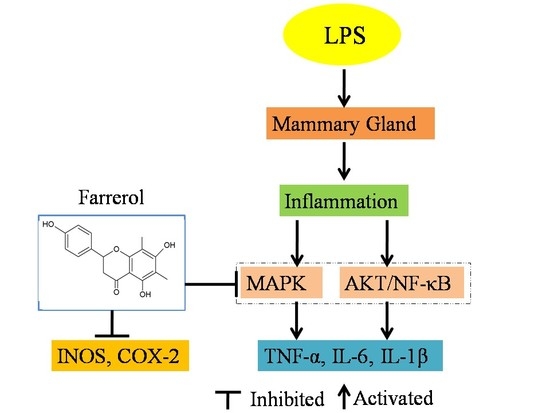
Farrerol Relieve Lipopolysaccharide (LPS)-Induced Mastitis by Inhibiting AKT/NF-κB p65, ERK1/2 and P38 Signaling Pathway
Abstract
:1. Introduction
2. Results
2.1. Effect of Farrerol on Histopathological Changes
2.2. Effect of Farrerol on Myeloperoxidase (MPO) Activity
2.3. Effect of Farrerol on the Production of Pro-Inflammatory Mediators in Mammary Gland
2.4. Effect of Farrerol on the Activity of AKT and NF-κB Signaling Pathways in LPS-Induced Mouse Mastitis
2.5. Effect of Farrerol on the Activity of MAPK Signaling Pathways in LPS-Induced Mouse Mastitis
2.6. Effect of Farrerol on mMECs Viability
2.7. Effect of Farrerol on Inflammatory Response in LPS-Stimulated mMECs
2.8. Effect of Farrerol on the Activity of NF-κB, AKT, and MAPK Signaling Pathways in LPS-Stimulated mMECs
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Experimental Model and Grouping Design
4.3. Histopathological Examination
4.4. Myeloperoxidase (MPO) Assay in Mammary Glands
4.5. Enzyme-Linked Immunosorbent Assay (ELISA)
4.6. Cell Culture
4.7. Cell Experimental Design
4.8. Cell Counting Kit-8 Assay
4.9. Real-Time PCR
4.10. Western Blot Analysis
4.11. Statistical Analyses
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| mMECs | mouse mammary epithelial cells |
| LPS | lipopolysaccharide |
References
- Viguier, C.; Arora, S.; Gilmartin, N.; Welbeck, K.; O’Kennedy, R. Mastitis detection: Current trends and future perspectives. Trends Biotechnol. 2009, 27, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Son, S.J.; Park, M.R.; Ryu, S.D.; Maburutse, B.E.; Oh, N.S.; Park, J.; Oh, S.; Kim, Y. Short communication: In vivo screening platform for bacteriocins using Caenorhabditis elegans to control mastitis-causing pathogens. J. Dairy Sci. 2016, 99, 8614–8621. [Google Scholar] [CrossRef] [PubMed]
- Ganda, E.K.; Bisinotto, R.S.; Decter, D.H.; Bicalho, R.C. Evaluation of an On-Farm Culture System (Accumast) for Fast Identification of Milk Pathogens Associated with Clinical Mastitis in Dairy Cows. PLoS ONE 2016, 11, e0155314. [Google Scholar] [CrossRef] [PubMed]
- Sadek, K.; Saleh, E.; Ayoub, M. Selective, reliable blood and milk bio-markers for diagnosing clinical and subclinical bovine mastitis. Trop. Anim. Health Prod. 2017, 49, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, R.A.; Fox, L.K.; Lysnyansky, I. Mycoplasma mastitis in cattle: To cull or not to cull. Vet. J. 2016, 216, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Sousaris, N.; Barr, R.G. Sonographic Elastography of Mastitis. J. Ultrasound Med. 2016, 35, 1791–1797. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Zerbe, H.; Petzl, W.; Brunner, R.M.; Guenther, J.; Draing, C.; von Aulocke, S.; Schuberth, H.J.; Seyfert, H.M. Bovine TLR2 and TLR4 properly transduce signals from Staphylococcus aureus and E. coli, but S. aureus fails to both activate NF-κB in mammary epithelial cells and to quickly induce TNFα and interleukin-8 (CXCL8) expression in the udder. Mol. Immunol. 2008, 45, 1385–1397. [Google Scholar] [CrossRef] [PubMed]
- Bougarn, S.; Cunha, P.; Gilbert, F.B.; Meurens, F.; Rainard, P. Technical note: Validation of candidate reference genes for normalization of quantitative PCR in bovine mammary epithelial cells responding to inflammatory stimuli. J. Dairy Sci. 2011, 94, 2425–2430. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhang, W.; Wang, T.; Jiang, H.; Zhang, Z.; Fu, Y.; Yang, Z.; Cao, Y.; Zhang, N. Geniposide plays an anti-inflammatory role via regulating TLR4 and downstream signaling pathways in lipopolysaccharide-induced mastitis in mice. Inflammation 2014, 37, 1588–1598. [Google Scholar] [CrossRef] [PubMed]
- Triantafilou, M.; Triantafilou, K. The dynamics of LPS recognition: Complex orchestration of multiple receptors. J. Endotoxin Res. 2005, 11, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Van Soest, F.J.S.; Abbeloos, E.; McDougall, S.; Hogeveen, H. Addition of meloxicam to the treatment of bovine clinical mastitis results in a net economic benefit to the dairy farmer. J. Dairy Sci. 2018, 101, 3387–3397. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, C.E.; Chapinal, N.; Petersson-Wolfe, C.S.; deVries, T.J.; Kelton, D.F.; Duffield, T.F.; Leslie, K.E. The effect of meloxicam on pain sensitivity, rumination time, and clinical signs in dairy cows with endotoxin-induced clinical mastitis. J. Dairy Sci. 2013, 96, 2847–2856. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.D.; Wang, M.Z.; Zhang, S.R. Studies on the quantitative determination of farrerol in Man-Shan-Hong (Rhododendron dauricum T.) leaves. Yao Xue Xue Bao 1980, 15, 736–740. [Google Scholar] [PubMed]
- Liu, E.; Liang, T.; Wang, X.; Ban, S.; Han, L.; Li, Q. Apoptosis induced by farrerol in human gastric cancer SGC-7901 cells through the mitochondrial-mediated pathway. Eur. J. Cancer Prev. 2015, 24, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Guo, C.; Wei, Z.; He, X.; Kou, J.; Zhou, E.; Yang, Z.; Fu, Y. Morin suppresses inflammatory cytokine expression by downregulation of nuclear factor-kappaB and mitogen-activated protein kinase (MAPK) signaling pathways in lipopolysaccharide-stimulated primary bovine mammary epithelial cells. J. Dairy Sci. 2016, 99, 3016–3022. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Fu, K.; Lv, X.; Li, W.; Zhang, N. Protective effects of kaempferol on lipopolysaccharide-induced mastitis in mice. Inflammation 2014, 37, 1453–1458. [Google Scholar] [CrossRef] [PubMed]
- Ci, X.; Chu, X.; Wei, M.; Yang, X.; Cai, Q.; Deng, X. Different effects of farrerol on an OVA-induced allergic asthma and LPS-induced acute lung injury. PLoS ONE 2012, 7, e34634. [Google Scholar] [CrossRef] [PubMed]
- Sordillo, L.M.; Streicher, K.L. Mammary gland immunity and mastitis susceptibility. J. Mammary Gland Biol. Neoplasia 2002, 7, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Alluwaimi, A.M. The cytokines of bovine mammary gland: Prospects for diagnosis and therapy. Res. Vet. Sci. 2004, 77, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Wang, T.; Zhang, Z.; Jiang, H.; Wang, W.; Cao, Y.; Zhang, N. Leonurine exerts anti-inflammatory effect by regulating inflammatory signaling pathways and cytokines in LPS-induced mouse mastitis. Inflammation 2015, 38, 79–88. [Google Scholar] [CrossRef] [PubMed]
- De Schepper, S.; de Ketelaere, A.; Bannerman, D.D.; Paape, M.J.; Peelman, L.; Burvenich, C. The toll-like receptor-4 (TLR-4) pathway and its possible role in the pathogenesis of Escherichia coli mastitis in dairy cattle. Vet. Res. 2008, 39, 5. [Google Scholar] [CrossRef] [PubMed]
- Ershun, Z.; Yunhe, F.; Zhengkai, W.; Yongguo, C.; Naisheng, Z.; Zhengtao, Y. Cepharanthine attenuates lipopolysaccharide-induced mice mastitis by suppressing the NF-κB signaling pathway. Inflammation 2014, 37, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, B.; Yu, J.L. Farrerol inhibits IL-6 and IL-8 production in LPS-stimulated human gingival fibroblasts by suppressing PI3K/AKT/NF-κB signaling pathway. Arch. Oral Biol. 2016, 62, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Saha, R.N.; Pahan, K. Regulation of inducible nitric oxide synthase gene in glial cells. Antioxid. Redox Signal. 2006, 8, 929–947. [Google Scholar] [CrossRef] [PubMed]
- Sil, S.; Ghosh, T.; Ghosh, R.; Gupta, P. Nitric oxide synthase inhibitor, aminoguanidine reduces intracerebroventricular colchicine induced neurodegeneration, memory impairments and changes of systemic immune responses in rats. J. Neuroimmunol. 2017, 303, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Bortolanza, M.; Cavalcanti-Kiwiatkoski, R.; Padovan-Neto, F.E.; da-Silva, C.A.; Mitkovski, M.; Raisman-Vozari, R.; Del-Bel, E. Glial activation is associated with l-DOPA induced dyskinesia and blocked by a nitric oxide synthase inhibitor in a rat model of Parkinson’s disease. Neurobiol. Dis. 2015, 73, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhou, E.; Wei, D.; Li, D.; Wei, Z.; Zhang, W.; Zhang, X. Emodin inhibits LPS-induced inflammatory response by activating PPAR-γ in mouse mammary epithelial cells. Int. Immunopharmacol. 2014, 21, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yan, J.; Zhuang, Y.; Han, G. Anti-inflammatory effects of farrerol on IL-1β-stimulated human osteoarthritis chondrocytes. Eur. J. Pharmacol. 2015, 764, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; May, M.J.; Kopp, E.B. NF-κB and Rel proteins: Evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 1998, 16, 225–260. [Google Scholar] [CrossRef] [PubMed]
- Manna, S.K. Double-edged sword effect of biochanin to inhibit nuclear factor κB: Suppression of serine/threonine and tyrosine kinases. Biochem. Pharmacol. 2012, 83, 1383–1392. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Yin, R.; Cong, Y.; Yang, Z.; Zhou, E.; Wei, Z.; Liu, Z.; Cao, Y.; Zhang, N. Oxymatrine lightened the inflammatory response of LPS-induced mastitis in mice through affecting NF-κB and MAPKs signaling pathways. Inflammation 2014, 37, 2047–2055. [Google Scholar] [CrossRef] [PubMed]
- Ruifeng, G.; Yunhe, F.; Zhengkai, W.; Ershun, Z.; Yimeng, L.; Minjun, Y.; Xiaojing, S.; Zhengtao, Y.; Naisheng, Z. Chlorogenic acid attenuates lipopolysaccharide-induced mice mastitis by suppressing TLR4-mediated NF-κB signaling pathway. Eur. J. Pharmacol. 2014, 729, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, N.; Cao, Y.; Zhang, W.; Su, G.; Sun, Y.; Liu, Z.; Li, F.; Liang, D.; Liu, B.; et al. Emodin ameliorates lipopolysaccharide-induced mastitis in mice by inhibiting activation of NF-κB and MAPKs signal pathways. Eur. J. Pharmacol. 2013, 705, 79–85. [Google Scholar] [CrossRef] [PubMed]









© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Gong, Q.; Guo, W.; Kan, X.; Xu, D.; Ma, H.; Fu, S.; Liu, J. Farrerol Relieve Lipopolysaccharide (LPS)-Induced Mastitis by Inhibiting AKT/NF-κB p65, ERK1/2 and P38 Signaling Pathway. Int. J. Mol. Sci. 2018, 19, 1770. https://doi.org/10.3390/ijms19061770
Li Y, Gong Q, Guo W, Kan X, Xu D, Ma H, Fu S, Liu J. Farrerol Relieve Lipopolysaccharide (LPS)-Induced Mastitis by Inhibiting AKT/NF-κB p65, ERK1/2 and P38 Signaling Pathway. International Journal of Molecular Sciences. 2018; 19(6):1770. https://doi.org/10.3390/ijms19061770
Chicago/Turabian StyleLi, Yanwei, Qian Gong, Wenjin Guo, Xingchi Kan, Dianwen Xu, He Ma, Shoupeng Fu, and Juxiong Liu. 2018. "Farrerol Relieve Lipopolysaccharide (LPS)-Induced Mastitis by Inhibiting AKT/NF-κB p65, ERK1/2 and P38 Signaling Pathway" International Journal of Molecular Sciences 19, no. 6: 1770. https://doi.org/10.3390/ijms19061770
APA StyleLi, Y., Gong, Q., Guo, W., Kan, X., Xu, D., Ma, H., Fu, S., & Liu, J. (2018). Farrerol Relieve Lipopolysaccharide (LPS)-Induced Mastitis by Inhibiting AKT/NF-κB p65, ERK1/2 and P38 Signaling Pathway. International Journal of Molecular Sciences, 19(6), 1770. https://doi.org/10.3390/ijms19061770