Synergistic Antitumor Effect of Oligogalacturonides and Cisplatin on Human Lung Cancer A549 Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effects of OGA and DDP on Cell Proliferation
2.2. Morphological Changes of Treated Cells
2.3. Combined Effects of OGA and DDP on Cell Cycle Distribution and Apoptosis
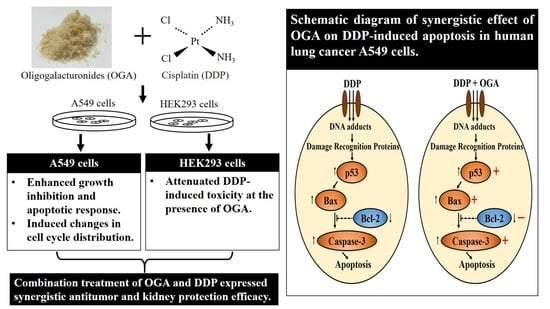
2.4. Mechanistic Studies of OGA-DDP Synergism
3. Materials and Methods
3.1. Materials
3.2. Preparation of Oligogalacturonides
3.3. Cell Culture and Treatments
3.4. Cell Viability Assay
3.5. Cell Morphology Assay
3.6. Detection of Lactate Dehydrogenase (LDH) Activity
3.7. Cell Cycle Analysis
3.8. Detection of Apoptosis by Annexin V-FITC
3.9. Western Blot Assay
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Quadrelli, S.; Lyons, G.; Colt, H.; Chimondeguy, D.; Silva, C. Lung cancer as a second primary malignancy: Increasing prevalence and its influence on survival. Ann. Surg. Oncol. 2009, 16, 1033–1038. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akinosoglou, K.S.; Karkoulias, K.; Marangos, M. Infectious complications in patients with lung cancer. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 8–18. [Google Scholar] [PubMed]
- Chang, A. Chemotherapy, chemoresistance and the changing treatment landscape for NSCLC. Lung Cancer 2011, 71, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Vasaikar, N.; Mahajan, U.; Patil, K.R.; Suchal, K.; Patil, C.R.; Ojha, S.; Goyal, S.N. D-pinitol attenuates cisplatin-induced nephrotoxicity in rats: Impact on proinflammatory cytokines. Chem. Biol. Interact. 2018, 290, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Florea, A.M.; Büsselberg, D. Cisplatin as an anti-tumor drug: Cellular mechanisms of activity, drug resistance and induced side effects. Cancers 2011, 3, 1351–1371. [Google Scholar] [CrossRef] [PubMed]
- Agudo, A.; Cabrera, L.; Amiano, P.; Ardanaz, E.; Barricarte, A.; Berenguer, T.; Chirlaque, M.D.; Dorronsoro, M.; Jakszyn, P.; Larrañaga, N.; et al. Fruit and vegetable intakes, dietary antioxidant nutrients, and total mortality in Spanish adults: Findings from the Spanish cohort of the European prospective investigation into cancer and nutrition (EPIC-Spain). Am. J. Clin. Nutr. 2007, 85, 1634–1642. [Google Scholar] [CrossRef] [PubMed]
- Feskanich, D.; Ziegler, R.; Michaud, D.; Giovannucci, E.; Speizer, F.; Willett, W.; Colditz, G. Prospective study of fruit and vegetable consumption and risk of lung cancer among men and women. J. Natl. Cancer Inst. 2000, 92, 1812–1823. [Google Scholar] [CrossRef] [PubMed]
- Kratchanova, M.; Nikolova, M.; Pavlova, E.; Yanakieva, I.; Kussovski, V. Composition and properties of biologically active pectic polysaccharides from leek (Allium porrum). J. Sci. Food Agric. 2010, 90, 2046–2051. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Sheu, M.T.; Chen, T.F.; Wang, Y.C.; Hou, W.C.; Liu, D.Z.; Chung, T.C.; Liang, Y.C. Suppression of endotoxin-induced proinflammatory responses by citrus pectin through blocking LPS signaling pathways. Biochem. Pharmacol. 2006, 72, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Park, H.R.; Park, S.B.; Hong, H.D.; Suh, H.J.; Shin, K.S. Structural elucidation of anti-metastatic rhamnogalacturonan II from the pectinase digest of citrus peels (Citrus unshiu). Int. J. Biol. Macromol. 2017, 94, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Leclere, L.; Fransolet, M.; Cote, F.; Cambier, P.; Arnould, T.; Van Cutsem, P.; Michiels, C. Heat-modified citrus pectin induces apoptosis-like cell death and autophagy in HepG2 and A549 cancer cells. PLoS ONE 2015, 10, e0115831. [Google Scholar] [CrossRef] [PubMed]
- Pienta, K.J.; Naik, H.; Akhtar, A.; Yamazaki, K.; Replogle, T.S.; Lehr, J.; Donat, T.L.; Tait, L.; Hogan, V.; Raz, A. Inhibition of spontaneous metastasis in a rat prostate cancer model by oral administration of modified citrus pectin. J. Natl. Cancer Inst. 1995, 87, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.H.; Fu, L.C.; Huang, C.S.; Wang, Y.T.; Wu, M.C. The uptake of oligogalacturonide and its effect on growth inhibition, lactate dehydrogenase activity and galactin-3 release of human cancer cells. Food Chem. 2012, 132, 1987–1995. [Google Scholar] [CrossRef]
- Wang, S.; Li, P.; Lu, S.M.; Ling, Z.Q. Chemoprevention of low-molecular-weight citrus pectin (LCP) in gastrointestinal cancer cells. Int. J. Biol. Sci. 2016, 12, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.H.; Lu, H.T.; Wang, Y.T.; Wu, M.C. Antioxidant activity and emulsion-stabilizing effect of pectic enzyme treated pectin in soy protein isolate-stabilized oil/water emulsion. J. Agric. Food. Chem. 2011, 59, 9623–9628. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.C.; Li, H.C.; Wu, P.H.; Huang, P.H.; Wang, Y.T. Assessment of oligogalacturonide from citrus pectin as a potential antibacterial agent against foodborne pathogens. J. Food Sci. 2014, 79, M1541–M1544. [Google Scholar] [CrossRef] [PubMed]
- Chirino, Y.I.; Pedraza-Chaverri, J. Role of oxidative and nitrosative stress in cisplatin-induced nephrotoxicity. Exp. Toxicol. Pathol. 2009, 61, 223–242. [Google Scholar] [CrossRef] [PubMed]
- Astolfi, L.; Ghiselli, S.; Guaran, V.; Chicca, M.; Simoni, E.; Olivetto, E.; Lelli, G.; Martini, A. Correlation of adverse effects of cisplatin administration in patients affected by solid tumours: A retrospective evaluation. Oncol. Rep. 2013, 29, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- Duan, G.; Tang, Q.; Yan, H.; Xie, L.; Wang, Y.; Zheng, X.E.; Zhuge, Y.; Shen, S.; Zhang, B.; Zhang, X.; et al. A strategy to delay the development of cisplatin resistance by maintaining a certain amount of cisplatin-sensitive cells. Sci. Rep. 2017, 7, 432. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.P.; Li, Z.; Guo, Z.Y.; Zhao, Y.M. The effects of vitamin C on DDP-induced anemia in rats. Toxicol. Mech. Methods 2013, 23, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Chan, F.K.; Moriwaki, K.; De Rosa, M.J. Detection of necrosis by release of lactate dehydrogenase activity. Methods Mol. Biol. 2013, 979, 65–70. [Google Scholar] [PubMed]
- Kosmider, B.; Zawlik, I.; Liberski, P.P.; Osiecka, R.; Zyner, E.; Ochocki, J.; Bartkowiak, J. Evaluation of P53 and BAX gene expression and induction of apoptosis and necrosis by the cis-Pt(II) complex of 3-aminoflavone in comparison with cis-diamminedichloroplatinum(II) (cis-DDP) in human lymphocytes. Mutat. Res. 2006, 604, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Chazotte, B. Labeling nuclear DNA using DAPI. Cold Spring Harb. Protocol. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Zeidan, Y.H.; Jenkins, R.W.; Hannun, Y.A. Remodeling of cellular cytoskeleton by the acid sphingomyelinase/ceramide pathway. J. Cell Biol. 2008, 181, 335–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vassilopoulos, A.; Xiao, C.; Chisholm, C.; Chen, W.; Xu, X.; Lahusen, T.J.; Bewley, C.; Deng, C.X. Synergistic therapeutic effect of cisplatin and phosphatidylinositol 3-kinase (PI3K) inhibitors in cancer growth and metastasis of Brca1 mutant tumors. J. Biol. Chem. 2014, 289, 24202–24214. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, J.; Hu, Y.; Qian, G. Cisplatin-induced apoptosis in human lung adenocarcinoma cell line A549. Zhongguo Fei Ai Za Zhi 2002, 5, 254–256. [Google Scholar] [PubMed]
- Vermes, I.; Haanen, C. Apoptosis and programmed cell death in health and disease. Adv. Clin. Chem. 1994, 31, 177–246. [Google Scholar] [PubMed]
- Zamble, D.B.; Jacks, T.; Lippard, S.J. p53-Dependent and -independent responses to cisplatin in mouse testicular teratocarcinoma cells. Proc. Natl. Acad. Sci. USA 1998, 95, 6163–6168. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.Y.; Lin, C.M.; Wu, M.C. Evaluation of the prebiotic effects of citrus pectin hydrolysate. J. Food Drug Anal. 2017, 25, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Y.; La, K.; Peng, L.; Song, X.; Shi, X.; Zhu, X.; Leung, P.; Ko, C.; Ye, C. Inhibitory effects of cocoa tea (Camellia ptilophylla) in human hepatocellular carcinoma HepG2 in vitro and in vivo through apoptosis. J. Nutr. Biochem. 2011, 23, 1051–1057. [Google Scholar] [CrossRef] [PubMed]






| (A) Growth Inhibition (%) on A549 Cells | ||||||
| Treatment | DDP (μg/mL) | |||||
| 0 | 2 | 4 | 6 | 8 | 10 | |
| OGA 12 h | 7.0 ± 1.2 | - | - | - | - | - |
| DDP 12 h | - | 8.1 ± 1.7 cE | 8.9 ± 1.0 cE | 11.5 ± 2.6 cD | 19.5 ± 2.6 bC | 34.1 ± 2.5 aD |
| DDP + OGA 12 h | - | 11.2 ± 0.8 cDE | 14.1 ± 2.9 cD | 20.7 ± 3.8 bBC | 38.3 ± 1.6 aB | 41.5 ± 1.7 aC |
| OGA 24 h | 12.7 ± 1.0 | - | - | - | - | - |
| DDP 24 h | - | 13.8 ± 2.8 cCD | 15.1 ± 2.5 cCD | 16.8 ± 3.6 bcC | 21.2 ± 2.3 bC | 39.8 ± 2.2 aC |
| DDP + OGA 24 h | - | 17.0 ± 1.0 dBC | 25.3 ± 2.9 cB | 22.3 ± 2.9 cB | 42.6 ± 1.2 bA | 61.5 ± 1.7 aA |
| DDP 12 h + OGA 12 h | - | 26.8 ± 3.1 dA | 30.5 ± 3.2 dA | 38.3 ± 1.2 cA | 45.5 ± 0.4 bA | 53.5 ± 1.2 aB |
| OGA 12 h + DDP12 h | - | 18.0 ± 2.1 bcB | 19.1 ± 0.5 bcC | 25.1 ± 3.0 aB | 22.4 ± 2.9 abC | 15.3 ± 3.0 cE |
| (B) Growth inhibition (%) on HEK293 cells | ||||||
| Treatment | DDP (μg/mL) | |||||
| 0 | 2 | 4 | 6 | 8 | 10 | |
| OGA 12 h | 6.0 ± 2.6 | - | - | - | - | - |
| DDP 12 h | - | 23.7 ± 2.8 cC | 28.3 ± 2.7 bcB | 29.8 ± 4.2 bB | 31.3 ± 2.4 abB | 35.4 ± 1.3 aC |
| DDP + OGA 12 h | - | 12.2 ± 2.5 dE | 17.8 ± 1.8 cC | 19.5 ± 3.7 cC | 24.1 ± 1.6 bC | 28.8 ± 0.7 aE |
| OGA 24 h | 9.4 ± 1.2 | - | - | - | - | - |
| DDP 24 h | - | 37.9 ± 0.5 dA | 43.2 ± 0.8 cA | 51.5 ± 2.7 bA | 52.2 ± 3.1 bA | 57.9 ± 1.0 aA |
| DDP + OGA 24 h | - | 27.1 ± 1.3 bB | 27.2 ± 0.6 bB | 24.1 ± 2.3 bC | 26.8 ± 3.3 bC | 37.8 ± 1.1 aB |
| DDP 12 h + OGA 12 h | - | 16.8 ± 2.1 cD | 21.8 ± 5.0 bC | 22.4 ± 1.0 bC | 32.4 ± 1.3 aB | 33.3 ± 1.0 aD |
| OGA 12 h + DDP12 h | - | 11.4 ± 0.8 dE | 19.9 ± 4.0 cC | 22.3 ± 1.1 cC | 27.1 ± 1.5 bC | 32.1 ± 1.2 aD |
| Treatment | LDH (Lactate Dehydrogenase) Activity (%) | |
|---|---|---|
| A549 | HEK293 | |
| Control 12 h | 0.0 ± 0.1 g | 0.0 ± 0.2 f |
| OGA 12 h | 4.3 ± 0.3 f | 0.2 ± 0.7 f |
| DDP 12 h | 11.2 ± 4.0 e | 14.5 ± 2.5 b |
| DDP + OGA 12 h | 15.6 ± 1.1 d | 5.9 ± 0.1 d |
| Control 24 h | 0.0 ± 1.0 g | 0.0 ± 0.1 f |
| OGA 24 h | 23.1 ± 2.6 c | 0.4 ± 0.0 f |
| DDP 24 h | 26.9 ± 4.3 c | 26.6 ± 2.6 a |
| DDP + OGA 24 h | 36.1 ± 2.3 b | 11.2 ± 0.3 c |
| DDP 12 h + OGA 12 h | 49.4 ± 2.2 a | 3.5 ± 0.9 e |
| OGA 12 h + DDP12 h | 35.2 ± 1.9 b | 3.1 ± 0.1 e |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.-S.; Huang, A.-C.; Huang, P.-H.; Lo, D.; Wang, Y.-T.; Wu, M.-C. Synergistic Antitumor Effect of Oligogalacturonides and Cisplatin on Human Lung Cancer A549 Cells. Int. J. Mol. Sci. 2018, 19, 1769. https://doi.org/10.3390/ijms19061769
Huang C-S, Huang A-C, Huang P-H, Lo D, Wang Y-T, Wu M-C. Synergistic Antitumor Effect of Oligogalacturonides and Cisplatin on Human Lung Cancer A549 Cells. International Journal of Molecular Sciences. 2018; 19(6):1769. https://doi.org/10.3390/ijms19061769
Chicago/Turabian StyleHuang, Cian-Song, Ai-Chun Huang, Ping-Hsiu Huang, Diana Lo, Yuh-Tai Wang, and Ming-Chang Wu. 2018. "Synergistic Antitumor Effect of Oligogalacturonides and Cisplatin on Human Lung Cancer A549 Cells" International Journal of Molecular Sciences 19, no. 6: 1769. https://doi.org/10.3390/ijms19061769
APA StyleHuang, C.-S., Huang, A.-C., Huang, P.-H., Lo, D., Wang, Y.-T., & Wu, M.-C. (2018). Synergistic Antitumor Effect of Oligogalacturonides and Cisplatin on Human Lung Cancer A549 Cells. International Journal of Molecular Sciences, 19(6), 1769. https://doi.org/10.3390/ijms19061769