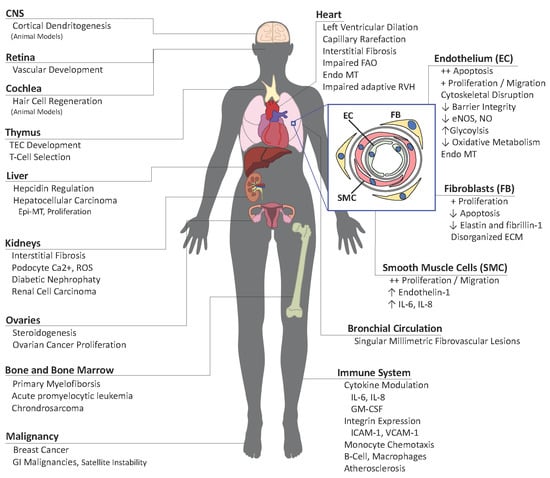
Consequences of BMPR2 Deficiency in the Pulmonary Vasculature and Beyond: Contributions to Pulmonary Arterial Hypertension
Abstract
:1. Introduction
2. Mechanisms for BMPR2 Deficiency
3. BMPR2 Deficiency in the Pulmonary Vasculature
3.1. Consequences of BMPR2 Deficiency in the Intimal Layer: Endothelial Cells
3.2. BMPR2 Deficiency in the Medial Layer: Smooth Muscle Cells
3.3. The Tunica Adventitia and BMPR2 Deficiency: Fibroblasts and Extracellular Matrix
4. BMPR2 Deficiency in the Systemic Circulation
5. BMPR2 Deficiency in the Heart
6. BMPR2 Deficiency in the Immune System
7. BMPR2 Deficiency in Other Organs
8. BMPR2 Deficiency in Bone Marrow
9. BMPR2 Deficiency in Malignancy
10. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ActR | Activin receptor |
| Akt | Protein kinase b |
| ALK | Activin-like receptor |
| ApoE | Apolipoprotein E |
| BAMBI | BMP and activin membrane-bound inhibitor |
| BMP | Bone morphogenetic protein |
| BMPR2 | Bone morphogenetic protein receptor type 2 |
| c-Src | Proto-oncogene tyrosine-protein kinase Src |
| Cav1 | Caveolin 1 |
| CLIC4 | Chloride intracellular channel 4 |
| Dvl | Disheveled |
| eNOS | Nitric oxide synthase 3 |
| ERK | Extracellular signal-regulated kinase |
| FGF | Fibroblast Growth Factor |
| GM-CSF | Granulocyte macrophage-colony stimulating factor |
| gp-120 | Envelope glycoprotein GP120 |
| GSK3-β | Glycogen synthase kinase 3-β |
| GDF | Growth differentiation factor |
| HIV | Human immunodeficiency virus |
| ICAM | Intracellular adhesion molecule |
| ID | Inhibitor of differentiation |
| IL | Interleukin |
| JNK | c-Jun N-terminal kinase |
| LIMK | Lin11, Isl-1, and Mec-3 domain kinase |
| MAPK | Mitogen-activated protein kinase |
| Nef | Negative factor |
| PAH | Pulmonary arterial hypertension |
| PDGF | Platelet derived growth factor |
| PPARγ | Peroxisome proliferator-activated receptor gamma |
| Rac1 | Ras-related C3 botulinum toxin substrate 1 |
| RAGE | Receptor for advanced glycation end products |
| RhoA | Ras homolog gene family member A |
| S100A4 | S100 Calcium Binding Protein A4 |
| SMAD | Mothers against decapentaplegic |
| SMURF | SMAD specific E3 ubiquitin protein ligase |
| STAT3 | Signal transducer and activator of transcription 3 |
| Tak1 | TGF-β activated kinase 1 |
| Tat | Trans-activator of transcription |
| TGF | Transforming Growth Factor |
| TNF | Tumo necrosis factor |
| VCAM | Vascular cell adhesion protein |
| VEGFR3 | Vascular endothelial growth factor receptor 3 |
| Wnt | Wingless |
References
- Deng, Z.; Morse, J.H.; Slager, S.L.; Cuervo, N.; Moore, K.J.; Venetos, G.; Kalachikov, S.; Cayanis, E.; Fischer, S.G.; Barst, R.J.; et al. Familial primary pulmonary hypertension (gene PPH1) is caused by mutations in the bone morphogenetic protein receptor-II gene. Am. J. Hum. Genet. 2000, 67, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Lane, K.B.; Machado, R.D.; Pauciulo, M.W.; Thomson, J.R.; Phillips, J.A., 3rd; Loyd, J.E.; Nichols, W.C.; Trembath, R.C. Heterozygous germline mutations in BMPR2, encoding a TGF-β receptor, cause familial primary pulmonary hypertension. Nat. Genet. 2000, 26, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Urist, M.R.; Strates, B.S. Bone morphogenetic protein. J. Dent. Res. 1971, 50, 1392–1406. [Google Scholar] [CrossRef] [PubMed]
- Salazar, V.S.; Gamer, L.W.; Rosen, V. BMP signalling in skeletal development, disease and repair. Nat. Rev. Endocrinol. 2016, 12, 203–221. [Google Scholar] [CrossRef] [PubMed]
- Garcia de Vinuesa, A.; Abdelilah-Seyfried, S.; Knaus, P.; Zwijsen, A.; Bailly, S. BMP signaling in vascular biology and dysfunction. Cytokine Growth Factor Rev. 2016, 27, 65–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez, G.; Loveland, K.L.; Clark, A.T.; Dziadek, M.; Bertram, J.F. Expression of bone morphogenetic protein receptors in the developing mouse metanephros. Exp. Nephrol. 2001, 9, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Jiramongkolchai, P.; Owens, P.; Hong, C.C. Emerging roles of the bone morphogenetic protein pathway in cancer: Potential therapeutic target for kinase inhibition. Biochem. Soc. Trans. 2016, 44, 1117–1134. [Google Scholar] [CrossRef] [PubMed]
- Cogan, J.D.; Pauciulo, M.W.; Batchman, A.P.; Prince, M.A.; Robbins, I.M.; Hedges, L.K.; Stanton, K.C.; Wheeler, L.A.; Phillips, J.A., 3rd; Loyd, J.E.; et al. High frequency of BMPR2 exonic deletions/duplications in familial pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2006, 174, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Aldred, M.A.; Vijayakrishnan, J.; James, V.; Soubrier, F.; Gomez-Sanchez, M.A.; Martensson, G.; Galie, N.; Manes, A.; Corris, P.; Simonneau, G.; et al. BMPR2 gene rearrangements account for a significant proportion of mutations in familial and idiopathic pulmonary arterial hypertension. Hum. Mutat. 2006, 27, 212–213. [Google Scholar] [CrossRef] [PubMed]
- Moustakas, A.; Souchelnytskyi, S.; Heldin, C.H. Smad regulation in TGF-β signal transduction. J. Cell Sci. 2001, 114, 4359–4369. [Google Scholar] [PubMed]
- Yang, J.; Li, X.; Morrell, N.W. Id proteins in the vasculature: From molecular biology to cardiopulmonary medicine. Cardiovasc. Res. 2014, 104, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Ishida, W.; Hamamoto, T.; Kusanagi, K.; Yagi, K.; Kawabata, M.; Takehara, K.; Sampath, T.K.; Kato, M.; Miyazono, K. Smad6 Is a Smad1/5-induced Smad Inhibitor. J. Biol. Chem. 2000, 275, 6075–6079. [Google Scholar] [CrossRef] [PubMed]
- Shintani, M.; Yagi, H.; Nakayama, T.; Saji, T.; Matsuoka, R. A new nonsense mutation of SMAD8 associated with pulmonary arterial hypertension. J. Med. Genet. 2009, 46, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Austin, E.D.; Ma, L.; LeDuc, C.; Berman Rosenzweig, E.; Borczuk, A.; Phillips, J.A., 3rd; Palomero, T.; Sumazin, P.; Kim, H.R.; Talati, M.H.; et al. Whole exome sequencing to identify a novel gene (caveolin-1) associated with human pulmonary arterial hypertension. Circ. Cardiovasc. Genet. 2012, 5, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Fan, R.; Ji, R.; Zou, W.; Penny, D.J.; Varghese, N.P.; Fan, Y. Novel homozygous BMP9 nonsense mutation causes pulmonary arterial hypertension: A case report. BMC Pulm. Med. 2016, 16, 17. [Google Scholar] [CrossRef] [PubMed]
- Girerd, B.; Montani, D.; Coulet, F.; Sztrymf, B.; Yaici, A.; Jaïs, X.; Tregouet, D.; Reis, A.; Drouin-Garraud, V.; Fraisse, A.; et al. Clinical outcomes of pulmonary arterial hypertension in patients carrying an ACVRL1 (ALK1) mutation. Am. J. Respir. Crit. Care Med. 2010, 181, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Trembath, R.C.; Thomson, J.R.; Machado, R.D.; Morgan, N.V.; Atkinson, C.; Winship, I.; Simonneau, G.; Galie, N.; Loyd, J.E.; Humbert, M.; et al. Clinical and molecular genetic features of pulmonary hypertension in patients with hereditary hemorrhagic telangiectasia. N. Engl. J. Med. 2001, 345, 325–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyle, M.A.; Fenstad, E.R.; McGoon, M.D.; Frantz, R.P.; Krowka, M.J.; Kane, G.C.; Swanson, K.L. Pulmonary Hypertension in Hereditary Hemorrhagic Telangiectasia. Chest 2016, 149, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Newman, J.H.; Trembath, R.C.; Morse, J.A.; Grunig, E.; Loyd, J.E.; Adnot, S.; Coccolo, F.; Ventura, C.; Phillips, J.A., 3rd; Knowles, J.A.; et al. Genetic basis of pulmonary arterial hypertension: Current understanding and future directions. J. Am. Coll. Cardiol. 2004, 43, S33–S39. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, C.; Stewart, S.; Upton, P.D.; Machado, R.; Thomson, J.R.; Trembath, R.C.; Morrell, N.W. Primary Pulmonary Hypertension Is Associated With Reduced Pulmonary Vascular Expression of Type II Bone Morphogenetic Protein Receptor. Circulation 2002, 105, 1672–1678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, N.Y.; Collum, S.D.; Luo, F.; Weng, T.; Le, T.T.; Hernandez, A.; Philip, K.; Molina, J.G.; Garcia-Morales, L.J.; Cao, Y.; et al. Macrophage bone morphogenic protein receptor 2 depletion in idiopathic pulmonary fibrosis and Group III pulmonary hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol. 2016, 311, L238–L254. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, J.R.; Ormiston, M.L.; Perez-Iratxeta, C.; Courtman, D.W.; Jiang, B.; Ferrer, E.; Caruso, P.; Southwood, M.; Foster, W.S.; Morrell, N.W.; et al. Proteomic analysis implicates translationally controlled tumor protein as a novel mediator of occlusive vascular remodeling in pulmonary arterial hypertension. Circulation 2014, 129, 2125–2135. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.R.; Tipney, H.; Painter, J.L.; Shen, J.; Nicoletti, P.; Shen, Y.; Floratos, A.; Sham, P.C.; Li, M.J.; Wang, J.; et al. The support of human genetic evidence for approved drug indications. Nat. Genet. 2015, 47, 856–860. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.M.; Xia, W.; Holmes, M.D.; Hodge, S.J.; Danilov, S.; Curiel, D.T.; Morrell, N.W.; Reynolds, P.N. Bone morphogenetic protein type 2 receptor gene therapy attenuates hypoxic pulmonary hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007, 292, L1182–L1192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spiekerkoetter, E.; Tian, X.; Cai, J.; Hopper, R.K.; Sudheendra, D.; Li, C.G.; El-Bizri, N.; Sawada, H.; Haghighat, R.; Chan, R.; et al. FK506 activates BMPR2, rescues endothelial dysfunction, and reverses pulmonary hypertension. J. Clin. Investig. 2013, 123, 3600–3613. [Google Scholar] [CrossRef] [PubMed]
- Long, L.; Ormiston, M.L.; Yang, X.; Southwood, M.; Gräf, S.; Machado, R.D.; Mueller, M.; Kinzel, B.; Yung, L.M.; Wilkinson, J.M.; et al. Selective enhancement of endothelial BMPR-II with BMP9 reverses pulmonary arterial hypertension. Nat. Med. 2015, 21, 777–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nickel, N.P.; Spiekerkoetter, E.; Gu, M.; Li, C.G.; Li, H.; Kaschwich, M.; Diebold, I.; Hennigs, J.K.; Kim, K.Y.; Miyagawa, K.; et al. Elafin Reverses Pulmonary Hypertension via Caveolin-1-Dependent Bone Morphogenetic Protein Signaling. Am. J. Respir. Crit. Care Med. 2015, 191, 1273–1286. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, X.; Al-Lamki, R.S.; Southwood, M.; Zhao, J.; Lever, A.M.; Grimminger, F.; Schermuly, R.T.; Morrell, N.W. Smad-dependent and smad-independent induction of id1 by prostacyclin analogues inhibits proliferation of pulmonary artery smooth muscle cells in vitro and in vivo. Circ. Res. 2010, 107, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, X.; Al-Lamki, R.S.; Wu, C.; Weiss, A.; Berk, J.; Schermuly, R.T.; Morrell, N.W. Sildenafil potentiates bone morphogenetic protein signaling in pulmonary arterial smooth muscle cells and in experimental pulmonary hypertension. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Orriols, M.; Gomez-Puerto, M.C.; Ten Dijke, P. BMP type II receptor as a therapeutic target in pulmonary arterial hypertension. Cell. Mol. Life Sci. 2017, 74, 2979–2995. [Google Scholar] [CrossRef] [PubMed]
- Richter, A.; Yeager, M.E.; Zaiman, A.; Cool, C.D.; Voelkel, N.F.; Tuder, R.M. Impaired transforming growth factor-beta signaling in idiopathic pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2004, 170, 1340–1348. [Google Scholar] [CrossRef] [PubMed]
- Rosenzweig, B.L.; Imamura, T.; Okadome, T.; Cox, G.N.; Yamashita, H.; Ten Dijke, P.; Heldin, C.H.; Miyazono, K. Cloning and characterization of a human type II receptor for bone morphogenetic proteins. Proc. Natl. Acad. Sci. USA 1995, 92, 7632–7636. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.D.; Girerd, B.; Montani, D.; Wang, X.J.; Galiè, N.; Austin, E.D.; Elliott, G.; Asano, K.; Grünig, E.; Yan, Y.; et al. BMPR2 mutations and survival in pulmonary arterial hypertension: An individual participant data meta-analysis. Lancet Respir. Med. 2016, 4, 129–137. [Google Scholar] [CrossRef]
- Machado, R.D.; Southgate, L.; Eichstaedt, C.A.; Aldred, M.A.; Austin, E.D.; Best, D.H.; Chung, W.K.; Benjamin, N.; Elliott, C.G.; Eyries, M.; et al. Pulmonary Arterial Hypertension: A Current Perspective on Established and Emerging Molecular Genetic Defects. Hum. Mutat. 2015, 36, 1113–1127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Austin, E.D.; Phillips, J.A.; Cogan, J.D.; Hamid, R.; Yu, C.; Stanton, K.C.; Phillips, C.A.; Wheeler, L.A.; Robbins, I.M.; Newman, J.H.; et al. Truncating and missense BMPR2 mutations differentially affect the severity of heritable pulmonary arterial hypertension. Respir. Res. 2009, 10, 87. [Google Scholar] [CrossRef] [PubMed]
- Girerd, B.; Coulet, F.; Jaïs, X.; Eyries, M.; Van Der Bruggen, C.; De Man, F.; Houweling, A.; Dorfmüller, P.; Savale, L.; Sitbon, O.; et al. Characteristics of pulmonary arterial hypertension in affected carriers of a mutation located in the cytoplasmic tail of bone morphogenetic protein receptor type 2. Chest 2015, 147, 1385–1394. [Google Scholar] [CrossRef] [PubMed]
- Aldred, M.A.; Comhair, S.A.; Varella-Garcia, M.; Asosingh, K.; Xu, W.; Noon, G.P.; Thistlethwaite, P.A.; Tuder, R.M.; Erzurum, S.C.; Geraci, M.W.; et al. Somatic chromosome abnormalities in the lungs of patients with pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2010, 182, 1153–1160. [Google Scholar] [CrossRef] [PubMed]
- Negi, V.; Chan, S.Y. Discerning functional hierarchies of microRNAs in pulmonary hypertension. JCI Insight 2017, 2, e91327. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.H.; Brazil, D.P. Bone morphogenetic proteins and their antagonists: Current and emerging clinical uses. Br. J. Pharmacol. 2014, 171, 3620–3632. [Google Scholar] [CrossRef] [PubMed]
- Sedlmeier, G.; Sleeman, J.P. Extracellular regulation of BMP signaling: Welcome to the matrix. Biochem. Soc. Trans. 2017, 45, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Shao, N.Y.; Sa, S.; Li, D.; Termglinchan, V.; Ameen, M.; Karakikes, I.; Sosa, G.; Grubert, F.; Lee, J.; et al. Patient-specific iPSC-derived endothelial cells uncover pathways that protect against pulmonary hypertension in BMPR2 mutation carriers. Cell Stem Cell 2017, 20, 490–504. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Kavsak, P.; Abdollah, S.; Wrana, J.L.; Thomsen, G.H. A SMAD ubiquitin ligase targets the BMP pathway and affects embryonic pattern formation. Nature 1999, 400, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chang, C.; Gehling, D.J.; Hemmati-Brivanlou, A.; Derynck, R. Regulation of Smad degradation and activity by Smurf2, an E3 ubiquitin ligase. Proc. Natl. Acad. Sci. USA 2001, 98, 974–979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwangbo, C.; Lee, H.W.; Kang, H.; Ju, H.; Wiley, D.S.; Papangeli, I.; Han, J.; Kim, J.D.; Dunworth, W.P.; Hu, X.; et al. Modulation of Endothelial Bone Morphogenetic Protein Receptor Type 2 Activity by Vascular Endothelial Growth Factor Receptor 3 in Pulmonary Arterial Hypertension. Circulation 2017, 135, 2288–2298. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, H.; Dewachter, C.; Belhaj, A.; Rondelet, B.; Sakai, S.; Remmelink, M.; Vachiery, J.L.; Naeije, R.; Dewachter, L. Endothelin-Bone morphogenetic protein type 2 receptor interaction induces pulmonary artery smooth muscle cell hyperplasia in pulmonary arterial hypertension. J. Heart Lung Transplant. 2015, 34, 468–478. [Google Scholar] [CrossRef] [PubMed]
- Meloche, J.; Courchesne, A.; Barrier, M.; Carter, S.; Bisserier, M.; Paulin, R.; Lauzon-Joset, J.F.; Breuils-Bonnet, S.; Tremblay, E.; Biardel, S.; et al. Critical role for the advanced glycation end-products receptor in pulmonary arterial hypertension etiology. J. Am. Heart. Assoc. 2013, 2, e005157. [Google Scholar] [CrossRef] [PubMed]
- Austin, E.D.; Hamid, R.; Hemnes, A.R.; Loyd, J.E.; Blackwell, T.; Yu, C.; Phillips Iii, J.A.; Gaddipati, R.; Gladson, S.; Gu, E.; et al. BMPR2 expression is suppressed by signaling through the estrogen receptor. Biol. Sex Differ. 2012, 3, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dannewitz Prosseda, S.; Tian, X.; Kuramoto, K.; Boehm, M.; Sudheendra, D.; Miyagawa, K.; Zhang, F.; Solow-Cordero, D.; Saldivar, J.C.; Austin, E.D.; et al. Fragile Histidine Triad (FHIT), a novel modifier gene in pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2018. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.W.; Song, H.; Kumar, S.; Nam, D.; Kwon, H.S.; Chang, K.H.; Son, D.J.; Kang, D.W.; Brodie, S.A.; Weiss, D.; et al. Anti-inflammatory and antiatherogenic role of BMP receptor II in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1350–1359. [Google Scholar] [CrossRef] [PubMed]
- Dalvi, P.; O’Brien-Ladner, A.; Dhillon, N.K. Downregulation of bone morphogenetic protein receptor axis during HIV-1 and cocaine-mediated pulmonary smooth muscle hyperplasia: Implications for HIV-related pulmonary arterial hypertension. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2585–2595. [Google Scholar] [CrossRef] [PubMed]
- Chinnappan, M.; Mohan, A.; Agarwal, S.; Dalvi, P.; Dhillon, N.K. Network of MicroRNAs Mediate Translational Repression of Bone Morphogenetic Protein Receptor-2: Involvement in HIV-Associated Pulmonary Vascular Remodeling. J. Am. Heart Assoc. 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Brock, M.; Trenkmann, M.; Gay, R.E.; Michel, B.A.; Gay, S.; Fischler, M.; Ulrich, S.; Speich, R.; Huber, L.C. Interleukin-6 modulates the expression of the bone morphogenic protein receptor type II through a novel STAT3-microRNA cluster 17/92 pathway. Circ. Res. 2009, 104, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- Hurst, L.A.; Dunmore, B.J.; Long, L.; Crosby, A.; Al-Lamki, R.; Deighton, J.; Southwood, M.; Yang, X.D.; Nikolic, M.Z.; Herrera, B.; et al. TNFalpha drives pulmonary arterial hypertension by suppressing the BMP type-II receptor and altering NOTCH signalling. Nat. Commun. 2017, 8, 14079. [Google Scholar] [CrossRef] [PubMed]
- Durrington, H.J.; Upton, P.D.; Hoer, S.; Boname, J.; Dunmore, B.J.; Yang, J.; Crilley, T.K.; Butler, L.M.; Blackbourn, D.J.; Nash, G.B.; et al. Identification of a lysosomal pathway regulating degradation of the bone morphogenetic protein receptor type II. J. Biol. Chem. 2010, 285, 37641–37649. [Google Scholar] [CrossRef] [PubMed]
- Stacher, E.; Graham, B.B.; Hunt, J.M.; Gandjeva, A.; Groshong, S.D.; McLaughlin, V.V.; Jessup, M.; Grizzle, W.E.; Aldred, M.A.; Cool, C.D.; et al. Modern age pathology of pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2012, 186, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Sztrymf, B.; Coulet, F.; Girerd, B.; Yaici, A.; Jais, X.; Sitbon, O.; Montani, D.; Souza, R.; Simonneau, G.; Soubrier, F.; et al. Clinical outcomes of pulmonary arterial hypertension in carriers of BMPR2 mutation. Am. J. Respir. Crit. Care Med. 2008, 177, 1377–1383. [Google Scholar] [CrossRef] [PubMed]
- Elliott, C.G.; Glissmeyer, E.W.; Havlena, G.T.; Carlquist, J.; McKinney, J.T.; Rich, S.; McGoon, M.D.; Scholand, M.B.; Kim, M.; Jensen, R.L.; et al. Relationship of BMPR2 mutations to vasoreactivity in pulmonary arterial hypertension. Circulation 2006, 113, 2509–2515. [Google Scholar] [CrossRef] [PubMed]
- Meyrick, B.; Clarke, S.W.; Symons, C.; Woodgate, D.J.; Reid, L. Primary pulmonary hypertension: A case report including electronmicroscopic study. Br. J. Dis. Chest 1974, 68, 11–20. [Google Scholar] [CrossRef]
- Rabinovitch, M.; Bothwell, T.; Hayakawa, B.N.; Williams, W.G.; Trusler, G.A.; Rowe, R.D.; Olley, P.M.; Cutz, E. Pulmonary artery endothelial abnormalities in patients with congenital heart defects and pulmonary hypertension. A correlation of light with scanning electron microscopy and transmission electron microscopy. Lab. Investig. 1986, 55, 632–653. [Google Scholar] [PubMed]
- Rabinovitch, M. Molecular pathogenesis of pulmonary arterial hypertension. J. Clin. Investig. 2012, 122, 4306–4313. [Google Scholar] [CrossRef] [PubMed]
- Regazzoni, C.; Winterhalter, K.H.; Rohrer, L. Type I collagen induces expression of bone morphogenetic protein receptor type II. Biochem. Biophys. Res. Commun. 2001, 283, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Teichert-Kuliszewska, K.; Kutryk, M.J.; Kuliszewski, M.A.; Karoubi, G.; Courtman, D.W.; Zucco, L.; Granton, J.; Stewart, D.J. Bone morphogenetic protein receptor-2 signaling promotes pulmonary arterial endothelial cell survival: Implications for loss-of-function mutations in the pathogenesis of pulmonary hypertension. Circ. Res. 2006, 98, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Awad, K.S.; Elinoff, J.M.; Wang, S.; Gairhe, S.; Ferreyra, G.A.; Cai, R.; Sun, J.; Solomon, M.A.; Danner, R.L. Raf/ERK drives the proliferative and invasive phenotype of BMPR2-silenced pulmonary artery endothelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2016, 310, L187–L201. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.A.; Hemnes, A.R.; Perrien, D.S.; Schuster, M.; Robinson, L.J.; Gladson, S.; Loibner, H.; Bai, S.; Blackwell, T.R.; Tada, Y.; et al. Cytoskeletal defects in Bmpr2-associated pulmonary arterial hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 302, L474–L484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Jesus Perez, V.A.; Alastalo, T.P.; Wu, J.C.; Axelrod, J.D.; Cooke, J.P.; Amieva, M.; Rabinovitch, M. Bone morphogenetic protein 2 induces pulmonary angiogenesis via Wnt-beta-catenin and Wnt-RhoA-Rac1 pathways. J. Cell Biol. 2009, 184, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Upton, P.D.; Davies, R.J.; Trembath, R.C.; Morrell, N.W. Bone morphogenetic protein (BMP) and activin type II receptors balance BMP9 signals mediated by activin receptor-like kinase-1 in human pulmonary artery endothelial cells. J. Biol. Chem. 2009, 284, 15794–15804. [Google Scholar] [CrossRef] [PubMed]
- Sawada, H.; Saito, T.; Nickel, N.P.; Alastalo, T.P.; Glotzbach, J.P.; Chan, R.; Haghighat, L.; Fuchs, G.; Januszyk, M.; Cao, A.; et al. Reduced BMPR2 expression induces GM-CSF translation and macrophage recruitment in humans and mice to exacerbate pulmonary hypertension. J. Exp. Med. 2014, 211, 263–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gangopahyay, A.; Oran, M.; Bauer, E.M.; Wertz, J.W.; Comhair, S.A.; Erzurum, S.C.; Bauer, P.M. Bone morphogenetic protein receptor II is a novel mediator of endothelial nitric-oxide synthase activation. J. Biol. Chem. 2011, 286, 33134–33140. [Google Scholar] [CrossRef] [PubMed]
- Diebold, I.; Hennigs, J.K.; Miyagawa, K.; Li, C.Y.G.; Nickel, N.P.; Kaschwich, M.; Cao, A.Q.; Wang, L.L.; Reddy, S.; Chen, P.I.; et al. BMPR2 preserves mitochondrial function and DNA during reoxygenation to promote endothelial cell survival and reverse pulmonary hypertension. Cell Metab. 2015, 21, 596–608. [Google Scholar] [CrossRef] [PubMed]
- Caruso, P.; Dunmore, B.J.; Schlosser, K.; Schoors, S.; Dos Santos, C.; Perez-Iratxeta, C.; Lavoie, J.R.; Zhang, H.; Long, L.; Flockton, A.R.; et al. Identification of MicroRNA-124 as a Major Regulator of Enhanced Endothelial Cell Glycolysis in Pulmonary Arterial Hypertension via PTBP1 (Polypyrimidine Tract Binding Protein) and Pyruvate Kinase M2. Circulation 2017, 136, 2451–2467. [Google Scholar] [CrossRef] [PubMed]
- Li, M.L.; Vattulainen, S.; Aho, J.; Orcholski, M.; Rojas, V.; Yuan, K.; Helenius, M.; Taimen, P.; Myllykangas, S.; Perez, V.D.; et al. Loss of bone morphogenetic protein receptor 2 is associated with abnormal DNA repair in pulmonary arterial hypertension. Am. J. Respir. Cell Mol. Biol. 2014, 50, 1118–1128. [Google Scholar] [CrossRef] [PubMed]
- Hopper, R.K.; Moonen, J.R.A.J.; Diebold, I.; Cao, A.Q.; Rhodes, C.J.; Tojais, N.F.; Hennigs, J.K.; Gu, M.X.; Wang, L.L.; Rabinovitch, M. In Pulmonary Arterial Hypertension, Reduced BMPR2 Promotes Endothelial-to-Mesenchymal Transition via HMGA1 and Its Target Slug. Circulation 2016, 133, 1783–1794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorai, H.; Vukicevic, S.; Sampath, T.K. Bone morphogenetic protein-7 (osteogenic protein-1) inhibits smooth muscle cell proliferation and stimulates the expression of markers that are characteristic of SMC phenotype in vitro. J. Cell. Physiol. 2000, 184, 37–45. [Google Scholar] [CrossRef]
- Zhang, S.; Fantozzi, I.; Tigno, D.D.; Yi, E.S.; Platoshyn, O.; Thistlethwaite, P.A.; Kriett, J.M.; Yung, G.; Rubin, L.J.; Yuan, J.X.J. Bone morphogenetic proteins induce apoptosis in human pulmonary vascular smooth muscle cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003, 285, L740–L754. [Google Scholar] [CrossRef] [PubMed]
- Morrell, N.W.; Yang, X.D.; Upton, P.D.; Jourdan, K.B.; Morgan, N.; Sheares, K.K.; Trembath, R.C. Altered growth responses of muscle cells from patients pulmonary artery smooth with primary pulmonary hypertension to transforming growth factor-β(1) and bone morphogenetic proteins. Circulation 2001, 104, 790–795. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.D.; Long, L.; Southwood, M.; Rudarakanchana, N.; Upton, P.D.; Jeffery, T.K.; Atkinson, C.; Chen, H.L.; Trembath, R.C.; Morrell, N.W. Dysfunctional smad signaling contributes to abnormal smooth muscle cell proliferation in familial pulmonary arterial hypertension. Circul. Res. 2005, 96, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Lagna, G.; Nguyen, P.H.; Ni, W.H.; Hata, A. BMP-dependent activation of caspase-9 and caspase-8 mediates apoptosis in pulmonary artery smooth muscle cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006, 291, L1059–L1067. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.B.; Deng, D.Y.; Beppu, H.; Hong, C.C.; Lai, C.; Hoyng, S.A.; Kawai, N.; Bloch, K.D. Bone morphogenetic protein (BMP) type II receptor is required for BMP-mediated growth arrest and differentiation in pulmonary artery smooth muscle cells. J. Biol. Chem. 2008, 283, 3877–3888. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, X.H.; Li, Y.; Southwood, M.; Ye, L.Y.; Long, L.; Al-Lamki, R.S.; Morrell, N.W. Id proteins are critical downstream effectors of BMP signaling in human pulmonary arterial smooth muscle cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2013, 305, L312–L321. [Google Scholar] [CrossRef] [PubMed]
- Davies, R.J.; Holmes, A.M.; Deighton, J.; Long, L.; Yang, X.D.; Barker, L.; Walker, C.; Budd, D.C.; Upton, P.D.; Morrell, N.W. BMP type II receptor deficiency confers resistance to growth inhibition by TGF-beta in pulmonary artery smooth muscle cells: Role of proinflammatory cytokines. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 302, L604–L615. [Google Scholar] [CrossRef] [PubMed]
- Upton, P.D.; Davies, R.J.; Tajsic, T.; Morrell, N.W. Transforming growth factor-beta(1) represses bone morphogenetic protein-mediated Smad signaling in pulmonary artery smooth muscle cells via Smad3. Am. J. Respir. Cell Mol. Biol. 2013, 49, 1135–1145. [Google Scholar] [CrossRef] [PubMed]
- Din, S.; Sarathchandra, P.; Yacoub, M.H.; Chester, A.H. Interaction between bone morphogenetic proteins and endothelin-1 in human pulmonary artery smooth muscle. Vascul. Pharmacol. 2009, 51, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Perez, V.A.D.; Ali, Z.; Alastalo, T.P.; Ikeno, F.; Sawada, H.; Lai, Y.J.; Kleisli, T.; Spiekerkoetter, E.; Qu, X.M.; Rubinos, L.H.; et al. BMP promotes motility and represses growth of smooth muscle cells by activation of tandem Wnt pathways. J. Cell Biol. 2011, 192, 171–188. [Google Scholar] [CrossRef] [PubMed]
- Hansmann, G.; de Jesus Perez, V.A.; Alastalo, T.P.; Alvira, C.M.; Guignabert, C.; Bekker, J.M.; Schellong, S.; Urashima, T.; Wang, L.; Morrell, N.W.; et al. An antiproliferative BMP-2/PPARγ/apoE axis in human and murine SMCs and its role in pulmonary hypertension. J. Clin. Investig. 2008, 118, 1846–1857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spiekerkoetter, E.; Guignabert, C.; de Jesus Perez, V.; Alastalo, T.P.; Powers, J.M.; Wang, L.; Lawrie, A.; Ambartsumian, N.; Schmidt, A.M.; Berryman, M.; et al. S100A4 and bone morphogenetic protein-2 codependently induce vascular smooth muscle cell migration via phospho-extracellular signal-regulated kinase and chloride intracellular channel 4. Circ. Res. 2009, 105, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.K.; Knowles, J.A.; Morse, J.H. Bone morphogenetic protein receptor type II C-terminus interacts with c-Src: Implication for a role in pulmonary arterial hypertension. Am. J. Respir. Cell Mol. Biol. 2005, 33, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Stenmark, K.R.; Nozik-Grayck, E.; Gerasimovskaya, E.; Anwar, A.; Li, M.; Riddle, S.; Frid, M. The adventitia: Essential role in pulmonary vascular remodeling. Compr. Physiol. 2011, 1, 141–161. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, T.K.; Upton, P.D.; Trembath, R.C.; Morrell, N.W. BMP4 inhibits proliferation and promotes myocyte differentiation of lung fibroblasts via Smad1 and JNK pathways. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005, 288, L370–L378. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Shen, J.; Pu, K.; Katus, H.A.; Ploger, F.; Tiefenbacher, C.P.; Chen, X.; Braun, T. GDF5 and BMP2 inhibit apoptosis via activation of BMPR2 and subsequent stabilization of XIAP. Biochim. Biophys. Acta 2009, 1793, 1819–1827. [Google Scholar] [CrossRef] [PubMed]
- Tojais, N.F.; Cao, A.; Lai, Y.J.; Wang, L.; Chen, P.I.; Alcazar, M.A.A.; de Jesus Perez, V.A.; Hopper, R.K.; Rhodes, C.J.; Bill, M.A.; et al. Codependence of bone morphogenetic protein receptor 2 and transforming growth factor-beta in elastic fiber assembly and its perturbation in pulmonary arterial hypertension. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1559–1569. [Google Scholar] [CrossRef] [PubMed]
- Denton, C.P.; Zheng, B.; Evans, L.A.; Xu, S.W.; Ong, V.H.; Fisher, I.; Lazaridis, K.; Abraham, D.J.; Black, C.M.; de Crombrugghe, B. Fibroblast-specific expression of a kinase-deficient type II transforming growth factor beta (TGFβ) receptor leads to paradoxical activation of TGFβ signaling pathways with fibrosis in transgenic mice. J. Biol. Chem. 2003, 278, 25109–25119. [Google Scholar] [CrossRef] [PubMed]
- Gilbane, A.J.; Derrett-Smith, E.; Trinder, S.L.; Good, R.B.; Pearce, A.; Denton, C.P.; Holmes, A.M. Impaired bone morphogenetic protein receptor II signaling in a transforming growth factor-β-dependent mouse model of pulmonary hypertension and in systemic sclerosis. Am. J. Respir. Crit. Care Med. 2015, 191, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Kendall, R.T.; Feghali-Bostwick, C.A. Fibroblasts in fibrosis: Novel roles and mediators. Front. Pharmacol. 2014, 5, 123. [Google Scholar] [CrossRef] [PubMed]
- Galambos, C.; Sims-Lucas, S.; Abman, S.H.; Cool, C.D. Intrapulmonary bronchopulmonary anastomoses and plexiform lesions in idiopathic pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2016, 193, 574–576. [Google Scholar] [CrossRef] [PubMed]
- Ghigna, M.R.; Guignabert, C.; Montani, D.; Girerd, B.; Jais, X.; Savale, L.; Herve, P.; de Montpreville, V.T.; Mercier, O.; Sitbon, O.; et al. BMPR2 mutation status influences bronchial vascular changes in pulmonary arterial hypertension. Eur. Respir. J. 2016, 48, 1668–1681. [Google Scholar] [CrossRef] [PubMed]
- Brittain, E.L.; Pugh, M.E.; Wheeler, L.A.; Robbins, I.M.; Loyd, J.E.; Newman, J.H.; Larkin, E.K.; Austin, E.D.; Hemnes, A.R. Shorter survival in familial versus idiopathic pulmonary arterial hypertension is associated with hemodynamic markers of impaired right ventricular function. Pulm. Circ. 2013, 3, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Van der Bruggen, C.E.; Happe, C.M.; Dorfmuller, P.; Trip, P.; Spruijt, O.A.; Rol, N.; Hoevenaars, F.P.; Houweling, A.C.; Girerd, B.; Marcus, J.T.; et al. Bone morphogenetic protein receptor type 2 mutation in pulmonary arterial hypertension: A view on the right ventricle. Circulation 2016, 133, 1747–1760. [Google Scholar] [CrossRef] [PubMed]
- Koitabashi, N.; Danner, T.; Zaiman, A.L.; Pinto, Y.M.; Rowell, J.; Mankowski, J.; Zhang, D.; Nakamura, T.; Takimoto, E.; Kass, D.A. Pivotal role of cardiomyocyte TGF-beta signaling in the murine pathological response to sustained pressure overload. J. Clin. Investig. 2011, 121, 2301–2312. [Google Scholar] [CrossRef] [PubMed]
- Izumi, M.; Fujio, Y.; Kunisada, K.; Negoro, S.; Tone, E.; Funamoto, M.; Osugi, T.; Oshima, Y.; Nakaoka, Y.; Kishimoto, T.; et al. Bone morphogenetic protein-2 inhibits serum deprivation-induced apoptosis of neonatal cardiac myocytes through activation of the Smad1 pathway. J. Biol. Chem. 2001, 276, 31133–31141. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.P.; Sagave, J.; Rutkovskiy, A.; Haugen, F.; Baysa, A.; Stale, N.; Czibik, G.; Dahl, C.P.; Gullestad, L.; Vaage, J.; et al. Expression of bone morphogenetic protein 4 and its receptors in the remodeling heart. Life Sci. 2014, 97, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Sutendra, G.; Michelakis, E.D. The metabolic basis of pulmonary arterial hypertension. Cell Metab. 2014, 19, 558–573. [Google Scholar] [CrossRef] [PubMed]
- Talati, M.H.; Brittain, E.L.; Fessel, J.P.; Penner, N.; Atkinson, J.; Funke, M.; Grueter, C.; Jerome, W.G.; Freeman, M.; Newman, J.H.; et al. Mechanisms of lipid accumulation in the bone morphogenetic protein receptor type 2 mutant right ventricle. Am. J. Respir. Crit. Care Med. 2016, 194, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Hemnes, A.R.; Brittain, E.L.; Trammell, A.W.; Fessel, J.P.; Austin, E.D.; Penner, N.; Maynard, K.B.; Gleaves, L.; Talati, M.; Absi, T.; et al. Evidence for right ventricular lipotoxicity in heritable pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2014, 189, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Nicolls, M.R.; Voelkel, N.F. The roles of immunity in the prevention and evolution of pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2017, 195, 1292–1299. [Google Scholar] [CrossRef] [PubMed]
- Soon, E.; Crosby, A.; Southwood, M.; Yang, P.; Tajsic, T.; Toshner, M.; Appleby, S.; Shanahan, C.M.; Bloch, K.D.; Pepke-Zaba, J.; et al. Bone morphogenetic protein receptor type II deficiency and increased inflammatory cytokine production. A gateway to pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2015, 192, 859–872. [Google Scholar] [CrossRef] [PubMed]
- Muller, W.A. Mechanisms of leukocyte transendothelial migration. Annu. Rev. Pathol. 2011, 6, 323–344. [Google Scholar] [CrossRef] [PubMed]
- Sorescu, G.P.; Song, H.; Tressel, S.L.; Hwang, J.; Dikalov, S.; Smith, D.A.; Boyd, N.L.; Platt, M.O.; Lassegue, B.; Griendling, K.K.; et al. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress induces monocyte adhesion by stimulating reactive oxygen species production from a nox1-based NADPH oxidase. Circ. Res. 2004, 95, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Burton, V.J.; Ciuclan, L.I.; Holmes, A.M.; Rodman, D.M.; Walker, C.; Budd, D.C. Bone morphogenetic protein receptor II regulates pulmonary artery endothelial cell barrier function. Blood 2011, 117, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Sato, A.Y.S.; Bub, G.L.; Campos, A.H. BMP-2 and -4 produced by vascular smooth muscle cells from atherosclerotic lesions induce monocyte chemotaxis through direct BMPRII activation. Atherosclerosis 2014, 235, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Talati, M.; West, J.; Zaynagetdinov, R.; Hong, C.C.; Han, W.; Blackwell, T.; Robinson, L.; Blackwell, T.S.; Lane, K. BMP pathway regulation of and by macrophages. PLoS ONE 2014, 9, e94119. [Google Scholar] [CrossRef] [PubMed]
- Marsh, L.M.; Jandl, K.; Grunig, G.; Foris, V.; Bashir, M.; Ghanim, B.; Klepetko, W.; Olschewski, H.; Olschewski, A.; Kwapiszewska, G. The inflammatory cell landscape in the lungs of patients with idiopathic pulmonary arterial hypertension. Eur. Respir. J. 2018, 51. [Google Scholar] [CrossRef] [PubMed]
- Tamosiuniene, R.; Tian, W.; Dhillon, G.; Wang, L.; Sung, Y.K.; Gera, L.; Patterson, A.J.; Agrawal, R.; Rabinovitch, M.; Ambler, K.; et al. Regulatory T cells limit vascular endothelial injury and prevent pulmonary hypertension. Circ. Res. 2011, 109, 867–879. [Google Scholar] [CrossRef] [PubMed]
- Graf, D.; Nethisinghe, S.; Palmer, D.B.; Fisher, A.G.; Merkenschlager, M. The developmentally regulated expression of Twisted gastrulation reveals a role for bone morphogenetic proteins in the control of T cell development. J. Exp. Med. 2002, 196, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Germain, R.N. T-cell development and the CD4-CD8 lineage decision. Nat. Rev. Immunol. 2002, 2, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Swann, J.B.; Krauth, B.; Happe, C.; Boehm, T. Cooperative interaction of BMP signalling and Foxn1 gene dosage determines the size of the functionally active thymic epithelial compartment. Sci. Rep. 2017, 7, 8492. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.; Jenkinson, W.E.; Jones, T.; Parnell, S.M.; Kinsella, F.A.; White, A.J.; Pongrac’z, J.E.; Rossi, S.W.; Jenkinson, E.J. Establishment and functioning of intrathymic microenvironments. Immunol. Rev. 2006, 209, 10–27. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Ten Dijke, P. Immunoregulation by members of the TGFbeta superfamily. Nat. Rev. Immunol. 2016, 16, 723–740. [Google Scholar] [CrossRef] [PubMed]
- Karsenty, G.; Luo, G.B.; Hofmann, C.; Bradley, A. BMP 7 is required for nephrogenesis, eye development, and skeletal patterning. Ann. N. Y. Acad. Sci. 1996, 785, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, Y.; Oshima, K.; Fogo, A.; Hogan, B.L.M.; Ichikawa, I. Bone morphogenetic protein 4 regulates the budding site and elongation of the mouse ureter. J. Clin. Investig. 2000, 105, 863–873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pache, G.; Schafer, C.; Wiesemann, S.; Springer, E.; Liebau, M.; Reinhardt, H.C.; August, C.; Pavenstadt, H.; Bek, M.J. Upregulation of Id-1 via BMP-2 receptors induces reactive oxygen species in podocytes. Am. J. Physiol. Renal Physiol. 2006, 291, F654–F662. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, J.; Hruska, K.; Guo, G.J.; Wang, S.; Chen, Q.; Klahr, S. Bone morphogenetic protein-7 improves renal fibrosis and accelerates the return of renal function. J. Am. Soc. Nephrol. 2002, 13, S14–S21. [Google Scholar] [PubMed]
- Yeh, C.H.; Chang, C.K.; Cheng, M.F.; Lin, H.J.; Cheng, J.T. Decrease of bone morphogenetic protein-7 (BMP-7) and its type II receptor (BMP-RII) in kidney of type 1-like diabetic rats. Horm. Metab. Res. 2009, 41, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.Y.; Lee, D.H.; Lee, D.K.; Kim, B.C.; Kim, H.T.; Leach, F.S.; Linehan, W.M.; Morton, R.A.; Kim, S.J. Decreased expression of bone morphogenetic protein (BMP) receptor type II correlates with insensitivity to BMP-6 in human renal cell carcinoma cells. Clin. Cancer Res. 2003, 9, 6046–6051. [Google Scholar] [PubMed]
- Mayeur, C.; Leyton, P.A.; Kolodziej, S.A.; Yu, B.L.; Bloch, K.D. BMP type II receptors have redundant roles in the regulation of hepatic hepcidin gene expression and iron metabolism. Blood 2014, 124, 2116–2123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Gu, X.; Weng, H.L.; Ghafoory, S.; Liu, Y.; Feng, T.; Dzieran, J.; Li, L.; IIkavets, I.; Kruithof-de Julio, M.; et al. Bone morphogenetic protein-9 induces epithelial to mesenchymal transition in hepatocellular carcinoma cells. Cancer Sci. 2013, 104, 398–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, R.Z.; Lei, L.; Cheng, L.; Jin, Z.F.; Zu, S.J.; Shan, Z.Y.; Wang, Z.D.; Zhang, J.X.; Liu, Z.H. Expression of GDF-9, BMP-15 and their receptors in mammalian ovary follicles. J. Mol. Histol. 2010, 41, 325–332. [Google Scholar] [CrossRef] [PubMed]
- De Resende, L.O.T.; Vireque, A.A.; Santana, L.F.; Moreno, D.A.; Silva, A.C.J.D.R.E.; Ferriani, R.A.; Scrideli, C.A.; Reis, R.M. Single-cell expression analysis of BMP15 and GDF9 in mature oocytes and BMPR2 in cumulus cells of women with polycystic ovary syndrome undergoing controlled ovarian hyperstimulation. J. Assist. Reprod. Genet. 2012, 29, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Herrera, B.; van Dinther, M.; ten Dijke, P.; Inman, G.J. Autocrine bone morphogenetic protein-9 signals through activin receptor-like kinase-2/Smad1/Smad4 to promote ovarian cancer cell proliferation. Cancer Res. 2009, 69, 9254–9262. [Google Scholar] [CrossRef] [PubMed]
- Iwata, N.; Hasegawa, T.; Fujita, S.; Nagao, S.; Nakano, Y.; Nada, T.; Nishiyama, Y.; Hosoya, T.; Otsuka, F. Effect of the interaction of metformin and bone morphogenetic proteins on ovarian steroidogenesis by human granulosa cells. Biochem. Biophys. Res. Commun. 2018, in press. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Chong, D.C.; Ola, R.; Dunworth, W.P.; Meadows, S.; Ka, J.; Kaartinen, V.M.; Qyang, Y.; Cleaver, O.; Bautch, V.L.; et al. Alk2/ACVR1 and Alk3/BMPR1A Provide Essential Function for Bone Morphogenetic Protein-Induced Retinal Angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.M.; Keller, J.J.; Wan, L.; Stone, J.S. Bone morphogenetic protein 4 antagonizes hair cell regeneration in the avian auditory epithelium. Hear. Res. 2018, 364, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lee-Hoeflich, S.T.; Causing, C.G.; Podkowa, M.; Zhao, X.; Wrana, J.L.; Attisano, L. Activation of LIMK1 by binding to the BMP receptor, BMPRII, regulates BMP-dependent dendritogenesis. EMBO J. 2004, 23, 4792–4801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adir, Y.; Elia, D.; Harari, S. Pulmonary hypertension in patients with chronic myeloproliferative disorders. Eur. Respir. Rev. 2015, 24, 400–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, E.; Frost, A.E.; Uday, R.P.L.; Josef, T.P. Dysregulation of BMPR2, Arginase II and HMGA2 expression in idiopathic myelofibrosis and secondary myelofibrosis. Blood 2004, 104, 792. [Google Scholar]
- Andrieux, J.; Roche-Lestienne, C.; Geffroy, S.; Desterke, C.; Grardel, N.; Plantier, I.; Selleslag, D.; Demory, J.L.; Lai, J.L.; Leleu, X.; et al. Bone morphogenetic protein antagonist gene NOG is involved in myeloproliferative disease associated with myelofibrosis. Cancer Genet. Cytogenet. 2007, 178, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Bock, O.; Hoftmann, J.; Theophile, K.; Hussein, K.; Wiese, B.; Schlue, J.; Kreipe, H. Bone morphogenetic proteins are overexpressed in the bone marrow of primary myelofibrosis and are apparently induced by fibrogenic cytokines. Am. J. Pathol. 2008, 172, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Howe, J.R.; Bair, J.L.; Sayed, M.G.; Anderson, M.E.; Mitros, F.A.; Petersen, G.M.; Velculescu, V.E.; Traverso, G.; Vogelstein, B. Germline mutations of the gene encoding bone morphogenetic protein receptor 1A in juvenile polyposis. Nat. Genet. 2001, 28, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Owens, P.; Pickup, M.W.; Novitskiy, S.V.; Chytil, A.; Gorska, A.E.; Aakre, M.E.; West, J.; Moses, H.L. Disruption of bone morphogenetic protein receptor 2 (BMPR2) in mammary tumors promotes metastases through cell autonomous and paracrine mediators. Proc. Natl. Acad. Sci. USA 2012, 109, 2814–2819. [Google Scholar] [CrossRef] [PubMed]
- Owens, P.; Pickup, M.W.; Novitskiy, S.V.; Giltnane, J.M.; Gorska, A.E.; Hopkins, C.R.; Hong, C.C.; Moses, H.L. Inhibition of BMP signaling suppresses metastasis in mammary cancer. Oncogene 2015, 34, 2437–2449. [Google Scholar] [CrossRef] [PubMed]
- Jiao, G.; Guo, W.; Ren, T.; Lu, Q.; Sun, Y.; Liang, W.; Ren, C.; Yang, K.; Sun, K. BMPR2 inhibition induced apoptosis and autophagy via destabilization of XIAP in human chondrosarcoma cells. Cell Death Dis. 2014, 5, e1571. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Ma, L.; Guo, Q.A.; Zhang, S.L. Expression of bone morphogenetic protein-2 and its receptors in epithelial ovarian cancer and their influence on the prognosis of ovarian cancer patients. J. Exp. Clin. Cancer Res. 2010, 29, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darby, S.; Cross, S.S.; Brown, N.J.; Hamdy, F.C.; Robson, C.N. BMP-6 over-expression in prostate cancer is associated with increased Id-1 protein and a more invasive phenotype. J. Pathol. 2008, 214, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Grcevic, D.; Marusic, A.; Grahovac, B.; Jaksic, B.; Kusec, R. Expression of bone morphogenetic proteins in acute promyelocytic leukemia before and after combined all trans-retinoic acid and cytotoxic treatment. Leukemia Res. 2003, 27, 731–738. [Google Scholar] [CrossRef]
- Park, S.W.; Hur, S.Y.; Yoo, N.J.; Lee, S.H. Somatic frameshift mutations of bone morphogenic protein receptor 2 gene in gastric and colorectal cancers with microsatellite instability. APMIS 2010, 118, 824–829. [Google Scholar] [CrossRef] [PubMed]
- Beppu, H.; Mwizerwa, O.N.; Beppu, Y.; Dattwyler, M.P.; Lauwers, G.Y.; Bloch, K.D.; Goldstein, A.M. Stromal inactivation of BMPRII leads to colorectal epithelial overgrowth and polyp formation. Oncogene 2008, 27, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.; Lim, A.; Zhao, C.; Sahoo, D.; Pan, Y.; Spiekerkoetter, E.; Liao, J.C.; Beachy, P.A. Hedgehog signaling restrains bladder cancer progression by eliciting stromal production of urothelial differentiation factors. Cancer Cell 2014, 26, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Claessen, G.; La Gerche, A.; Petit, T.; Gillijns, H.; Bogaert, J.; Claeys, M.; Dymarkowski, S.; Claus, P.; Delcroix, M.; Heidbuchel, H. Right ventricular and pulmonary vascular reserve in asymptomatic BMPR2 mutation carriers. J. Heart Lung Transplant. 2017, 36, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Mikhak, Z.; Strassner, J.P.; Luster, A.D. Lung dendritic cells imprint T cell lung homing and promote lung immunity through the chemokine receptor CCR4. J. Exp. Med. 2013, 210, 1855–1869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeager, M.E.; Halley, G.R.; Golpon, H.A.; Voelkel, N.F.; Tuder, R.M. Microsatellite instability of endothelial cell growth and apoptosis genes within plexiform lesions in primary pulmonary hypertension. Circul. Res. 2001, 88, e2–e11. [Google Scholar] [CrossRef]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andruska, A.; Spiekerkoetter, E. Consequences of BMPR2 Deficiency in the Pulmonary Vasculature and Beyond: Contributions to Pulmonary Arterial Hypertension. Int. J. Mol. Sci. 2018, 19, 2499. https://doi.org/10.3390/ijms19092499
Andruska A, Spiekerkoetter E. Consequences of BMPR2 Deficiency in the Pulmonary Vasculature and Beyond: Contributions to Pulmonary Arterial Hypertension. International Journal of Molecular Sciences. 2018; 19(9):2499. https://doi.org/10.3390/ijms19092499
Chicago/Turabian StyleAndruska, Adam, and Edda Spiekerkoetter. 2018. "Consequences of BMPR2 Deficiency in the Pulmonary Vasculature and Beyond: Contributions to Pulmonary Arterial Hypertension" International Journal of Molecular Sciences 19, no. 9: 2499. https://doi.org/10.3390/ijms19092499
APA StyleAndruska, A., & Spiekerkoetter, E. (2018). Consequences of BMPR2 Deficiency in the Pulmonary Vasculature and Beyond: Contributions to Pulmonary Arterial Hypertension. International Journal of Molecular Sciences, 19(9), 2499. https://doi.org/10.3390/ijms19092499