Inhibitory Effects of Human Primary Intervertebral Disc Cells on Human Primary Osteoblasts in a Co-Culture System
Abstract
:1. Introduction
2. Results
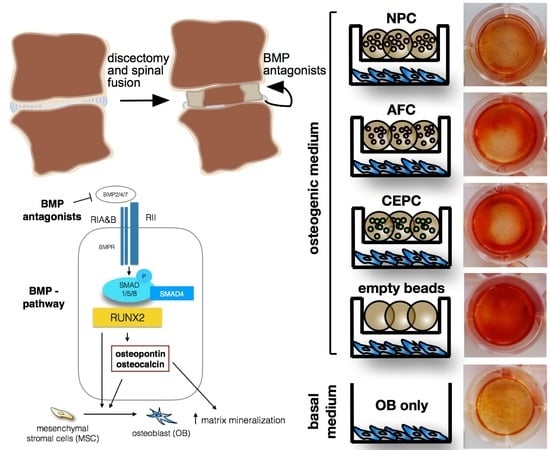
2.1. Matrix Mineralization and Alkaline Phosphatase (ALP) Activity of Human Primary OB
2.2. Expression of Major Bone Genes in Human Primary OB
2.3. Inhibition of Intracellular Signaling
2.4. Expression of Major IVD/Bone Marker and Several BMP Antagonist Genes in 3D IVD Cell Alginate Culture
3. Discussion
4. Materials and Methods
4.1. IVD and OB Donor Materials and Cell Isolation
4.2. Cell Encapsulation and Co-Culture
4.3. Histological Staining of Cell Mineralization
4.4. Alkaline Phosphatase Activity and Total Protein Content
4.5. Analysis of Specific Gene Expression in Human Primary OB Monolayer and Human Primary IVD 3D Culture with Quantitative Polymerase Chain Reaction (qPCR)
4.6. SMAD1/5/8 Protein Quantification by Western Blot Analysis
4.7. Statistics
5. Conclusions
- Dose-dependent inhibitory effects of IVD cell number on primary OB could not be statistically confirmed at the RNA and protein levels.
- A strong donor variation was found in the clinically derived cells for the inhibition of OBs.
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ACAN | Aggrecan |
| AF | Annulus fibrosus |
| AFC | Annulus fibrosus cells |
| ALP | Alkaline phosphatase |
| ALZR | Alizarin red S |
| BGLAP | Osteocalcin |
| BMP | Bone morphogenetic protein |
| CEP | Cartilaginous endplate |
| CEPC | Cartilaginous endplate cells |
| CHRD | Chordin |
| COL1 | Collagen type 1 |
| COL2 | Collagen type 2 |
| FBS | Fetal bovine serum |
| FST | Follistatin |
| GREM1 | Gremlin 1 |
| GREM2 | Gremlin 2 |
| IVD | Intervertebral disc |
| KRT18 | Cyto-keratin 18 |
| LG-DMEM | Low glucose-Dulbecco’s Modified Eagle Medium |
| MSC | Mesenchymal stromal cells |
| NP | Nucleus pulposus |
| NPC | Nucleus pulposus cells |
| OB | Osteoblast |
| P/S | Penicillin/streptomycin |
| qPCR | Quantitative polymerase chain reaction |
| RUNX2 | Runt-related transcription factor 2 |
| SD | Standard deviation |
| SEM | Standard error of mean |
| SPP1 | Osteopontin |
| TWSG1 | Twisted gastrulation BMP signaling modulator 1 |
References
- Manchikanti, L. Epidemiology of low back pain. Pain Physician 2000, 3, 167–192. [Google Scholar] [PubMed]
- Rubin, D.I. Epidemiology and risk factors for spine pain. Neurol. Clin. 2007, 25, 353–371. [Google Scholar] [CrossRef] [PubMed]
- Hoy, D.; March, L.; Brooks, P.; Blyth, F.; Woolf, A.; Bain, C.; Williams, G.; Smith, E.; Vos, T.; Barendregt, J.; et al. The global burden of low back pain: Estimates from the Global Burden of Disease 2010 study. Ann. Rheum. Dis. 2014, 73, 968–974. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, N.N.; Mandalia, S.; Raasch, J.; Knezevic, I.; Candido, K.D. Treatment of chronic low back pain—New approaches on the horizon. J. Pain Res. 2017, 10, 1111–1123. [Google Scholar] [CrossRef] [PubMed]
- Bodalia, P.N.; Balaji, V.; Kaila, R.; Wilson, L. Effectiveness and safety of recombinant human bone morphogenetic protein-2 for adults with lumbar spine pseudarthrosis following spinal fusion surgery: A systematic review. Bone Jt. Res. 2016, 5, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Berjano, P.; Langella, F.; Damilano, M.; Pejrona, M.; Buric, J.; Ismael, M.; Villafañe, J.H.; Lamartina, C. Fusion rate following extreme lateral lumbar interbody fusion. Eur. Spine J. 2015, 24, S369–S371. [Google Scholar] [CrossRef] [PubMed]
- Chun, D.S.; Baker, K.C.; Hsu, W.K. Lumbar pseudarthrosis: A review of current diagnosis and treatment. Neurosurg. Focus 2015, 39, E10. [Google Scholar] [CrossRef] [PubMed]
- DePalma, A.F.; Rothman, R.H. The nature of pseudoarthrosis. 1968. Clin. Orthop. Relat. Res. 1968, 284, 3–9. [Google Scholar]
- Chan, S.C.; Tekari, A.; Benneker, L.M.; Heini, P.F.; Gantenbein, B. Osteogenic differentiation of bone marrow stromal cells is hindered by the presence of intervertebral disc cells. Arthritis Res. Ther. 2015, 18, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Zou, X.; Laursen, M.; Egund, N.; Lind, M.; Bünger, C. The influence of intervertebral disc tissue on anterior spinal interbody fusion: An experimental study on pigs. Eur. Spine J. 2002, 11, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Rosen, V. BMP and BMP inhibitors in bone. Ann. N. Y. Acad. Sci. 2006, 1068, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Deng, C.; Li, Y.P. TGF-β and BMP signaling in osteoblast differentiation and bone formation. Int. J. Biol. Sci. 2012, 8, 272–288. [Google Scholar] [CrossRef] [PubMed]
- Canalis, E.; Economides, A.N.; Gazzerro, E. Bone morphogenetic proteins, their antagonists, and the skeleton. Endocr. Rev. 2003, 24, 218–235. [Google Scholar] [CrossRef] [PubMed]
- Van Bezooijen, R.L.; Svensson, J.P.; Eefting, D.; Visser, A.; van der Horst, G.; Karperien, M.; Quax, P.H.; Vrieling, H.; Papapoulos, S.E.; ten Dijke, P.; et al. Wnt but not BMP signaling is involved in the inhibitory action of sclerostin on BMP-stimulated bone formation. J. Bone Miner. Res. 2007, 22, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.C.; Wang, S.H.; Xiong, F.; Zhao, C.P.; Peng, F.N.; Feng, S.W.; Li, M.S.; Li, Y.; Zhang, C. Wnt3a signaling promotes proliferation, myogenic differentiation, and migration of rat bone marrow mesenchymal stem cells. Acta Pharmacol. Sin. 2007, 28, 1761–1774. [Google Scholar] [CrossRef] [PubMed]
- Matta, A.; Karim, M.Z.; Isenman, D.E.; Erwin, W.M. Molecular Therapy for Degenerative Disc Disease: Clues from Secretome Analysis of the Notochordal Cell-Rich Nucleus Pulposus. Sci. Rep. 2017, 7, 45623. [Google Scholar] [CrossRef] [PubMed]
- Hiyama, A.; Sakai, D.; Tanaka, M.; Arai, F.; Nakajima, D.; Abe, K.; Mochida, J. The relationship between the Wnt/β-catenin and TGF-β/BMP signals in the intervertebral disc cell. J. Cell. Physiol. 2011, 226, 1139–1148. [Google Scholar] [CrossRef] [PubMed]
- Vaibhav, B.; Nilesh, P.; Vikram, S.; Anshul, C. Bone morphogenic protein and its application in trauma cases: A current concept update. Injury 2007, 38, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Walsh, D.W.; Godson, C.; Brazil, D.P.; Martin, F. Extracellular BMP-antagonist regulation in development and disease: Tied up in knots. Trends Cell Biol. 2010, 20, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Chen, G.; Li, Y.-P. TGF-β and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res. 2016, 4, 16009. [Google Scholar] [CrossRef] [PubMed]
- Nolan, K.; Thompson, T.B. The DAN family: Modulators of TGF-β signaling and beyond. Protein Sci. 2014, 23, 999–1012. [Google Scholar] [CrossRef] [PubMed]
- Brazil, D.P.; Church, R.H.; Surae, S.; Godson, C.; Martin, F. BMP signalling: Agony and antagony in the family. Trends Cell Biol. 2015, 25, 249–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Worthley, D.L.; Churchill, M.; Compton, J.T.; Tailor, Y.; Rao, M.; Si, Y.; Levin, D.; Schwartz, M.G.; Uygur, A.; Hayakawa, Y.; et al. Gremlin 1 identifies a skeletal stem cell with bone, cartilage, and reticular stromal potential. Cell 2015, 160, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Merino, R.; Rodriguez-Leon, J.; Macias, D.; Gañan, Y.; Economides, A.N.; Hurle, J.M. The BMP antagonist Gremlin regulates outgrowth, chondrogenesis and programmed cell death in the developing limb. Development 1999, 126, 5515–5522. [Google Scholar] [PubMed]
- Dudarić, L.; Cvek, S.Z.; Cvijanović, O.; Santić, V.; Marić, I.; Crncević-Orlić, Z.; Bobinac, D. Expression of the BMP-2, -4 and -7 and their antagonists gremlin, chordin, noggin and follistatin during ectopic osteogenesis. Coll. Antropol. 2013, 37, 1291–1298. [Google Scholar] [PubMed]
- Church, R.H.; Krishnakumar, A.; Urbanek, A.; Geschwindner, S.; Meneely, J.; Bianchi, A.; Basta, B.; Monaghan, S.; Elliot, C.; Strömstedt, M.; et al. Gremlin1 preferentially binds to bone morphogenetic protein-2 (BMP-2) and BMP-4 over BMP-7. Biochem. J. 2015, 466, 55–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tekari, A.; May, R.D.; Frauchiger, D.A.; Chan, S.C.; Benneker, L.M.; Gantenbein, B. The BMP2 variant L51P restores the osteogenic differentiation of human mesenchymal stromal cells in the presence of intervertebral disc cells. Eur. Cells Mater. 2017, 33, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.Y.; Yan, J.J.; Hsieh, C.C.; Chang, M.S.; Lin, R.M. The in vivo biological effects of intradiscal recombinant human bone morphogenetic protein-2 on the injured intervertebral disc: An animal experiment. Spine 2007, 32, 1174–1180. [Google Scholar] [CrossRef] [PubMed]
- Albers, C.E.; Hofstetter, W.; Sebald, H.J.; Sebald, W.; Siebenrock, K.A.; Klenke, F.M. L51P—A BMP2 variant with osteoinductive activity via inhibition of Noggin. Bone 2012, 51, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Hulsart-Billström, G.; Dawson, J.I.; Hofmann, S.; Müller, R.; Stoddart, M.J.; Alini, M.; Redl, H.; El Haj, A.; Brown, R.; Salih, V.; et al. A surprisingly poor correlation between in vitro and in vivo testing of biomaterials for bone regeneration: Results of a multicentre analysis. Eur. Cells Mater. 2016, 31, 312–322. [Google Scholar] [CrossRef]
- Brown, S.; Turner, S.; Hunt, A.; Birender, B.; Davidson, N.; Roberts, S. Is osteogenic differentiation of human nucleus pulposus cells a possibility for biological spinal fusion? Cartilage 2018, in press. [Google Scholar] [CrossRef] [PubMed]
- Tekari, A.; Chan, S.C.; Sakai, D.; Grad, S.; Gantenbein, B. Angiopoietin-1 receptor Tie2 distinguishes multipotent differentiation capability in bovine coccygeal nucleus pulposus cells. Stem Cell Res. Ther. 2016, 7, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haschtmann, D.; Ferguson, S.J.; Stoyanov, J.V. BMP-2 and TGF-β3 do not prevent spontaneous degeneration in rabbit disc explants but induce ossification of the annulus fibrosus. Eur. Spine J. 2012, 21, 1724–1733. [Google Scholar] [CrossRef] [PubMed]
- Wan, D.C.; Pomerantz, J.H.; Brunet, L.J.; Kim, J.-B.; Chou, Y.-F.; Wu, B.M.; Harland, R.; Blau, H.M.; Longaker, M.T. Noggin suppression enhances in vitro osteogenesis and accelerates in vivo bone formation. J. Biol. Chem. 2007, 282, 26450–26459. [Google Scholar] [CrossRef] [PubMed]
- Takayama, K.; Suzuki, A.; Manaka, T.; Taguchi, S.; Hashimoto, Y.; Imai, Y.; Wakitani, S.; Takaoka, K. RNA interference for noggin enhances the biological activity of bone morphogenetic proteins in vivo and in vitro. J. Bone Miner. Metab. 2009, 27, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Uludağ, H.; Wang, Z.; Jiang, H. Noggin suppression decreases BMP-2-induced osteogenesis of human bone marrow-derived mesenchymal stem cells in vitro. J. Cell Biochem. 2012, 113, 3672–3680. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Park, H.; Tan, S.; Lee, M. Enhanced osteogenesis of adipose derived stem cells with Noggin suppression and delivery of BMP-2. PLoS ONE 2013, 8, e72474. [Google Scholar] [CrossRef] [PubMed]
- Cornejo, M.C.; Cho, S.K.; Giannarelli, C.; Iatridis, J.C.; Purmessur, D. Soluble Factors from the Notochordal-rich Intervertebral Disc Inhibit Endothelial Cell Invasion and Vessel Formation in the Presence and Absence of Pro-inflammatory cytokines. Osteoarthr. Cartil. 2014, 23, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, K.B.; Frost, A.; Nilsson, O.; Ljunghall, S.; Ljunggren, O. Three isolation techniques for primary culture of human osteoblast-like cells: A comparison. Acta Orthop. Scand. 1999, 70, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, B.A.; Oegema, T.R. Initial characterization of the metabolism of intervertebral disc cells encapsulated in microspheres. J. Orthop. Res. 1992, 10, 677–690. [Google Scholar] [CrossRef] [PubMed]
- Gantenbein-Ritter, B.; Chan, S.C. The evolutionary importance of cell ratio between notochordal and nucleus pulposus cells: An experimental 3-D co-culture study. Eur. Spine J. 2011, 21, S819–S825. [Google Scholar] [CrossRef] [PubMed]
- Reno, C.; Marchuk, L.; Sciore, P.; Frank, C.B.; Hart, D.A. Rapid isolation of total RNA from small samples of hypocellular, dense connective tissues. Biotechniques 1997, 22, 1082–1086. [Google Scholar] [PubMed]
- May, R.D.; Tekari, A.; Frauchiger, D.A.; Krismer, A.; Benneker, L.M.; Gantenbein, B. Efficient non-viral transfection of primary intervertebral disc cells by electroporation for tissue engineering application. Tissue Eng. Part C Methods 2017, 23, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Risbud, M.V.; Schoepflin, Z.R.; Mwale, F.; Kandel, R.A.; Grad, S.; Iatridis, J.C.; Sakai, D.; Hoyland, J.A. Defining the Phenotype of Young Healthy Nucleus Pulposus Cells: Recommendations of the Spine Research Interest Group at the 2014 Annual ORS Meeting. J. Orthop. Res. 2014. [Google Scholar] [CrossRef] [PubMed]
- Ginzinger, D.G. Gene quantification using real-time quantitative PCR: An emerging technology hits the mainstream. Exp. Hematol. 2002, 30, 503–512. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]





| No. | OBs | NPC/AFC/CEPC | Pfirrmann Grade | Comments | ||||
|---|---|---|---|---|---|---|---|---|
| Passage | Age | Sex | Passage | Age | Sex | |||
| 1 | 1 | 65 | F | 1 | 51 | F | 3 | Monotrauma, surgery 10 days post injury, osteoporotic patient |
| 2 | 1 | 66 | F | 1 | 56 | M | 2–3 | Polytrauma, surgery 8 days post injury, healthy athlete |
| 3 | 1 | 76 | F | 2 | 32 | M | 1 | Polytrauma, surgery 9 days post injury, healthy patient |
| 4 | 1 | 88 | F | 2 | 57 | F | 3 | Monotrauma, surgery 3 days post injury, injury of endplates, healthy patient |
| 5 | 1 | 67 | M | 2 | 23 | M | 1–2 | Monotrauma, surgery 2 days post injury |
| 6 | 1 | 24 | F | 2 | 61 | M | 3 | No trauma, connection instability |
| 7 | 1 | 69 | M | 2 | 59 | M | 2 | Polytrauma, surgery 11 days post injury, injury of endplates, healthy athlete |
| Name | Description | Accession No. | Primer forward | Primer reverse |
|---|---|---|---|---|
| 18S | Ribosomal 18S RNA gene | NR_145820.1 | CGA TGC GGC GGC GTT ATT C | TCT GTC AAT CCT GTC CGT GTCC |
| ACAN | Aggrecan | XM_017021987.1 | AAG GCT GCT ATG GAG ACA A | ACT CAT TGG CTG CTT CCT |
| BGLAP (OCN) | Osteocalcin | NM_199173.5 | GCA GAG TCC AGCAAA GGT G | CCA GCC ATT GATACA GGT AGC |
| COL1A2 | Collagen type I α2 chain | NM_000089.3 | GTG GCA GTG ATG GAA GTG | CAC CAG TAA GGC CGT TTG |
| COL2A1 | Collagen type II α1 chain | XM_017018831.1 | AGC AGC AAG AGC AAG GAG AA | GTA GGA AGG TCA TCT GGA |
| CHRD | Chordin | XM_017007394.1 | GCC TCC GCT TCT CTA TCT | AAC AGG ACA CTG CCA TTG |
| FST | Follistatin | NM_006350.3 | GGA CCA GAC CAA TAA TGC | CTC ATA GGC TAA TCC AAT AGA T |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase | NM_001289745.2 | ATC TTC CAG GAGCGA GAT | GGA GGC ATT GCTGAT GAT |
| GREM1 | Gremlin1, DAN family BMP antagonist | NM_001191322.1 | GAG AAG ACG ACG AGA GTA AGG AA | CCA ACC AGT AGC AGA TGA ACA G |
| GREM2 | Gremlin2, DAN family BMP antagonist | XM_011544249.2 | CCA TCC TCA ACC GCT TCT | GAC TCC TCC TCC TTC TTC AC |
| KRT18 | Cyto-Keratin 18 | NM_199187.1 | TCT TGC TGC TGA TGA CTT | CCT CTT CGT GGT TCT TCT |
| RUNX2 | Runt-related transcription factor 2 | NM_001024630 | AGC AGC ACT CCATAT CTC T | TTC CAT CAG CGTCAA CAC |
| SPP1 (OPN) | Osteopontin | NM_001251830.1 | ACG CCG ACC AAGGAA AAC TC | GTC CAT AAA CCACAC TAT CAC CTC G |
| TSWG1 | Twisted gastrulation BMP signaling modulator 1 | NM_020648.5 | CCA GCC ACA CCA CCA GAA | ACT CGC AGC AGG CAT TAT GA |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
May, R.D.; Frauchiger, D.A.; Albers, C.E.; Benneker, L.M.; Kohl, S.; Gantenbein, B. Inhibitory Effects of Human Primary Intervertebral Disc Cells on Human Primary Osteoblasts in a Co-Culture System. Int. J. Mol. Sci. 2018, 19, 1195. https://doi.org/10.3390/ijms19041195
May RD, Frauchiger DA, Albers CE, Benneker LM, Kohl S, Gantenbein B. Inhibitory Effects of Human Primary Intervertebral Disc Cells on Human Primary Osteoblasts in a Co-Culture System. International Journal of Molecular Sciences. 2018; 19(4):1195. https://doi.org/10.3390/ijms19041195
Chicago/Turabian StyleMay, Rahel D., Daniela A. Frauchiger, Christoph E. Albers, Lorin M. Benneker, Sandro Kohl, and Benjamin Gantenbein. 2018. "Inhibitory Effects of Human Primary Intervertebral Disc Cells on Human Primary Osteoblasts in a Co-Culture System" International Journal of Molecular Sciences 19, no. 4: 1195. https://doi.org/10.3390/ijms19041195
APA StyleMay, R. D., Frauchiger, D. A., Albers, C. E., Benneker, L. M., Kohl, S., & Gantenbein, B. (2018). Inhibitory Effects of Human Primary Intervertebral Disc Cells on Human Primary Osteoblasts in a Co-Culture System. International Journal of Molecular Sciences, 19(4), 1195. https://doi.org/10.3390/ijms19041195