Gestational Diabetes Alters the Metabolomic Profile in 2nd Trimester Amniotic Fluid in a Sex-Specific Manner
Abstract
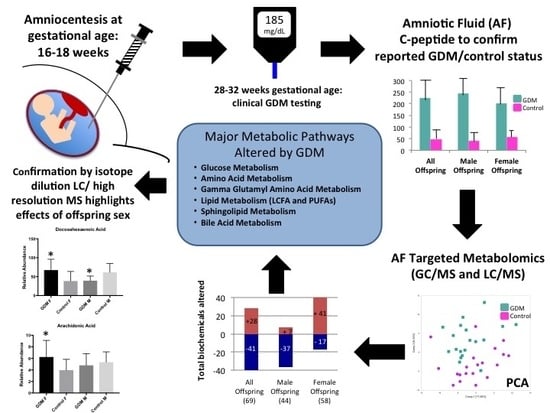
:1. Introduction
2. Results
2.1. Clinical Characteristics of Women and Infants
2.2. Confirmation of GDM Classification
2.3. Global Assessment of Metabolomic Data
2.4. Metabolic Pathways Altered in AF Exposed to GDM
2.4.1. Glucose Metabolism
2.4.2. Amino Acid Metabolism
2.4.3. Lipid Metabolism
2.4.4. Sphingolipid Metabolism
2.4.5. Bile Acid Metabolism
3. Discussion
4. Materials and Methods
4.1. Amniotic Fluid Samples
4.2. C-Peptide Measurement
4.3. Targeted Metabolomic Analysis
4.4. Confirmatory PUFA Analysis by Isotope Dilution LC/High Resolution MS
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 1,5-AG | 1,5-anhydroglucitol |
| AA | amino acid |
| AF | amniotic fluid |
| AHB | 2-hydroxybutyrate |
| ANOVA | analysis of variance |
| BA | bile acids |
| BMI | body mass index |
| DOAJ | directory of open access journals |
| FC | fold change |
| GA | gestational age |
| GLUT | glucose transporter protein |
| GC/MS | gas chromatography/mass spectrometry |
| GDM | gestational diabetes mellitus |
| LC/MS | liquid chromatography/mass spectrometry |
| MDPI | Multidisciplinary Digital Publishing Institute |
| PCA | principal component analysis |
| RF | random forest |
| T2DM | type 2 diabetes mellitus |
| TCA | tricarboxylic acid |
References
- Martin, A.O.; Simpson, J.L.; Ober, C.; Freinkel, N. Frequency of diabetes mellitus in mothers of probands with gestational diabetes: Possible maternal influence on the predisposition to gestational diabetes. Am. J. Obstet. Gynecol. 1985, 151, 471–475. [Google Scholar] [CrossRef]
- Pettitt, D.J.; Aleck, K.A.; Baird, H.R.; Carraher, M.J.; Bennett, P.H.; Knowler, W.C. Congenital susceptibility to niddm. Role of intrauterine environment. Diabetes 1988, 37, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Pettitt, D.J.; Baird, H.R.; Aleck, K.A.; Bennett, P.H.; Knowler, W.C. Excessive obesity in offspring of pima indian women with diabetes during pregnancy. N. Engl. J. Med. 1983, 308, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Plagemann, A. Perinatal programming and functional teratogenesis: Impact on body weight regulation and obesity. Physiol. Behav. 2005, 86, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Silverman, B.L.; Metzger, B.E.; Cho, N.H.; Loeb, C.A. Impaired glucose tolerance in adolescent offspring of diabetic mothers. Relationship to fetal hyperinsulinism. Diabetes Care 1995, 18, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Clausen, T.D.; Mathiesen, E.R.; Hansen, T.; Pedersen, O.; Jensen, D.M.; Lauenborg, J.; Damm, P. High prevalence of type 2 diabetes and pre-diabetes in adult offspring of women with gestational diabetes mellitus or type 1 diabetes: The role of intrauterine hyperglycemia. Diabetes Care 2008, 31, 340–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crume, T.L.; Ogden, L.; West, N.A.; Vehik, K.S.; Scherzinger, A.; Daniels, S.; McDuffie, R.; Bischoff, K.; Hamman, R.F.; Norris, J.M.; et al. Association of exposure to diabetes in utero with adiposity and fat distribution in a multiethnic population of youth: The exploring perinatal outcomes among children (epoch) study. Diabetologia 2011, 54, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Egeland, G.M.; Meltzer, S.J. Following in mother’s footsteps? Mother-daughter risks for insulin resistance and cardiovascular disease 15 years after gestational diabetes. Diabet. Med. 2010, 27, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, M.; Arata, N.; Ogawa, Y. Obesity and abnormal glucose tolerance in the offspring of mothers with diabetes. Curr. Opin. Obstet. Gynecol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Tam, W.H.; Ma, R.C.W.; Ozaki, R.; Li, A.M.; Chan, M.H.M.; Yuen, L.Y.; Lao, T.T.H.; Yang, X.; Ho, C.S.; Tutino, G.E.; et al. In utero exposure to maternal hyperglycemia increases childhood cardiometabolic risk in offspring. Diabetes Care 2017, 40, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Lawlor, D.A.; Lichtenstein, P.; Langstrom, N. Association of maternal diabetes mellitus in pregnancy with offspring adiposity into early adulthood: Sibling study in a prospective cohort of 280,866 men from 248,293 families. Circulation 2011, 123, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Metzger, B.E.; Silverman, B.L.; Freinkel, N.; Dooley, S.L.; Ogata, E.S.; Green, O.C. Amniotic fluid insulin concentration as a predictor of obesity. Arch. Dis. Child. 1990, 65, 1050–1052. [Google Scholar] [CrossRef] [PubMed]
- Silverman, B.L.; Rizzo, T.A.; Cho, N.H.; Metzger, B.E. Long-term effects of the intrauterine environment. The northwestern university diabetes in pregnancy center. Diabetes Care 1998, 21 (Suppl. 2), B142–B149. [Google Scholar] [PubMed]
- Tokarz, J.; Haid, M.; Cecil, A.; Prehn, C.; Artati, A.; Moller, G.; Adamski, J. Endocrinology meets metabolomics: Achievements, pitfalls, and challenges. Trends Endocrinol. Metab. 2017, 28, 705–721. [Google Scholar] [CrossRef] [PubMed]
- Bock, J.L. Metabolic profiling of amniotic fluid by proton nuclear magnetic resonance spectroscopy: Correlation with fetal maturation and other clinical variables. Clin. Chem. 1994, 40, 56–61. [Google Scholar] [PubMed]
- Chen, X.; Scholl, T.O. Oxidative stress: Changes in pregnancy and with gestational diabetes mellitus. Curr. Diabetes Rep. 2005, 5, 282–288. [Google Scholar] [CrossRef]
- Underwood, M.A.; Gilbert, W.M.; Sherman, M.P. Amniotic fluid: Not just fetal urine anymore. J. Perinatol. 2005, 25, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Diaz, S.O.; Pinto, J.; Graca, G.; Duarte, I.F.; Barros, A.S.; Galhano, E.; Pita, C.; Almeida Mdo, C.; Goodfellow, B.J.; Carreira, I.M.; et al. Metabolic biomarkers of prenatal disorders: An exploratory nmr metabonomics study of second trimester maternal urine and blood plasma. J. Proteome Res. 2011, 10, 3732–3742. [Google Scholar] [CrossRef] [PubMed]
- Graca, G.; Duarte, I.F.; Barros, A.S.; Goodfellow, B.J.; Diaz, S.O.; Pinto, J.; Carreira, I.M.; Galhano, E.; Pita, C.; Gil, A.M. Impact of prenatal disorders on the metabolic profile of second trimester amniotic fluid: A nuclear magnetic resonance metabonomic study. J. Proteome Res. 2010, 9, 6016–6024. [Google Scholar] [CrossRef] [PubMed]
- Spellacy, W.N.; Buhi, W.C.; Bradley, B.; Holsinger, K.K. Maternal, fetal and amniotic fluid levels of glucose, insulin and growth hormone. Obstet. Gynecol. 1973, 41, 323–331. [Google Scholar] [PubMed]
- Stanirowski, P.J.; Szukiewicz, D.; Pazura-Turowska, M.; Sawicki, W.; Cendrowski, K. Expression of glucose transporter proteins in human diabetic placenta. Can. J. Diabetes 2017, 42, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Gall, W.E.; Beebe, K.; Lawton, K.A.; Adam, K.P.; Mitchell, M.W.; Nakhle, P.J.; Ryals, J.A.; Milburn, M.V.; Nannipieri, M.; Camastra, S.; et al. Alpha-hydroxybutyrate is an early biomarker of insulin resistance and glucose intolerance in a nondiabetic population. PLoS ONE 2010, 5, e10883. [Google Scholar] [CrossRef] [PubMed]
- Bogavac, M.; Lakic, N.; Simin, N.; Nikolic, A.; Sudji, J.; Bozin, B. Biomarkers of oxidative stress in amniotic fluid and complications in pregnancy. J. Matern. Fetal Neonatal Med. 2012, 25, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Wright, L.A.; Hirsch, I.B.; Gooley, T.A.; Brown, Z. 1,5-anhydroglucitol and neonatal complications in pregnancy complicated by diabetes. Endocr. Pract. 2015, 21, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Wikoff, W.R.; Anfora, A.T.; Liu, J.; Schultz, P.G.; Lesley, S.A.; Peters, E.C.; Siuzdak, G. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc. Natl. Acad. Sci. USA 2009, 106, 3698–3703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Mello, V.D.; Paananen, J.; Lindstrom, J.; Lankinen, M.A.; Shi, L.; Kuusisto, J.; Pihlajamaki, J.; Auriola, S.; Lehtonen, M.; Rolandsson, O.; et al. Indolepropionic acid and novel lipid metabolites are associated with a lower risk of type 2 diabetes in the finnish diabetes prevention study. Sci. Rep. 2017, 7, 46337. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.A.; Kang, S.I.; Shin, H.S.; Kang, S.W.; Kim, J.H.; Ko, H.C.; Kim, S.J. P-coumaric acid modulates glucose and lipid metabolism via amp-activated protein kinase in l6 skeletal muscle cells. Biochem. Biophys. Res. Commun. 2013, 432, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Mizugishi, K.; Inoue, T.; Hatayama, H.; Bielawski, J.; Pierce, J.S.; Sato, Y.; Takaori-Kondo, A.; Konishi, I.; Yamashita, K. Sphingolipid pathway regulates innate immune responses at the fetomaternal interface during pregnancy. J. Biol. Chem. 2015, 290, 2053–2068. [Google Scholar] [CrossRef] [PubMed]
- Holland, W.L.; Brozinick, J.T.; Wang, L.P.; Hawkins, E.D.; Sargent, K.M.; Liu, Y.; Narra, K.; Hoehn, K.L.; Knotts, T.A.; Siesky, A.; et al. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 2007, 5, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Brouwers, L.; Koster, M.P.; Page-Christiaens, G.C.; Kemperman, H.; Boon, J.; Evers, I.M.; Bogte, A.; Oudijk, M.A. Intrahepatic cholestasis of pregnancy: Maternal and fetal outcomes associated with elevated bile acid levels. Am. J. Obstet. Gynecol. 2015, 212, e1–e7. [Google Scholar] [CrossRef] [PubMed]
- Retnakaran, R.; Kramer, C.K.; Ye, C.; Kew, S.; Hanley, A.J.; Connelly, P.W.; Sermer, M.; Zinman, B. Fetal sex and maternal risk of gestational diabetes mellitus: The impact of having a boy. Diabetes Care 2015, 38, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Lingwood, B.E.; Henry, A.M.; d’Emden, M.C.; Fullerton, A.M.; Mortimer, R.H.; Colditz, P.B.; Le Cao, K.A.; Callaway, L.K. Determinants of body fat in infants of women with gestational diabetes mellitus differ with fetal sex. Diabetes Care 2011, 34, 2581–2585. [Google Scholar] [CrossRef] [PubMed]
- Persson, M.; Fadl, H. Perinatal outcome in relation to fetal sex in offspring to mothers with pre-gestational and gestational diabetes—A population-based study. Diabet. Med. 2014, 31, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.; Teague, A.M.; Chen, J.; Aston, C.E.; Leung, Y.K.; Chernausek, S.; Simmons, R.A.; Pinney, S.E. Offspring sex impacts DNA methylation and gene expression in placentae from women with diabetes during pregnancy. PLoS ONE 2018, 13, e0190698. [Google Scholar] [CrossRef] [PubMed]
- Stenman, L.K.; Holma, R.; Eggert, A.; Korpela, R. A novel mechanism for gut barrier dysfunction by dietary fat: Epithelial disruption by hydrophobic bile acids. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 304, G227–G234. [Google Scholar] [CrossRef] [PubMed]
- Susiarjo, M.; Xin, F.; Stefaniak, M.; Mesaros, C.; Simmons, R.A.; Bartolomei, M.S. Bile acids and tryptophan metabolism are novel pathways involved in metabolic abnormalities in bpa-exposed pregnant mice and male offspring. Endocrinology 2017, 158, 2533–2542. [Google Scholar] [CrossRef] [PubMed]
- Gomez de Aguero, M.; Ganal-Vonarburg, S.C.; Fuhrer, T.; Rupp, S.; Uchimura, Y.; Li, H.; Steinert, A.; Heikenwalder, M.; Hapfelmeier, S.; Sauer, U.; et al. The maternal microbiota drives early postnatal innate immune development. Science 2016, 351, 1296–1302. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Koulman, A.; Petry, C.J.; Jenkins, B.; Matthews, L.; Hughes, I.A.; Acerini, C.L.; Ong, K.K.; Dunger, D.B. An unbiased lipidomics approach identifies early second trimester lipids predictive of maternal glycemic traits and gestational diabetes mellitus. Diabetes Care 2016, 39, 2232–2239. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Lambert, J.E.; Hovhannisyan, Y.; Ramos-Roman, M.A.; Trombold, J.R.; Wagner, D.A.; Parks, E.J. Palmitoleic acid is elevated in fatty liver disease and reflects hepatic lipogenesis. Am. J. Clin. Nutr. 2015, 101, 34–43. [Google Scholar] [CrossRef] [PubMed]
- De Souza, C.O.; Teixeira, A.A.S.; Biondo, L.A.; Lima Junior, E.A.; Batatinha, H.A.P.; Rosa Neto, J.C. Palmitoleic acid improves metabolic functions in fatty liver by pparalpha-dependent ampk activation. J. Cell Physiol. 2017, 232, 2168–2177. [Google Scholar] [CrossRef] [PubMed]
- Regnault, N.; Gillman, M.W.; Rifas-Shiman, S.L.; Eggleston, E.; Oken, E. Sex-specific associations of gestational glucose tolerance with childhood body composition. Diabetes Care 2013, 36, 3045–3053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffith, O.W.; Bridges, R.J.; Meister, A. Transport of gamma-glutamyl amino acids: Role of glutathione and gamma-glutamyl transpeptidase. Proc. Natl. Acad. Sci. USA 1979, 76, 6319–6322. [Google Scholar] [CrossRef] [PubMed]
- Coughlan, M.T.; Vervaart, P.P.; Permezel, M.; Georgiou, H.M.; Rice, G.E. Altered placental oxidative stress status in gestational diabetes mellitus. Placenta 2004, 25, 78–84. [Google Scholar] [CrossRef]
- Cetin, I.; de Santis, M.S.; Taricco, E.; Radaelli, T.; Teng, C.; Ronzoni, S.; Spada, E.; Milani, S.; Pardi, G. Maternal and fetal amino acid concentrations in normal pregnancies and in pregnancies with gestational diabetes mellitus. Am. J. Obstet. Gynecol. 2005, 192, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Metzger, B.E.; Phelps, R.L.; Freinkel, N.; Navickas, I.A. Effects of gestational diabetes on diurnal profiles of plasma glucose, lipids, and individual amino acids. Diabetes Care 1980, 3, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Scholtens, D.M.; Muehlbauer, M.J.; Daya, N.R.; Stevens, R.D.; Dyer, A.R.; Lowe, L.P.; Metzger, B.E.; Newgard, C.B.; Bain, J.R.; Lowe, W.L., Jr.; et al. Metabolomics reveals broad-scale metabolic perturbations in hyperglycemic mothers during pregnancy. Diabetes Care 2014, 37, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Hui, L.; Muggli, E.E.; Halliday, J.L. Population-based trends in prenatal screening and diagnosis for aneuploidy: A retrospective analysis of 38 years of state-wide data. BJOG 2016, 123, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.M.; Bridgewater, B.R.; Liu, Q.; Mitchell, M.W.; Robinson, R.J.; Dai, H.; Stewart, S.J.; DeHaven, C.D.; Miller, L.A.D. High resolution mass spectrometry improves data quantity and quality as compared to unit mass resolution mass spectrometry in high throughput profiling metabolomics. Metabolomics 2014, 4. [Google Scholar] [CrossRef]
- DeHaven, C.D.; Evans, A.M.; Dai, H.; Lawton, K.A. Software Techniques for Enabling High-Throughput Analysis of Metabolomics Datasets. Available online: http://www.intechopen.com/books/metabolomics/softwaretechniques-for-enabling-high-throughput-analysis-on-metabolomic-datasets (accessed on 6 September 2018).
- Storey, J.D.; Tibshirani, R. Statistical significance for genomewide studies. Proc. Natl. Acad. Sci. USA 2003, 100, 9440–9445. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Maternal and Infant Characteristics | GDM n = 20 | Control n = 20 | p Value |
|---|---|---|---|
| Maternal Age (years, mean ± SD) | 37.4 ± 3.6 | 37.3 ± 2.8 | NS |
| Gestational Age at Amniocentesis (weeks, mean ± SD) | 16.2 ± 0.6 | 16.2 ± 0.5 | NS |
| Gestational Age at Birth (weeks, mean ± SD) | 39.1 ± 1.4 | 38.9 ± 1.6 | NS |
| Birthweight (grams, mean ± SD) | |||
| All Offspring Female Offspring Male Offspring | 3389 ± 526 3079 ± 411 3733 ± 422 | 3419 ± 480 3345 ± 553 3493 ± 410 | 0.83 0.16 0.08 |
| Glucose Metabolism | GDM | ||
|---|---|---|---|
| Control | |||
| All | Female | Male | |
| 1,5-anhydroglucitol (1,5-AG) | 0.73 | 0.86 | 0.61 |
| glucose | 1.19 | 1.30 | 1.07 |
| pyruvate | 0.82 | 0.71 | 1.00 |
| lactate | 1.11 | 0.95 | 1.28 |
| glycerate | 1.10 | 1.36 | 0.92 |
| 2-hydroxybutyrate (AHB) | 1.50 | 1.52 | 1.47 |
| Amino Acid Metabolis | Amino Acid | GDM | ||
|---|---|---|---|---|
| Control | ||||
| All | Female | Male | ||
| Glycine Metabolism | glycine | 0.81 | 0.81 | 0.81 |
| betaine | 0.89 | 0.92 | 0.87 | |
| serine | 0.86 | 1.04 | 0.67 | |
| Glutamate Metabolism | glutamate | 0.92 | 1.02 | 0.84 |
| pyroglutamine | 0.68 | 0.85 | 0.56 | |
| Histidine Metabolism | histidine | 0.84 | 0.88 | 0.79 |
| N-acetyl-1-methylhistidine | 0.86 | 0.7 | 1.03 | |
| Lysine Metabolism | glutarylcarnitine (C5) | 0.83 * | 0.86 | 0.80 |
| 3-methylglutarylcarnitine | 0.36 * | 0.40 | 0.33 | |
| Phenylalanine Metabolism | phenylacetylglutamine | 0.72 * | 0.77 | 0.68 |
| Tyrosine Metabolism | p-cresol sulfate | 0.75 * | 0.79 | 0.71 |
| 3-(3-hydroxyphenyl)- propionate | 1.53 | 0.91 | 2.71 | |
| 3-(4-hydroxyphenyl)- propionate | 1.25 | 0.54 | 5.3 | |
| 3-phenylpropionate- (hydrocinnamate) | 0.48 | 0.39 | 0.59 | |
| p-cresol-glucuronide | 0.61 | 0.49 | 0.75 | |
| Tryptophan Metabolism | indolepropionate | 0.26 | 0.12 | 0.82 |
| N-acetylkynurenine | 0.87 | 0.57 | 1.08 | |
| Leucine Metabolism | 4-methyl-2-oxopentanoate | 1.15 | 1.19 | 1.11 |
| isovalerate | 1.33 | 1.94 | 0.95 | |
| isovalerylglycine | 0.61 | 0.53 | 0.73 | |
| alpha-hydroxyisovalerate | 1.19 | 1.13 | 1.25 | |
| 3-methyl-2-oxovalerate | 1.28 | 1.29 | 1.26 | |
| 2-hydroxy-3-methylvalerate | 1.31 | 1.26 | 1.35 | |
| isobutyrylglycine | 0.59 | 0.54 | 0.67 | |
| Methionine Metabolism | methionine sulfoxide | 1.11 | 1.49 | 0.82 |
| 2-aminobutyrate | 1.14 | 1.26 | 1.01 | |
| 2-hydroxybutyrate (AHB) | 1.5 | 1.52 | 1.47 | |
| cysteine | 0.92 | 0.99 | 0.84 | |
| Arginine Metabolism | arginine | 0.91 | 0.97 | 0.84 |
| urea | 0.81 | 0.81 | 0.81 | |
| ornithine | 0.90 | 0.96 | 0.84 | |
| citrulline | 0.91 | 1.08 | 0.75 | |
| N2,N5-diacetylornithine | 0.36 | 0.44 | 0.28 | |
| N-acetylcitrulline | 0.49 | 0.51 | 0.46 | |
| Polyamine Metabolism | 5-methylthioadenosine (MTA) | 0.83 | 0.93 | 0.74 |
| Gamma-Glutamyl Amino Acid | GDM | ||
|---|---|---|---|
| Control | |||
| All | Female | Male | |
| gamma-glutamylalanine | 0.81 | 0.16 | 1.88 |
| gamma-glutamylglutamate | 0.86 | 0.23 | 1.67 |
| gamma-glutamylglycine | 0.55 | 0.26 | 0.84 |
| gamma-glutamylisoleucine | 0.78 | 0.87 | 0.69 |
| gamma-glutamylleucine | 0.59 | 0.63 | 0.54 |
| gamma-glutamyllysine | 0.51 | 0.18 | 1.29 |
| gamma-glutamylmethionine | 0.79 | 0.19 | 1.75 |
| gamma-glutamylphenylalanine | 0.55 | 0.54 | 0.57 |
| gamma-glutamylthreonine | 0.6 | 0.66 | 0.55 |
| gamma-glutamyltyrosine | 0.5 | 0.48 | 0.53 |
| gamma-glutamylvaline | 0.69 | 0.72 | 0.67 |
| Fatty Acid Subtype | Fatty Acid Species | GDM | ||
|---|---|---|---|---|
| Control | ||||
| All | Female | Male | ||
| Medium Chain Fatty Acids | caproate (6:0) | 0.93 | 1.08 | 0.8 |
| heptanoate (7:0) | 0.99 | 1.06 | 0.94 | |
| caprylate (8:0) | 1.13 | 2.02 | 0.62 | |
| pelargonate (9:0) | 0.94 | 1.09 | 0.81 | |
| caprate (10:0) | 1.04 | 1.08 | 1.01 | |
| 10-undecenoate (11:1n1) | 0.86 | 1.30 | 0.53 | |
| Long Chain Fatty Acids | palmitoleate (16:1n7) | 3.32 | 5.23 | 1.37 |
| 10-heptadecenoate (17:1n7) | 3.44 | 5.81 | 1.14 | |
| stearate (18:0) | 1.19 | 1.4 | 1.02 | |
| arachidate (20:0) | 1.05 | 1.22 | 0.90 | |
| eicosenoate (20:1n9 or 11) | 1.88 | 2.82 | 1.15 | |
| Polyunsaturated Fatty Acids (n3 and n6) | eicosapentaenoate (EPA; 20:5n3) | 1.46 | 1.99 | 1.07 |
| docosapentaenoate (DPA; 22:5n3) | 1.49 | 1.76 | 1.18 | |
| docosahexaenoate (DHA; 22:6n3) | 1.45 | 2.29 | 0.90 | |
| linoleate (18:2n6) | 1.73 | 2.66 | 1.12 | |
| linolenate (alpha or gamma; (18:3n3 or 6)) | 2.05 | 3.47 | 1.22 | |
| dihomo-linolenate (20:3n3 or n6) | 1.97 | 3.1 | 1.30 | |
| arachidonate (20:4n6) | 1.61 | 2.13 | 1.23 | |
| docosapentaenoate (n6 DPA; 22:5n6) | 1.45 | 1.98 | 1.02 | |
| dihomo-linoleate (20:2n6) | 1.26 | 1.52 | 1.08 | |
| Sphingolipid Subtypes | GDM | ||
|---|---|---|---|
| Control | |||
| All | Female | Male | |
| palmitoyl sphingomyelin (C16:0-SM) | 1.21 | 1.39 | 1.05 |
| stearoyl sphingomyelin (C18:0-SM) | 1.21 | 1.51 | 1.00 |
| nervonoyl sphingomyelin (C24:1-SM) | 1.21 | 1.40 | 1.03 |
| palmitoleoyl sphingomyelin (C16:1-SM) | 1.26 | 1.65 | 0.96 |
| Bile Acid Subtype | Bile Acid Species | GDM | ||
|---|---|---|---|---|
| Control | ||||
| All | Female | Male | ||
| Primary Bile Acid Metabolism | cholate | 0.9 | 0.77 | 1.04 |
| glycocholate | 1.31 | 1.47 | 1.01 | |
| taurocholate | 1.02 | 1.23 | 0.76 | |
| chenodeoxycholate | 1.3 | 1.64 | 1.02 | |
| glycochenodeoxycholate | 1.29 | 1.36 | 1.17 | |
| taurochenodeoxycholate | 1.11 | 1.14 | 1.04 | |
| tauro-beta-muricholate | 0.98 | 1.01 | 0.95 | |
| Secondary Bile Acid Metabolism | deoxycholate | 1.03 | 0.99 | 1.08 |
| glycolithocholate sulfate | 0.58 | 0.79 | 0.46 | |
| taurolithocholate 3-sulfate | 0.72 | 1.02 | 0.57 | |
| glycoursodeoxycholate | 0.99 | 0.7 | 3.26 | |
| tauroursodeoxycholate | 0.82 | 1.02 | 0.58 | |
| glycohyocholate | 1.29 | 1.98 | 0.62 | |
| glycocholenate sulfate | 1.06 | 1.47 | 0.83 | |
| taurocholenate sulfate | 0.9 | 1.38 | 0.66 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Neill, K.; Alexander, J.; Azuma, R.; Xiao, R.; Snyder, N.W.; Mesaros, C.A.; Blair, I.A.; Pinney, S.E. Gestational Diabetes Alters the Metabolomic Profile in 2nd Trimester Amniotic Fluid in a Sex-Specific Manner. Int. J. Mol. Sci. 2018, 19, 2696. https://doi.org/10.3390/ijms19092696
O’Neill K, Alexander J, Azuma R, Xiao R, Snyder NW, Mesaros CA, Blair IA, Pinney SE. Gestational Diabetes Alters the Metabolomic Profile in 2nd Trimester Amniotic Fluid in a Sex-Specific Manner. International Journal of Molecular Sciences. 2018; 19(9):2696. https://doi.org/10.3390/ijms19092696
Chicago/Turabian StyleO’Neill, Kathleen, Jacqueline Alexander, Rikka Azuma, Rui Xiao, Nathaniel W. Snyder, Clementina A. Mesaros, Ian A. Blair, and Sara E. Pinney. 2018. "Gestational Diabetes Alters the Metabolomic Profile in 2nd Trimester Amniotic Fluid in a Sex-Specific Manner" International Journal of Molecular Sciences 19, no. 9: 2696. https://doi.org/10.3390/ijms19092696
APA StyleO’Neill, K., Alexander, J., Azuma, R., Xiao, R., Snyder, N. W., Mesaros, C. A., Blair, I. A., & Pinney, S. E. (2018). Gestational Diabetes Alters the Metabolomic Profile in 2nd Trimester Amniotic Fluid in a Sex-Specific Manner. International Journal of Molecular Sciences, 19(9), 2696. https://doi.org/10.3390/ijms19092696