic leukemic stimulators using oligonucleotide arrays. Exp. Hematol. 2005, 33, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Hongeng, S.; Petvises, S.; Worapongpaiboon, S.; Rerkamnuaychoke, B.; Pakakasama, S.; Jootar, S. Generation of CD3+CD56+ cytokine-induced killer cells and their in vitro cytotoxicity against pediatric cancer cells. Int. J. Hematol. 2003, 77, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Kuci, S.; Rettinger, E.; Voss, B.; Weber, G.; Stais, M.; Kreyenberg, H.; Willasch, A.; Kuci, Z.; Koscielniak, E.; Kloss, S.; et al. Efficient lysis of rhabdomyosarcoma cells by cytokine-induced killer cells: Implications for adoptive immunotherapy after allogeneic stem cell transplantation. Haematologica 2010, 95, 1579–1586. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.F.; Kunda, P.E.; Lin, J.W.; Wang, H.; Chen, X.M.; Liu, Q.L.; Liu, T. Cytokine-induced killer cells co-cultured with complete tumor antigen-loaded dendritic cells, have enhanced selective cytotoxicity on carboplatin-resistant retinoblastoma cells. Oncol. Rep. 2013, 29, 1841–1850. [Google Scholar] [CrossRef] [PubMed]
- Lopez, R.D.; Waller, E.K.; Lu, P.H.; Negrin, R.S. CD58/LFA-3 and IL-12 provided by activated monocytes are critical in the in vitro expansion of CD56+ T cells. Cancer Immunol. Immunother. 2001, 49, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Wolf, I.G.; Lefterova, P.; Johnston, V.; Scheffold, C.; Csipai, M.; Mehta, B.A.; Tsuruo, T.; Huhn, D.; Negrin, R.S. Sensitivity of multidrug-resistant tumor cell lines to immunologic effector cells. Cell. Immunol. 1996, 169, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Mehta, B.A.; Schmidt-Wolf, I.G.; Weissman, I.L.; Negrin, R.S. Two pathways of exocytosis of cytoplasmic granule contents and target cell killing by cytokine-induced CD3+CD56+ killer cells. Blood 1995, 86, 3493–3499. [Google Scholar] [PubMed]
- Houchins, J.P.; Yabe, T.; McSherry, C.; Bach, F.H. DNA sequence analysis of NKG2, a family of related cDNA clones encoding type II integral membrane proteins on human natural killer cells. J. Exp. Med. 1991, 173, 1017–1020. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, A.M.; Diefenbach, A.; McMahon, C.W.; Xiong, N.; Carlyle, J.R.; Raulet, D.H. The role of the NKG2D immunoreceptor in immune cell activation and natural killing. Immunity 2002, 17, 19–29. [Google Scholar] [CrossRef]
- Diefenbach, A.; Jamieson, A.M.; Liu, S.D.; Shastri, N.; Raulet, D.H. Ligands for the murine NKG2D receptor: Expression by tumor cells and activation of NK cells and macrophages. Nat. Immunol. 2000, 1, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Verneris, M.R.; Karami, M.; Baker, J.; Jayaswal, A.; Negrin, R.S. Role of NKG2D signaling in the cytotoxicity of activated and expanded CD8+ T cells. Blood 2004, 103, 3065–3072. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.; Cao, T.M.; Baker, J.A.; Verneris, M.R.; Soares, L.; Negrin, R.S. Silencing human NKG2D, DAP10, and DAP12 reduces cytotoxicity of activated CD8+ T cells and NK cells. J. Immunol. 2005, 175, 7819–7828. [Google Scholar] [CrossRef] [PubMed]
- Rettinger, E.; Kuci, S.; Naumann, I.; Becker, P.; Kreyenberg, H.; Anzaghe, M.; Willasch, A.; Koehl, U.; Bug, G.; Ruthardt, M.; et al. The cytotoxic potential of interleukin-15-stimulated cytokine-induced killer cells against leukemia cells. Cytotherapy 2012, 14, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Pievani, A.; Borleri, G.; Pende, D.; Moretta, L.; Rambaldi, A.; Golay, J.; Introna, M. Dual-functional capability of CD3+CD56+ CIK cells, a T-cell subset that acquires NK function and retains TCR-mediated specific cytotoxicity. Blood 2011, 118, 3301–3310. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Grzywacz, B.; Wang, H.; Kataria, N.; Cao, Q.; Wagner, J.E.; Blazar, B.R.; Miller, J.S.; Verneris, M.R. Umbilical cord blood T cells express multiple natural cytotoxicity receptors after IL-15 stimulation, but only NKp30 is functional. J. Immunol. 2008, 181, 4507–4515. [Google Scholar] [CrossRef] [PubMed]
- Marten, A.; Ziske, C.; Schottker, B.; Renoth, S.; Weineck, S.; Buttgereit, P.; Schakowski, F.; von Rucker, A.; Sauerbruch, T.; Schmidt-Wolf, I.G. Interactions between dendritic cells and cytokine-induced killer cells lead to an activation of both populations. J. Immunother. 2001, 24, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, C.; Yu, J.; Cao, S.; Wei, F.; Zhang, W.; Han, Y.; Ren, X.B. Dendritic cell-activated cytokine-induced killer cells enhance the anti-tumor effect of chemotherapy on non-small cell lung cancer in patients after surgery. Cytotherapy 2009, 11, 1076–1083. [Google Scholar] [CrossRef] [PubMed]
- Wongkajornsilp, A.; Wamanuttajinda, V.; Kasetsinsombat, K.; Duangsa-ard, S.; Sa-ngiamsuntorn, K.; Hongeng, S.; Maneechotesuwan, K. Sunitinib indirectly enhanced anti-tumor cytotoxicity of cytokine-induced killer cells and CD3+CD56+ subset through the co-culturing dendritic cells. PLoS ONE 2013, 8, e78980. [Google Scholar] [CrossRef] [PubMed]
- Ferlazzo, G.; Tsang, M.L.; Moretta, L.; Melioli, G.; Steinman, R.M.; Munz, C. Human dendritic cells activate resting natural killer (NK) cells and are recognized via the NKp30 receptor by activated NK cells. J. Exp. Med. 2002, 195, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Vitale, M.; Della Chiesa, M.; Carlomagno, S.; Pende, D.; Arico, M.; Moretta, L.; Moretta, A. NK-dependent DC maturation is mediated by TNFalpha and IFNgamma released upon engagement of the NKp30 triggering receptor. Blood 2005, 106, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Walzer, T.; Dalod, M.; Robbins, S.H.; Zitvogel, L.; Vivier, E. Natural-killer cells and dendritic cells: “l’union fait la force”. Blood 2005, 106, 2252–2258. [Google Scholar] [CrossRef] [PubMed]
- Joshi, P.S.; Liu, J.Q.; Wang, Y.; Chang, X.; Richards, J.; Assarsson, E.; Shi, F.D.; Ljunggren, H.G.; Bai, X.F. Cytokine-induced killer T cells kill immature dendritic cells by TCR-independent and perforin-dependent mechanisms. J. Leukoc. Biol. 2006, 80, 1345–1353. [Google Scholar] [CrossRef] [PubMed]
- Valgardsdottir, R.; Capitanio, C.; Texido, G.; Pende, D.; Cantoni, C.; Pesenti, E.; Rambaldi, A.; Golay, J.; Introna, M. Direct involvement of CD56 in cytokine-induced killer-mediated lysis of CD56+ hematopoietic target cells. Exp. Hematol. 2014, 42, 1013–1021.e1. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.S.; Liu, M.X.; Zhang, B.; Shi, M.; Lei, Z.Y.; Sun, W.B.; Du, Q.Y.; Chen, J.M. Antitumor activities of human autologous cytokine-induced killer (CIK) cells against hepatocellular carcinoma cells in vitro and in vivo. World J. Gastroenterol. 2002, 8, 464–468. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Wolf, I.G.; Negrin, R.S.; Kiem, H.P.; Blume, K.G.; Weissman, I.L. Use of a SCID mouse/human lymphoma model to evaluate cytokine-induced killer cells with potent antitumor cell activity. J. Exp. Med. 1991, 174, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Verneris, M.R.; Ito, M.; Baker, J.; Arshi, A.; Negrin, R.S.; Shizuru, J.A. Engineering hematopoietic grafts: Purified allogeneic hematopoietic stem cells plus expanded CD8+ NK-T cells in the treatment of lymphoma. Biol. Blood Marrow Transplant. 2001, 7, 532–542. [Google Scholar] [CrossRef]
- Baker, J.; Verneris, M.R.; Ito, M.; Shizuru, J.A.; Negrin, R.S. Expansion of cytolytic CD8+ natural killer T cells with limited capacity for graft-versus-host disease induction due to interferon γ production. Blood 2001, 97, 2923–2931. [Google Scholar] [CrossRef] [PubMed]
- Edinger, M.; Cao, Y.A.; Verneris, M.R.; Bachmann, M.H.; Contag, C.H.; Negrin, R.S. Revealing lymphoma growth and the efficacy of immune cell therapies using in vivo bioluminescence imaging. Blood 2003, 101, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, R.; Baker, J.; Beilhack, A.; Zeiser, R.; Olson, J.A.; Sega, E.I.; Karimi, M.; Negrin, R.S. In vivo trafficking and survival of cytokine-induced killer cells resulting in minimal GVHD with retention of antitumor activity. Blood 2008, 112, 2563–2574. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, T.J.; Mailander, V.; Tucker, A.A.; Olomu, A.B.; Zhang, W.; Cao, Y.; Negrin, R.S.; Contag, C.H. Visualizing the kinetics of tumor-cell clearance in living animals. Proc. Natl. Acad. Sci. USA 1999, 96, 12044–12049. [Google Scholar] [CrossRef] [PubMed]
- Hontscha, C.; Borck, Y.; Zhou, H.; Messmer, D.; Schmidt-Wolf, I.G. Clinical trials on CIK cells: First report of the international registry on CIK cells (IRCC). J. Cancer Res. Clin. Oncol. 2011, 137, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Leemhuis, T.; Wells, S.; Scheffold, C.; Edinger, M.; Negrin, R.S. A phase I trial of autologous cytokine-induced killer cells for the treatment of relapsed Hodgkin disease and non-Hodgkin lymphoma. Biol. Blood Marrow Transplant. 2005, 11, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Olioso, P.; Giancola, R.; Di Riti, M.; Contento, A.; Accorsi, P.; Iacone, A. Immunotherapy with cytokine induced killer cells in solid and hematopoietic tumours: A pilot clinical trial. Hematol. Oncol. 2009, 27, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Linn, Y.C.; Yong, H.X.; Niam, M.; Lim, T.J.; Chu, S.; Choong, A.; Chuah, C.; Goh, Y.T.; Hwang, W.; Loh, Y.; et al. A phase I/II clinical trial of autologous cytokine-induced killer cells as adjuvant immunotherapy for acute and chronic myeloid leukemia in clinical remission. Cytotherapy 2012, 14, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Lu, X.C.; Yu, R.L.; Chi, X.H.; Liu, Y.; Wang, Y.; Dai, H.R.; Zhu, H.L.; Cai, L.L.; Han, W.D. Repeated transfusions of autologous cytokine-induced killer cells for treatment of haematological malignancies in elderly patients: A pilot clinical trial. Hematol. Oncol. 2012, 30, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.C.; Yang, B.; Yu, R.L.; Chi, X.H.; Tuo, S.; Tuo, C.W.; Zhu, H.L.; Wang, Y.; Jiang, C.G.; Fu, X.B.; et al. Clinical study of autologous cytokine-induced killer cells for the treatment of elderly patients with diffuse large B-cell lymphoma. Cell Biochem. Biophys. 2011, 62, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bo, J.; Dai, H.R.; Lu, X.C.; Lv, H.Y.; Yang, B.; Wang, T.; Han, W.D. CIK cells from recurrent or refractory AML patients can be efficiently expanded in vitro and used for reduction of leukemic blasts in vivo. Exp. Hematol. 2013, 41, 241–252.e3. [Google Scholar] [CrossRef] [PubMed]
- Introna, M.; Borleri, G.; Conti, E.; Franceschetti, M.; Barbui, A.M.; Broady, R.; Dander, E.; Gaipa, G.; D’Amico, G.; Biagi, E.; et al. Repeated infusions of donor-derived cytokine-induced killer cells in patients relapsing after allogeneic stem cell transplantation: A phase I study. Haematologica 2007, 92, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Laport, G.G.; Sheehan, K.; Baker, J.; Armstrong, R.; Wong, R.M.; Lowsky, R.; Johnston, L.J.; Shizuru, J.A.; Miklos, D.; Arai, S.; et al. Adoptive immunotherapy with cytokine-induced killer cells for patients with relapsed hematologic malignancies after allogeneic hematopoietic cell transplantation. Biol. Blood Marrow Transplant. 2011, 17, 1679–1687. [Google Scholar] [CrossRef] [PubMed]
- Linn, Y.C.; Niam, M.; Chu, S.; Choong, A.; Yong, H.X.; Heng, K.K.; Hwang, W.; Loh, Y.; Goh, Y.T.; Suck, G.; et al. The anti-tumour activity of allogeneic cytokine-induced killer cells in patients who relapse after allogeneic transplant for haematological malignancies. Bone Marrow Transplant. 2012, 47, 957–966. [Google Scholar] [CrossRef] [PubMed]
- Introna, M.; Lussana, F.; Algarotti, A.; Gotti, E.; Valgardsdottir, R.; Mico, C.; Grassi, A.; Pavoni, C.; Ferrari, M.L.; Delaini, F.; et al. Phase II Study of Sequential Infusion of Donor Lymphocyte Infusion and Cytokine-Induced Killer Cells for Patients Relapsed after Allogeneic Hematopoietic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2017, 23, 2070–2078. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, H.; Liu, C.; Jiao, X.; Liu, D.; Du, W.; He, Y.; Zhang, Z.; Wu, X.; Wang, J.; et al. Human leukocyte antigen-haploidentical donor-derived cytokine-induced killer cells are safe and prolong the survival of patients with advanced non-small cell lung cancer. Oncol. Lett. 2014, 8, 2727–2733. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhu, J.; Sun, H.; Shao, L.; Xu, M.; Guo, H. Family haploidentical donor-derived cytokine-induced killer cell biotherapy combined with bortezomib in two patients with relapsed multiple myeloma in a non-allogeneic transplant setting. Leuk. Lymphoma 2013, 54, 209–211. [Google Scholar] [CrossRef] [PubMed]
- Rettinger, E.; Huenecke, S.; Bonig, H.; Merker, M.; Jarisch, A.; Soerensen, J.; Willasch, A.; Bug, G.; Schulz, A.; Klingebiel, T.; et al. Interleukin-15-activated cytokine-induced killer cells may sustain remission in leukemia patients after allogeneic stem cell transplantation: Feasibility, safety and first insights on efficacy. Haematologica 2016, 101, e153–e156. [Google Scholar] [CrossRef] [PubMed]
- Rettinger, E.; Bonig, H.; Wehner, S.; Lucchini, G.; Willasch, A.; Jarisch, A.; Soerensen, J.; Esser, R.; Rossig, C.; Klingebiel, T.; et al. Feasibility of IL-15-activated cytokine-induced killer cell infusions after haploidentical stem cell transplantation. Bone Marrow Transplant. 2013, 48, 1141–1143. [Google Scholar] [CrossRef] [PubMed]
- Golay, J.; D’Amico, A.; Borleri, G.; Bonzi, M.; Valgardsdottir, R.; Alzani, R.; Cribioli, S.; Albanese, C.; Pesenti, E.; Finazzi, M.C.; et al. A novel method using blinatumomab for efficient, clinical-grade expansion of polyclonal T cells for adoptive immunotherapy. J. Immunol. 2014, 193, 4739–4747. [Google Scholar] [CrossRef] [PubMed]
- Pfirrmann, V.; Oelsner, S.; Rettinger, E.; Huenecke, S.; Bonig, H.; Merker, M.; Wels, W.S.; Cinatl, J.; Schubert, R.; Klingebiel, T.; et al. Cytomegalovirus-specific cytokine-induced killer cells: Concurrent targeting of leukemia and cytomegalovirus. Cytotherapy 2015, 17, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Biondi, A.; Magnani, C.F.; Tettamanti, S.; Gaipa, G.; Biagi, E. Redirecting T cells with Chimeric Antigen Receptor (CAR) for the treatment of childhood acute lymphoblastic leukemia. J. Autoimmun. 2017, 85, 141–152. [Google Scholar] [CrossRef] [PubMed]

| # Patients | Median Cells Infused | Acute Toxicity | GVHD | Reference |
|---|---|---|---|---|
| 11 | 12.4 × 109/kg | - | 4 aGvHD I + II | [50] |
| 2 cGvHD | ||||
| 18 | from 1 × 107/kg to 1 × 108/kg | - | 2 aGvHD I + II 1 cGvHD mild | [51] |
| 24 | - | - | 3 aGvHD I | [52] |
| 74 | 20 × 106/kg | - | 12 aGvHD (4 grade I; 3 grade II; 4 grade III; 1 grade IV) | [53] |
| 11 cGvHD (4 mild; 5 moderate; 2 severe) |
| # Patients | Median Cells Infused | Acute Toxicity | GvHD | Reference |
|---|---|---|---|---|
| 170 (solid cancers) | 3–5 administrations × 5 × 109 | 11 insomnia; 10 fever; 1 nausea; 1 vomiting | - | [54] |
| 2 | from 1 × 107/kg to 1 × 108/kg | - | - | [55] |
| 13 | from 0.1 × 106/kg to 100 × 106/kg | - | 3 aGvHD III | [56] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Introna, M.; Correnti, F. Innovative Clinical Perspectives for CIK Cells in Cancer Patients. Int. J. Mol. Sci. 2018, 19, 358. https://doi.org/10.3390/ijms19020358
Introna M, Correnti F. Innovative Clinical Perspectives for CIK Cells in Cancer Patients. International Journal of Molecular Sciences. 2018; 19(2):358. https://doi.org/10.3390/ijms19020358
Chicago/Turabian StyleIntrona, Martino, and Fabio Correnti. 2018. "Innovative Clinical Perspectives for CIK Cells in Cancer Patients" International Journal of Molecular Sciences 19, no. 2: 358. https://doi.org/10.3390/ijms19020358
APA StyleIntrona, M., & Correnti, F. (2018). Innovative Clinical Perspectives for CIK Cells in Cancer Patients. International Journal of Molecular Sciences, 19(2), 358. https://doi.org/10.3390/ijms19020358